Microbial Fuel Cells - Harvesting clean energy from the ground?
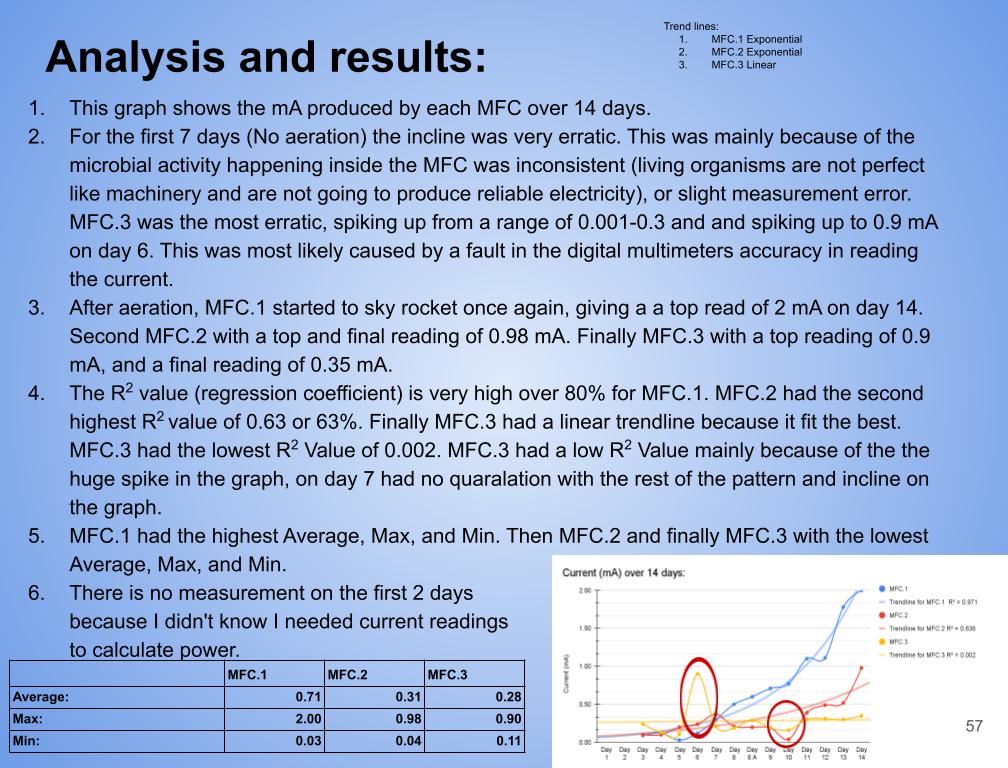
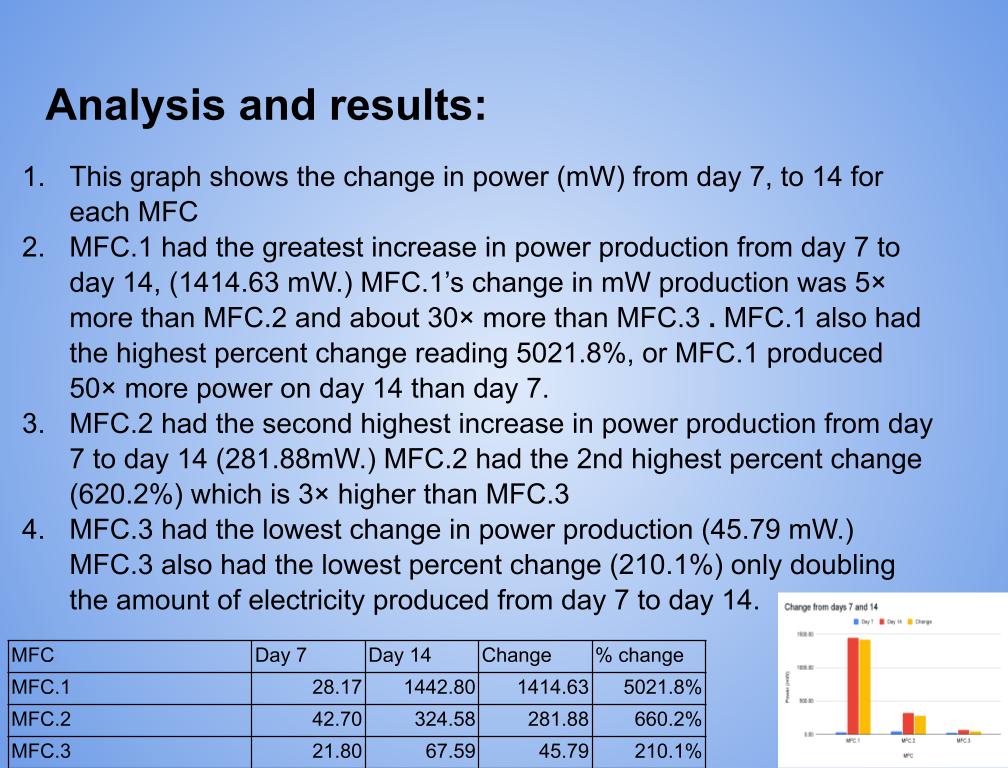
Grade 8
Presentation
Hypothesis
Main Question?
Can microbial fuel cells produce renewable electricity from mud/soil samples?
Sub questions:
How much electricity can be generated from a microbial fuel cell?
Which substrates help with producing the most electricity?
Can aeration to a cathode chamber help with electricity production in an microbial fuel cell?
