Zoe.Z & Sadie.O
Grade 5
Presentation
No video provided
Hypothesis
Zoe: I think the denser the liquid the higher the bottles will fly. I also keep in mind that the denser the liquid is the heavier the bottle will be so it’s a little bit of a fifty-fifty chance.
Sadie: I agree. I also think that the weather will affect how high the bottle goes along with how the density of the liquid affects how high it goes.
So…If the liquid is denser then the liquid will go higher because there’s more potential energy in a denser substance.
Research
Water rockets:
- A water rocket is a type of model rocket using water as its reaction mass. The water is forced out by a pressurized gas, typically compressed air. Like all rockets, it operates on the principle of Newton's third law of motion. Newton's third law of motion states that every action has an equal and opposite reaction. By creating a controlled explosion, bottle rockets make a powerful thrust that propels them into the air.
- Liquid is an essential ingredient in bottle rockets. It serves as a reaction mass, providing the necessary weight and stability during the initial launch. As the water is expelled, the rocket becomes lighter, allowing it to gain altitude.
- Peak launch velocities can easily reach 30 meters per second (60 miles per hour), and sometimes it's possible for a rocket to reach heights in excess of 30 m.
Density:
Here is a photo that shows corn syrup, water, and oil. The volumes were all the same. You can see which liquid is the most heavy/light based on what floats to the top.
Variables
Our variables are type of liquids, volume of liquids, airtime, and pressure. The liquids are stated below. The manipulated variable is the type of liquid and its density. The responding variable was the airtime. The controlled variables were the volume of the liquids, which was 100 mL for each trial, and the air pressure which was 100 PSI for each trial. Our gas used for the pressure in the launching process was air because it was the easiest material to use and it was the most efficient way of launching particularly because it's wherever you go and easy to find as well as use.
Procedure
MATERIALS FOR ROCKET
- Liquids (milk, water, corn syrup, rubbing alcohol, coke, canola oil)
- Soda bottles (2 liter)
- Strong plastic
- Bicycle pump
- String
MATERIALS FOR LAUNCHER
https://www.youtube.com/watch?v=hJf7DHApz2Y
- PVC Pipe cement.
- A tire schrader valve.
- A 1/2" PVC Slip to Slip Elbow.
- A 3/4" Female Thread to Slip adapter.
- A Hose quick connector with male threads.
- A 1/2" slip to 3/4" slip adapter.
- A 1/2" female threaded cap.
- A 1/2" make thread to 1/2" slip adapter.
- Teflon thread tape
- Electrical Tape.
- About 18" of 1/2" PVC pipe.
- Some scrap wood.
- Two screw eyelets.
- Two U bolts.
- Thin rope
PROCEDURE PART 1 (BUILDING THE ROCKET)
- Find a bottle. This will be your rocket
- Measure out 100 mL and pour into rocket
- Attach water rocket nozzles
PROCEDURE PART 2 (BUILDING THE LAUNCHER)
- Begin by cutting two pieces of PVC pipe. One pipe should be about 10 inches’-12’ inches long and the other pipe should be about 2’-3’ long.
- Assemble the PVC pipes and fittings together.
- Mount the schrader valve so you have a place to connect the air supply to the launcher. Take the ½ inch female threaded cap and drill an 8-10 millimeter hole through the center of the end. When installing the schrader valve, take a rod or a Phillips screwdriver and push it into the hole at the bottom of the schrader valve. Push it hard from inside until it snaps in place. Then, screw the cap onto the launcher.
- Use the two U-bolts to secure the PVC pipes of the launcher to the base. You can use a piece of scrap lumber for the base, or you can build a more elaborate launcher base from wood or other materials.
- Put a screw eye on either side of the gardena release mechanism to contain and direct the release string
- Use electrical tape to secure a length of string to either side of the release mechanism and through each screw eye. You can tie the remaining string to the release string to extend it so you can launch the rocket from a safe distance.