Hypothesis 1.0
If three substrates are put in a MFC to produce electricity; and bacteria are given a chance to break down the substrate, then the Compost soil will produce the most electricity from the MFC because, the compost soil will attract more bacteria for a number of reasons.
- First compost provides a great abundance of organic matter and nutrients for the bacteria to feast on.
- Second as compost biodegrades, it generates heat. This temperature increase will be beneficial for bacteria to live in.
- Last, compost is usually created from organic manner. The majority of the moisture from all the organic matter improves living conditions for the bacteria.
Also the topsoil and benthic mud samples may not have as much of organic matter as the soil because soil has other components in it such as minerals. Compost on the other hand consists of 70% and more organic matter. While it is uncertain with the 2 other soils.
_____________________________________________________________________________________________________________
Hypothesis 2.0
If a microbial fuel cell is built and is aerated for 7 days, and not aerated for another 7 days, then the time period where the microbial fuel cell is aerated will produce more electricity than the non aerated time period because, when the cathode chamber is aerated, the oxygen availability increases. This will then enhance the amounts reactions at the cathode. In MFCs, electrons flow from the anode to the cathode, oxygen acts as the final electron acceptor. Higher oxygen levels at the cathode chamber lead to a more efficient reduction of oxygen to water, thus producing more electricity.
Research
Vocabulary
- Catalyst: A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed or changed during the reaction³.
- ‘Bio’catalyst: A substance, such as an enzyme or hormone, that initiates or increases the rate of a chemical reaction.
- Salt bridge: (PEM)-[Proton exchange membrane] It is a tube filled with an electrolyte/conductive solution. The purpose of the salt bridge is to keep the chambers of an MFC electrically neutral and allow the free flow of ions to exchange with 2 or (More) cells. If there is no salt bridge, positive and negative charges will build up around the electrodes causing the reaction to stop.
- Electrodes: An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (Sometimes power source).
- Oxidation: A chemical reaction that takes place when a substance comes into contact with oxygen or another oxidizing substance. (Ex. Rust)7
- Electrochemical cell: An electrochemical cell is a device that either generates electricity from chemical reactions or uses electricity to cause chemical reactions.9
- Bacteria: Single celled microorganisms that are found almost everywhere on earth; like the soil, water, and even the human body.
- Conductivity: The measure of the ease at which an electric charge or heat can pass through a material.
- Biofilm: A community of living microorganisms embedded in a slimy or protective substance that provides protection against external aggressors or predators, like antibiotics, or disinfectants, as well as a host's immune system
- Electrogen: Special family of bacteria that decompose organic matter in anaerobic conditions and can break down the matter into electrons and protons (etc.)
Background Research
What is a Microbial Fuel cell?A microbial fuel cell (MFC) is a bio-electrochemical system (or a fuel cell) that converts biochemical energy to electrical energy through reactions catalyzed by microorganisms under anaerobic conditions.¹
What are the main components of soil?
Soil is a material made of five ingredients. These are minerals, soil organic matter, living organisms, gas, and water.⁴
Who invented microbial fuel cells?
The idea of using microbes to produce electricity was created in the early 20th century. Michael Cressé Potter investigated the subject in 1911. But his findings received very little attention.
What is an anode and a cathode?
A cathode and an anode are the two electrodes found in a battery or an electrochemical cell, which help the flow of electric charge. A cathode is the positive electrode, where reduction (gain of electrons) occurs. An anode is the negative electrode, where oxidation (loss of electrons) takes place.6
What is a Double Chamber MFC?
A double-chamber MFC consists of an anode and cathode compartment separated by a proton exchange membrane (PEM) to avoid electron movement between the compartments.
What is a Single Chamber MFC?
In single compartment microbial fuel cells (SCMFC), there is only one anode chamber in which wastewater to be treated is placed along with the microbial catalysts*.
* A process of utilizing microbes for CO2 transformation to organic molecules along with electrons that are obtained from cathode.
What things can power a microbial fuel cell?
- Organic substrates: Wastewater, food scraps, agricultural waste, and complex and simple organic sugars (e.g. cellulose/glucose).
- Sunlight: In photosynthetic MFCs, where algae or cyanobacteria* generate organic matter through photosynthesis.
- Urine: Organic compounds in human or animal urine.
- Sediment: Natural organic matter in marine, river, or lake sediments.
- Wastewater: Organic pollutants in wastewater from households, industries, or agriculture.
The above are only a few examples.
*Cyanobacteria are a type of bacteria that can perform photosynthesis, just like plants.
What is ion exchange?
The exchange of ions of the same charge between an insoluble solid and a solution in contact with it.12
What is a polysaccharide?
A polysaccharide is a long chain of molecules (usually carbohydrates) that are followed by the smaller molecules that are called monosaccharides. Polysaccharides are usually insoluble in water and will make colloidal suspensions.14
What are monomers?
Monomers are atoms or small molecules that bond together to form more complex structures such as polymers.
What is the chemical compound for agar?
Agar is a mixture of polysaccharides, typically extracted from the cell walls of red algae. They are composed of agarose and agaropectin. Agarose is made up of d-galactose and 3,6 anhydro-l-galactose units. These are 2 types of sugar molecules (monomers) that form agarose. D-galactose is a simple sugar form, while Anhydro-L-Galactose is a certain form of galactose. The 3-6 means the 3rd and 6th carbon atoms in the galactose molecules. 16, 15
What is the chemical compound for agar? (P.T 2)
G-Galactose and 3,6 anhydro-l-galactose are connected by alternate α-(1,3) and β-(1,4) glycosidic bonds. (This describes the type and pattern of chemical bonds connecting sugar molecules together. A glycosidic bond is a type of bond that links a sugar molecules to other molecules) Agarose is the gelling part of agars chemical composition and makes up 85% of agar. While agrospectin provides viscous properties. Agrospectin is made of D-Galactose and L-galactose (Basic sugar molecules that make the majority of Agaropectin), Sulfate groups (they contain sulfate esters* that give some gelling capabilities), and D-gluconic acid.
The molecular formula for agar is C14H24O9.15, 16 ,17
What allows Agar to be a proton exchange membrane?
Many reasons allow agar to be a proton exchange membrane, including:
Proton conductivity: Agarose, the main component of agar has hydroxyl groups (-OH) that help with the flow of protons/H+
Chemical stability: Agar is chemically stable and can handle acidic and basic conditions found in MFCs. Also it's tough structure protects OH groups from splitting apart.22
Biocompatibility: Agar is normally extracted from red algae, making it suitable for biological systems like MFCs 20
Cons* Agar is a gel at room temperature, remaining firm at temperature as high as 65°C. Agar melts at approximately 85°C, a different temperature from that at which it solidifies, 32-40°C. Agar thickens harder the cold it gets.21
Where do bacteria come from in an MFC?
Many types of bacteria are found in benthic areas. When these bacteria eat, their by products can be Carbon, Hydrogen+, and electrons. When a soil sample is put into an anode chamber/or a anaerobic environment, this allows the bacteria to form a bigger colony. This increases the production for the Carbon, Hydrogen+, and electrons.
What are some species of electrogens?
Some species are:
- Escherichia coli
- Shewanella
- Enterococcus faecalis
- Rhodoferax ferrireducens
- Saccharomyces cerevisiae
Are Electrons present in compost?
Compost create a rich habitat for electrogenic bacteria because they provide the bacteria with plentiful amounts of organic matter for the bacteria to consume. Many studies have shown that compost substrate will increase the by products of electrogens.
How can activated charcoal collect electrons?
Activated charcoal/activated carbon can attract/collect electrons for 2 reasons:
- Graphite microcrystals: Within Activated charcoal, there are regions of graphitic microcrystals. These crystals have a structure similar to graphite, where carbon atoms are arranged in in hexagonal layers. In these hexagonal layers, some electrons are delocalized (Allow free flowing electrons)
- When Activated charcoal is “activated” there are many pours on the carbon structure. This is the biggest reason why they attract electrons.
- Chemical structure: When Activated Charcoal is “activated” (activation is when the carbon structure is put through a chemical process) the activation bonds hydroxyl, carboxyl, and other oxygen groups to the carbon. These groups can interact with electrons, enhancing the carbon's ability to hold electrons 24,25
What are the components of a microbial fuel cell?
- Anode compartment: The compartment where organic substrates are introduced, and the bacteria act as the biocatalysts.
- Cathode compartment: The compartment where the positively charged hydrogen ions and protons attach themselves with oxygen to create water.
- Salt bridge (Proton exchange membrane): A semipermeable membrane that permits a certain amount of protons, from the anode to the cathode compartment.
- Electrodes: anode and cathode, which help with the transfer of electrons (Creating electricity)
- External circuit: This includes wires connecting to both electrodes and a battery (Electrical storage) or a digital multimeter (Measure voltage and current)
How does a double chamber microbial fuel cell work?
First, organic substrates are introduced into the anaerobic anode compartment, where bacteria colonize the anode and substrate. This forms a biofilm and acts as a biocatalyst. Then the bacteria consume and metabolize the organic substrates by anaerobic respiration and decomposition. The bacteria decomposes complex organic molecules (EX. sugars) into simpler compounds (products), mainly carbon, electrons and hydrogen ions+. The electrons present in the chamber are attracted to the anode because of pores and a conductive material. The electrons then go through the external circuit (wires). Meanwhile on the other side, the hydrogen ions+ (or protons) are forced in the opposite direction (because they are the opposite force) through the PEM. The PEM is made of a neutral and conductive substance so the hydrogen ions+ flow through the PEM and go to the cathode compartment. In this compartment the hydrogen ions+ bond with oxygen to create water. This then completes the reaction. The flow of electrons through the external circuit influences the current which can be used to calculate electricity.
How to calculate power?
To calculate the power of a microbial fuel cell, It is needed for 2 things:
The formula for power is, Power = Current (I) x Voltage (V)
Current is Joules per second or Amps. This means the flow of electricity.
Voltage is the measure of difference in electric potential energy, between 2 points of a circuit.26
Variables
Variables:
Manipulated: Different types of soil/mud, and building the microbial fuel cell
Responding: Amount of electricity produced
Controlled: The control in this experiment was topsoil (referred to as MFC.3 throughout the experiment).
Benthic mud, and compost are rich in aquatic and decomposed nutrients. This is because benthic mud contains rich sediment carried from the river bed, and is eroded into fine minerals because the water hits the benthic mud (thus eroding it). Compost is made of decomposed organic matter which is high in nutrients.
Topsoil on the other hand, has a less nutrient high composition and provides moderate minerals and organic matter (etc.) so it serves as a baseline. Also topsoil is a generic, and most basic soil that is found everywhere.
Constants: same benthic mud, same benthic mud source same anode and cathode material (activated carbon), Same Digital multimeter, Same environment of experiment, type of microorganism/bacteria, distance between electrodes, same PEM membrane.
Why have a control?
Control variables are needed to ensure the reliability and what stays the same in an experiment.