PROCEDURE PART 3 (LAUNCHING THE ROCKET)
1. Measure out 100 mL of the liquid and pour into the rocket
2. Attach rocket to apparatus
3. Pump till bubbles appear in liquid (min 80 psi)
4. Lay pump down and clear the area for launch
Observations
-Filling the bottles and attaching then to the launcher
It was difficult to get all of the liquid in the bottle without a little spillage because sometimes the bottle that we were pouring from had a bigger opening than the bottle we were pouring the liquid into. It was also difficult to see whether the bottle was properly attached or not.
-Pressurizing the liquid
We had a few leaks even from the launcher that we made ourselves, so we had to do a few retries on some of the liquids. We also noticed that some liquids bubbled more than others did even though they got the same amount of pressure. This was due to the air surfacing through the water creating bubbles.
-Launching the rockets and measuring height
We tried measuring the height with string but it didn't work because the string kept getting tangled so we decided to measure the height using a technique called proportional reasoning. That didn't work because with some of the videos we took, the rocket went off the screen. So that's when we decided to do airtime.
Analysis
COLLECTING THE DATA
We collected the data by timing from launch to landing as airtime. Our previous method was using the altimeter (which would have been more accurate) but due to the fact that the altimeter didn’t work, we had to use other methods. After the Proportional Reasoning equation proved unsuccessful, (because on some of the videos that we filmed, the bottle went higher then the camera was directed and while watching was off screen) we decided to use airtime instead. We counted the seconds that the bottle was in the air on camera and that gave us our answer.
Factors |
WATER |
CORN SYRUP |
COKE |
CANOLA OIL |
RUBBINGALCOHOL |
MILK |
Density(g/mL) |
1g/mL |
1.4g/mL |
1.042g/mL |
0.909g/mL |
0.785g/mL |
1.03g/mL |
|
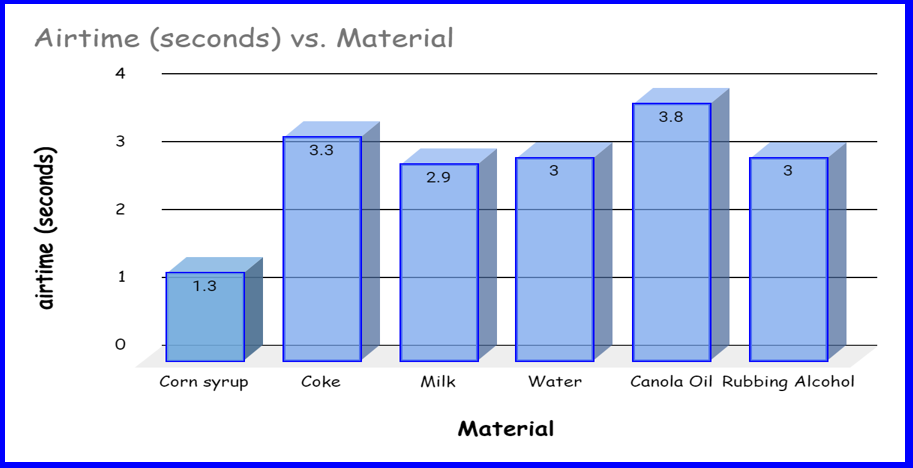
Airtime (in seconds) |
3.0sec |
1.3sec |
3.3 sec |
3.8sec |
3.0sec |
2.9 sec |
|
PSI |
100 PSI |
100 PSI |
100 PSI |
100 PSI |
100 PSI | 100 PSI |
|
Volume of liquid per trial (mL) |
100mL |
100mL |
100mL |
100mL |
100mL |
100mL |
Explaining factors
-Density is a comparison between the mass and volume of a substance.
-Airtime means how long the rocket stays in the air.
-PSI means pounds per square inch, which is used to measure the pressure
-mL stands for milliliters, which is used to measure the volume of the liquids
-Viscosity is how fast/slow something pours
-cP stands for centipoise, and is used to
measure viscosity
ANALYSIS
●Corn syrup is significantly more dense than all the others and the airtime was a lot less. This is likely because the airtime and the density could be related.
●Water, coke and milk all had similar densities (since they are all water based), and all the airtimes were similar.
●Canola oil and rubbing alcohol both have densities less than water. The airtimes were not what we expected. This might be because they are not made of water at all.
●Heavy liquids aren't a good rocket fuel as they would just weigh the rocket down so it can’t fly.