Controlled variables are important because you can compare them to other trials, when a control variable is implemented then it becomes easier to compare other trials to the control group. Then I can see the (mathematical) difference between different trials, which in short can help draw conclusions.
Procedure
MATERIALS & PROCEDURE:
MATERIALS:
Chambers
- Compression fitting
- Sandpaper, medium-grit
- Permanent marker
- Ruler
- Lab notebook
- Containers (Acrylic or plastic)(3.2L)(6)
- Safety goggles
- Drill or drill press with 3/4-in. spade drill bit, 2-millimeter (mm) drill bit
- Adhesive (like acrylic cement or DevCon Plastic Welder)
- Paper towel
- Popsicle sticks, or any unwanted skinny stick.
Making the Electrodes
- Activated charcoal
- Copper mesh
- Wood
- Monkey wrench
- Wire strippers
- Nickel epoxy or other conductive epoxy
- Copper wire, 12-gauge (12 pieces, 18 in. each)
- Digital multimeter.
- Electrical tape
PEM
- Baking tray
- Plastic wrap
- Aluminum foil
- Measuring cup
- Tap water
- Pot
- Glass rod
- Spoon
- Digital kitchen scale
- Agar, 30 g
- Table salt, 6 g
Getting the benthic mud samples
- Buckets (3)
- Plastic wrap
- Shovel
- Hoe
- Pitch/Fork
Assembling the Fuel Cell
- Measuring cup
- Large bowl
- 72 tbsp of salt
- Spoon
- Aquarium air pump/aerator with tubing
- Safety goggles
- Mud sample
Testing the MFC
- Alligator clip cables 6
PROCEDURE:
Making the electrodes:
- With scissors, cut the copper mesh into 6 rectangles with the dimensions of 6 cm x 18cm. Fold these into 6cm x 6cm squares into 3rds.
- Cut 6 strips of wire with lengths of around 1 ft ½ and strip 6in of insulator on one side, and 1-2 cm on the other.
- Layer Activated charcoal, Nickel epoxy, and copper mesh into this specific pattern: Copper mesh, epoxy, and then charcoal (E.t.c). Do this quickly and push down onto the electrode.
- Before pushing on the electrodes, put a wire in one of the folds with the 6in side, layer with as much epoxy as needed.
- After completing steps 1 and 2, put the electrode on top of a wooden piece (Chunk of a 2x4 or scrap wood).
- Next, add another nearly identical piece of wood and place it on top of the electrode. Place a monkey wrench onto the 2 wooden pieces and tighten as much as possible.
- Leave this for 6-12 hrs
- After 6-12 min, take the monkey wrench off. (Note* if the electrode layers are not stuck together, try the process again, only adding a little more glue)
- When the electrode is in one assembly, take each of the four pieces of copper wire and with the wire strippers, strip off 6 inches of the insulator on one end of each piece.
- Repeat steps above as much as possible until there are 6 electrodes.
Making the Salt Bridges
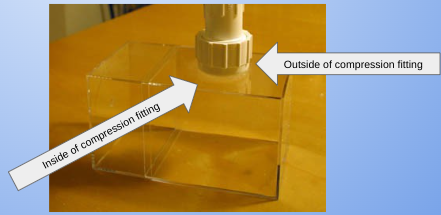
- Place and stand up vertically all 3 compression fittings on a baking sheet, put aluminum foil around one side of the compression fitting tightly so no liquid can escape the tube. Repeat this to all compression fittings.
- Measure 300 milliliters (mL) of water and pour it into the pot.
- Using the scale, measure out 30 g of agar. Set the measured agar aside. Now measure out 6 g of salt.
- Place the pot of water on the stove and bring it to a boil. When the water is boiling, add the agar and stir it with the glass rod until it is dissolved.
- Once the agar is dissolved, take the pot off of the heat and add 6 g of salt. Stir with a spoon until the salt is dissolved.
- While the solution is still hot, carefully pour the solution into the tubes/compression fitting. If the tubes leak, or tighten, remove the foil and refill them.
- Once the tubes are filled and stable (i.e. haven't fallen over, leaked liquid, etc.) for 10 minutes, carefully move the backing sheet to the refrigerator. Let the tubes sit in the refrigerator overnight. These tubes are the salt bridges.
- The next day, come back and place the salt bridges into a plastic baggie and seal it. This prevents the salt bridges from drying out. They should be a firm, jelly-like substance but not completely solid.
- Take the bridges out when is is ready to be used.
Building the anode & cathode chambers:
- Unscrew the two ends of the compression fitting and throw out the rubber fitting and discard end caps. Using sandpaper, roughen the endcaps of the compression fitting.
- Take the sandpaper and roughen two opposite sides of two of the plastic containers. (Roughen two patches the circumference of the compression fitting, across from each other.)
- Using the permanent marker, make a mark in the center of the roughened side of one of the plastic containers. Also create 2 lines crossing through exactly half of the container (vertically, and horizontally)
- With a sharpie, place the center compression fitting on the center of the mark, and trace a circle around the compression fitting.
- Measure the location of where the circles are on the container (EX. the circle is 6 cm above the bottom of container, and 4 cm left of the center line)
- Apply all markings to all containers.
- Put on the safety goggles. Drill a hole 8-2 millimeters in diameter on top of two of the plastic container lids.
- Using the drill, drill a hole on the permanent marker marks on the sides on all
- containers. (Note: Drill slowly or the acrylic might crack. Brush off any plastic debris. There should now have two plastic containers that each have a hole in one side.)
P.T 2
- Screw in compression fitting into 2 containers so that there is a tiny bit of room to squeeze adhesive on both sides of the container. Squeeze epoxy around out/inner edges of the container that are around compression fitting, apply with a stick (popsicle stick) around the part of the compression fitting that is out/in of the container.
- After 2 hours repeat step one, only with acrylic cement. Apply the acrylic cement with fingers to close any gaps more precisely. Repeat this process until all microbial fuel cells are made. Leave for another 2 hours.
- After the time has passed, check to see if the two joints are watertight. Fill the containers with water past the holes/joints. Wait for 5 minutes. If there is no water leaking out, then proceed to the next section.
- If there is excess water coming out of a joint, empty the containers and dry them off completely with paper towels. Carefully squeeze acrylic cement around the endcap joint that leaked. Squeeze out enough cement that a seal is make. Wait for 10 hours and retest the watertightness. Try again if this doesn't work. (If it still doesn't work, remake the assembly with fresh parts.)
Obtaining the Benthic Mud Samples/Substrate
- Go to the location of your stream where there is a rich patch of mud.
- Fill buckets (with shovel hoe, and pitch fork), and make sure to get enough of the benthic sample to fill the anode chamber.
*NOTE: if there are lots of rocks or grass use the pitch fork to separate them.
- Collect some of the stream water and put it into the bucket (Only if mud/soil is dry)
- (repeat steps 1 -3 to only collect topsoil)
- Collect some compost soil near available sources
- After all samples are collected and put into buckets, then put plastic wrap onto them (so they don't dry out.)
Assembling the Microbial Fuel Cells
- Make a conductive salt solution using the water samples. Measure out 12 cups of tap water into the large bowl. Add 6 Tbsp. of salt to the bowl and stir with a spoon until the salt has been dissolved. Fill the cathode chambers of the three microbial fuel cells with the salt solution. (Add more solution according to the size of containers.
- Take an electrode (this will be a cathode) and thread it through the smaller hole of one of the lids with two
- holes. Place the lid with the holes and the connected cathode back onto the cathode chamber. Make sure the electrode is submerged. Repeat this step with another electrode and the other lids with the holes. Seal each cathode chamber with a lid.
- Connect the tubing to the aquarium pump. Push the tubing into the cathode chambers under the lid. Be sure to submerge the end of the tubing under water. (NOTE* only do this step after 7 days of observations)