We came to this analysis because we took the data from the chart and graph and made a hypothesis. Our hypothesis is no longer based on density but on viscosity. As we know, viscosity is how fast or slow something pours. Items with a lower viscosity flow better and fly higher. An interesting factor is that although the airtime and densities are similar with rubbing alcohol and canola oil, the viscosity is very different.
Conclusion
We know density is a comparison between the mass and volume of a substance. So if we keep the volume the same for each substance so that we can compare their masses. The heavier the substance for a set volume, the higher the density. Corn syrup is significantly more dense than all the other liquids that we tested due to its composition and the process by which it is made. This is due to density. Water, coke and milk all had similar densities (since they are all water based), and all the air times were similar because their densities are similar. Our hypothesis was incorrect because the liquids with less density flew higher. We think this is because the liquid was light and that helped it fly.
Application
We want to find out how different densities of different liquids affect how high a bottle rocket will go. We will see if the substance that is in the air for the longest (Canola Oil) can be a future fuel for humankind. People can use Canola Oil for things like fueling your car, heating, and cooling. One thing about Canola Oil is that it is reusable, recyclable, and renewable which makes it a flexible liquid.
Sources Of Error
Problems with measuring height
-The Altimeter
The altimeter that we were going to use to measure the height did not work, we set it to 0' and it came down at 0’. So then we decided to record ourselves. It’s easy to access and to see visually or time if we need to.
-The measuring string
The string method that we used to measure the height of the rocket did not work because the string kept getting tangled and was not an efficient way to do it. Then we decided to use the proportional reasoning technique because it was a simple and efficient way to measure height.
Problems with the launching
-The Quest water rocket kit
This kit was an absolute failure for many reasons. It was low quality and did not have the structural support to be able to launch rockets. The support legs kept breaking, the latch that let the bottle rocket go also kept breaking, the string was not thick enough and kept snapping, the wings (that were not useful anyway) kept flying off when we launched the rocket, we had many leaking issues and the bottles kept losing air. So we decided to make our own launchers and bottle designs because it was more cost efficient.
-Proportional reasoning technique
We used this technique to try and measure the height by watching the videos but on some of the videos the bottle rocket went higher than the camera was filming. So we decided to measure by air time so that we didn't have to see the rockets on the screen.
Citations
1. Water rocket booklet. (n.d.). https://www.npl.co.uk/skills-learning/outreach/water-rockets/wr_booklet_print.pdf
2. Viscosity Chart .https://www.dixonvalve.com/sites/default/files/product/files/brochures-literature/viscosity%20chart.pdf
3. Isopropanol Alcohol Solvent Properties https://macro.lsu.edu/howto/solvents/ipa.htm
4.Product Viscosity Chart https://prosysfill.com/product-viscosity-chart/
5 BlackCat Fireworks - What Are Bottle Rockets? https://blackcatfireworks.com/what-are-bottle-rockets/#:~:text=Chinese%20pyrotechnicians%20developed%20bottle%20rockets,the%20air%20attached%20to%20arrows
6. KiwiCo https://www.kiwico.com/blog/the-science-behind/the-science-behindbottle-rockets
7. Denseme https://denseme.com/density-of-corn-syrup
8. Department of Chemistry
https://chem.washington.edu/lecture-demos/density-column-coke-and-diet-coke
9. Material Match Search Platform https://matmatch.com/learn/property/density-of-milk-weight-per-gallon
10. US Geological Survey Water Density https://www.usgs.gov/special-topics/water-science-school/science/water-density
11. Densme https://denseme.com/density-of-vegetable-oil/
12. Denseme https://denseme.com/density-of-rubbing-alcohol
Acknowledgement
-Jason Zalmanowitz (Zoe’s dad)
Thank you for guiding us through this project specifically with science.
-Lisa Yellin (Zoe’s mom)
Thank you for being there to help us with questions and answers.
-Robyn Oppenheim (Sadie’s mom)
Thank you for guiding us as far as math is concerned.
-Joe Oppenheim (Sadie’s dad)
Thank you for making us laugh along our journey.
-Mr E. Gelman
Thank you for supporting and drawing out the guidelines of this project.