- Now, wearing gloves and safety goggles, fill half of the anode chamber of a microbial fuel cell with the benthic mud sample. Make sure that there are no bubbles in the mud. Push the mud sample down or gently tap to remove any bubbles. Take one of the electrodes (this will be an anode) and bury it in the mud. Then place more of the mud into the anode chamber, covering the anode. Push the free end of the electrode copper wire into the 2-mm holes in the container lids. Replace the lid onto the container to make sure that the electrode is hanging freely without hitting any of the walls or the bottom.
- Repeat filling the anode chamber with the benthic mud sample and inserting the anode with the other two microbial fuel cells by repeating step 5 for the two microbial fuel cells.
- The microbial fuel cells are now complete and you should have three MFCs in total.
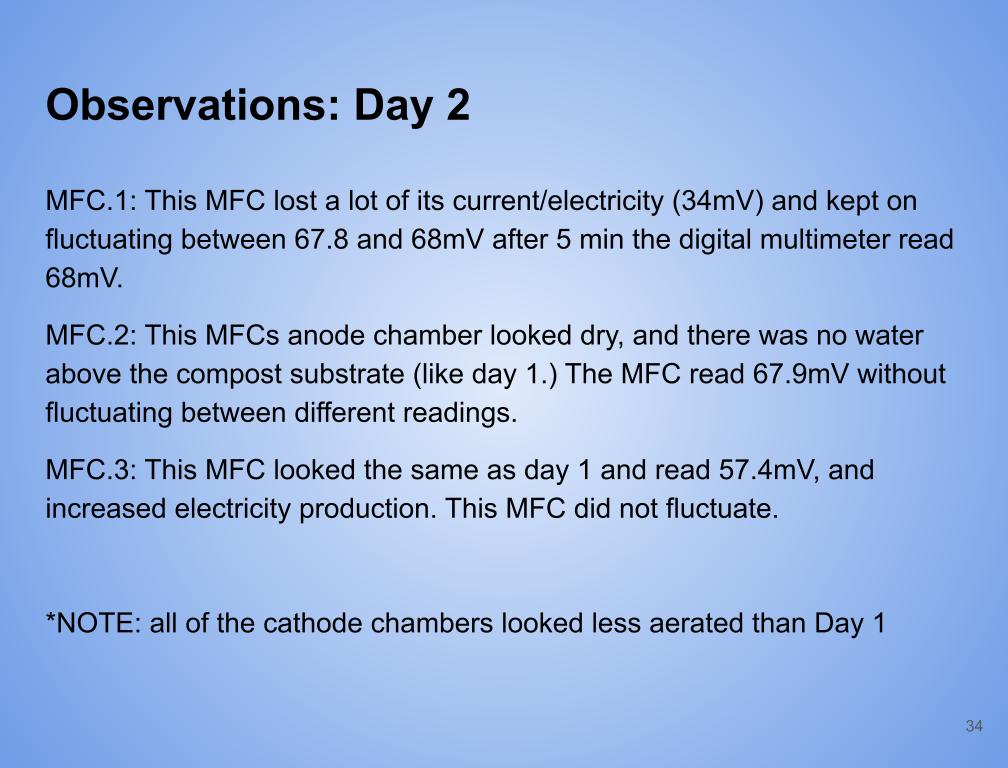
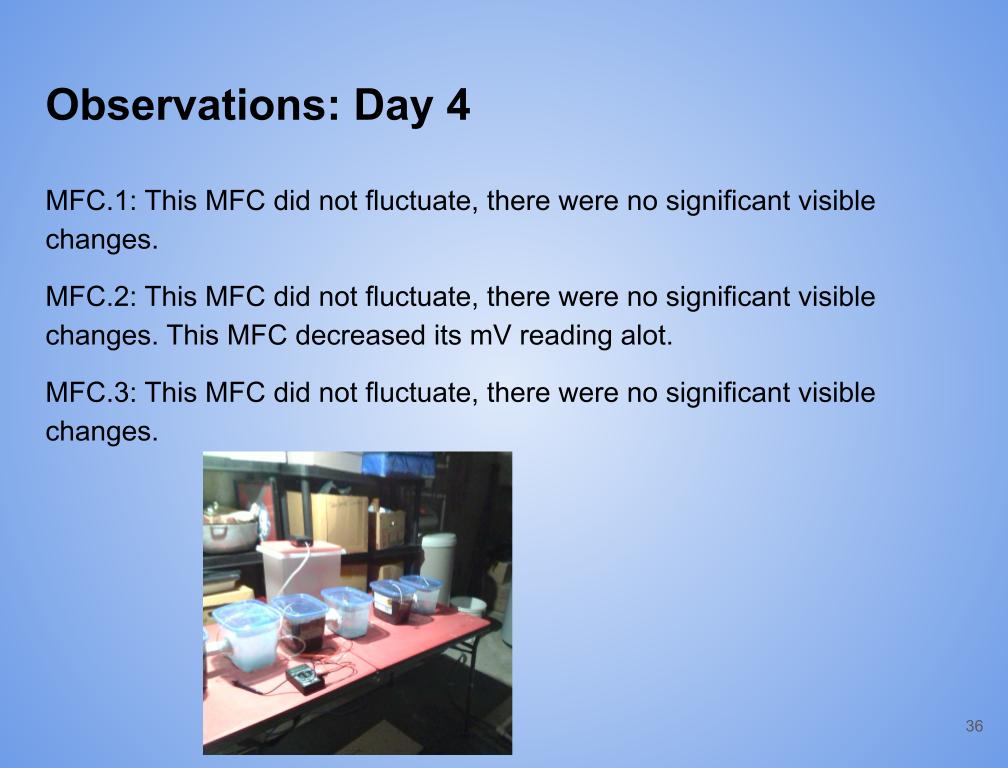
Observations















Analysis
Analysis plan:
- First it was needed to enter 2 different readings of data, voltage and current into the spreadsheet.
Voltage:
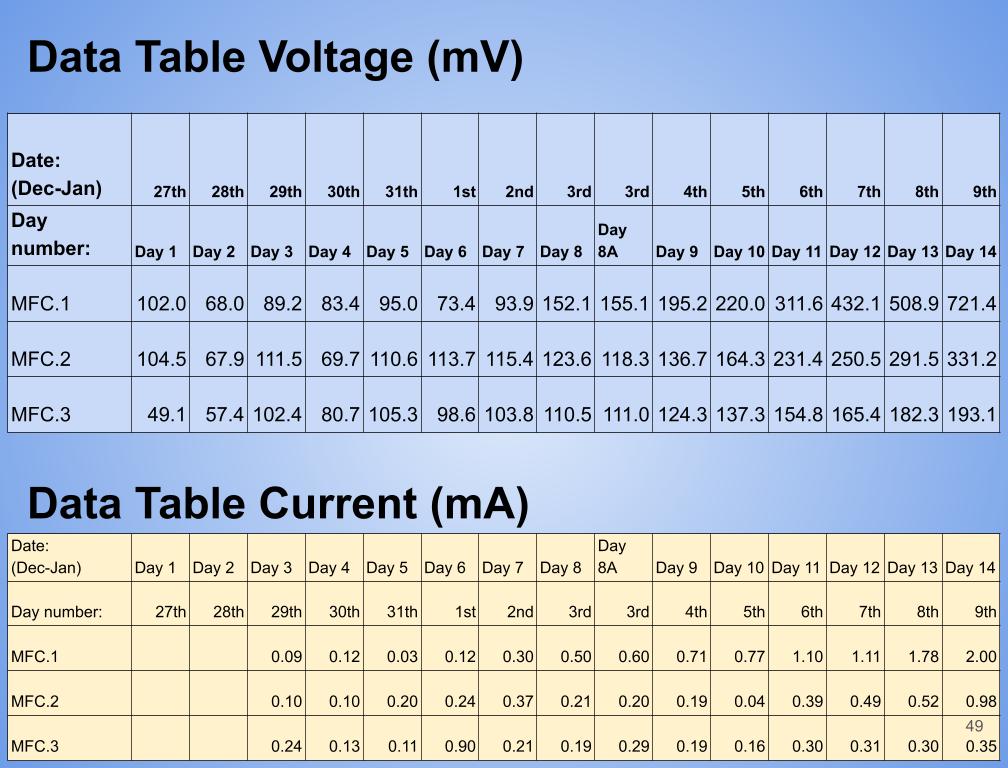
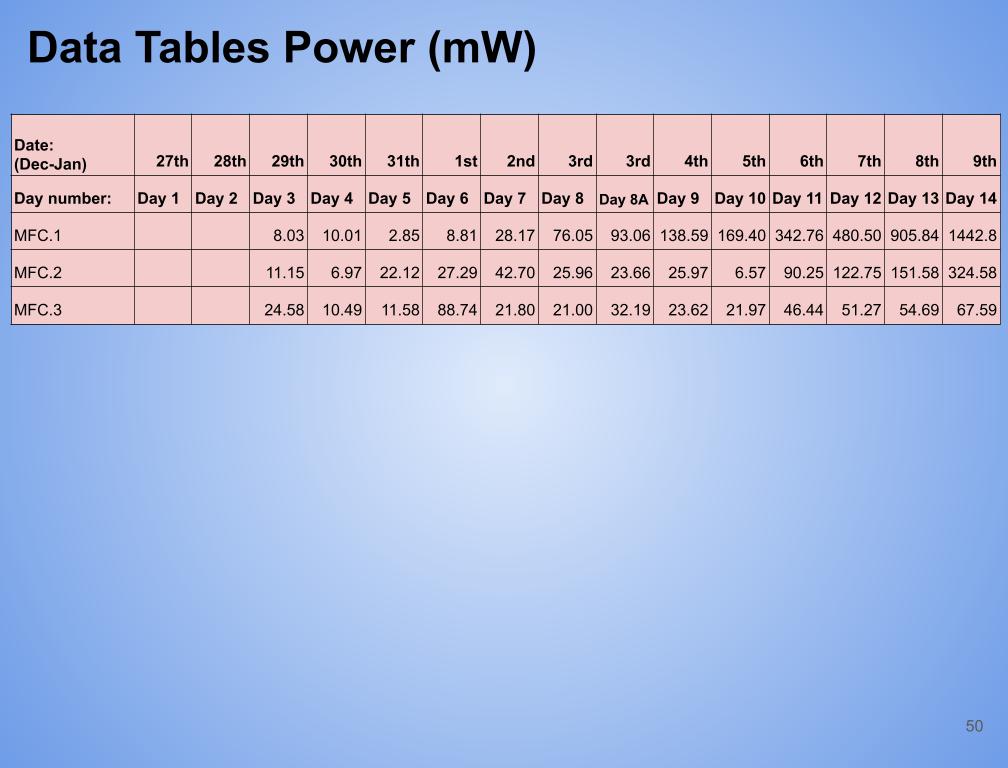
- Created a table with daily voltage readings (mV) for each MFC for days 1-14.
- Then calculated the average voltage for each set of data for an MFC over 14 days.
- Determined the maximum and minimum readings for each MFC for days 1 to 14.
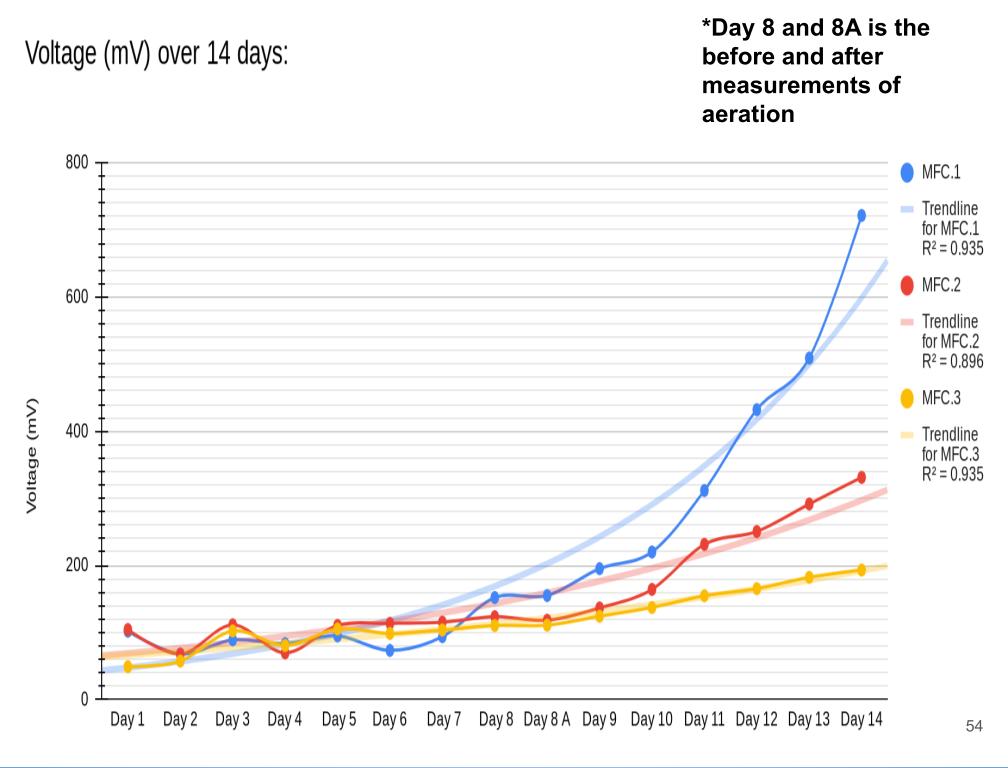
- Created a graph with the data collected above (Voltage vs Time)
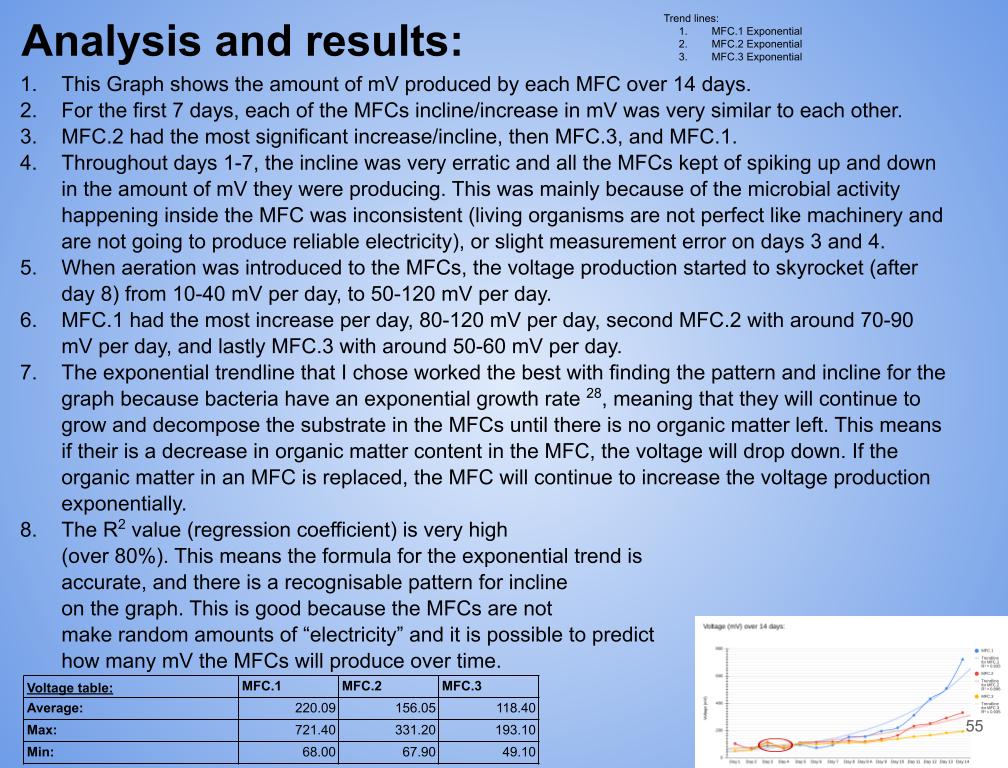
- Plotted the most accurate trendline (I experimented with all the different types, and settled on exponential*)
Current:
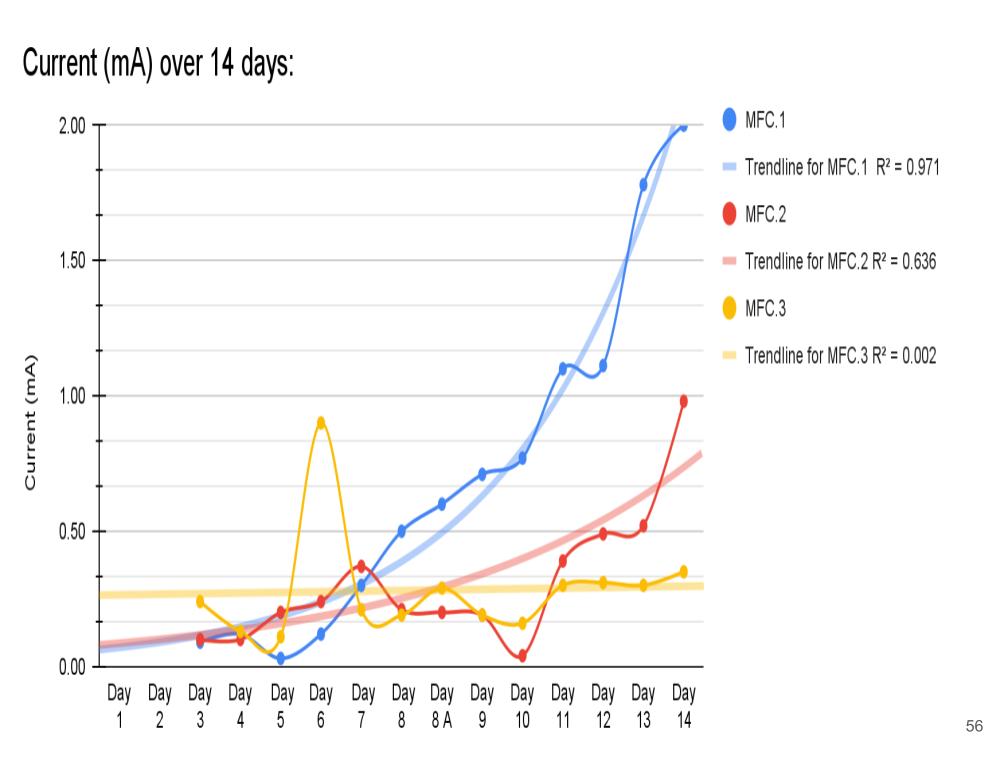
- Created a table with daily current readings (mA) for each MFC for days 1-14.
- Then calculated the average current for each set of data for an MFC over 14 days.
- Determined the maximum and minimum readings for each MFC for days 1 to 14.
- Created a graph with the data collected above (Current vs Time)
- Plotted the most accurate trendline (I experimented with all the different types, and settled on exponential* for MFC.1 and 2, linear for MFC.3)
Power:
- First it was needed to calculate the power production for the MFCs (Power=V×I, or Power = Voltage × Current)
- Then it was needed create a spreadsheet for the power generated (mW) from the MFCs for 14 day
- Then calculated the average power for each set of data for an MFC over 14 days.
- Determined the maximum and minimum readings for each MFC for days 1 to 14.
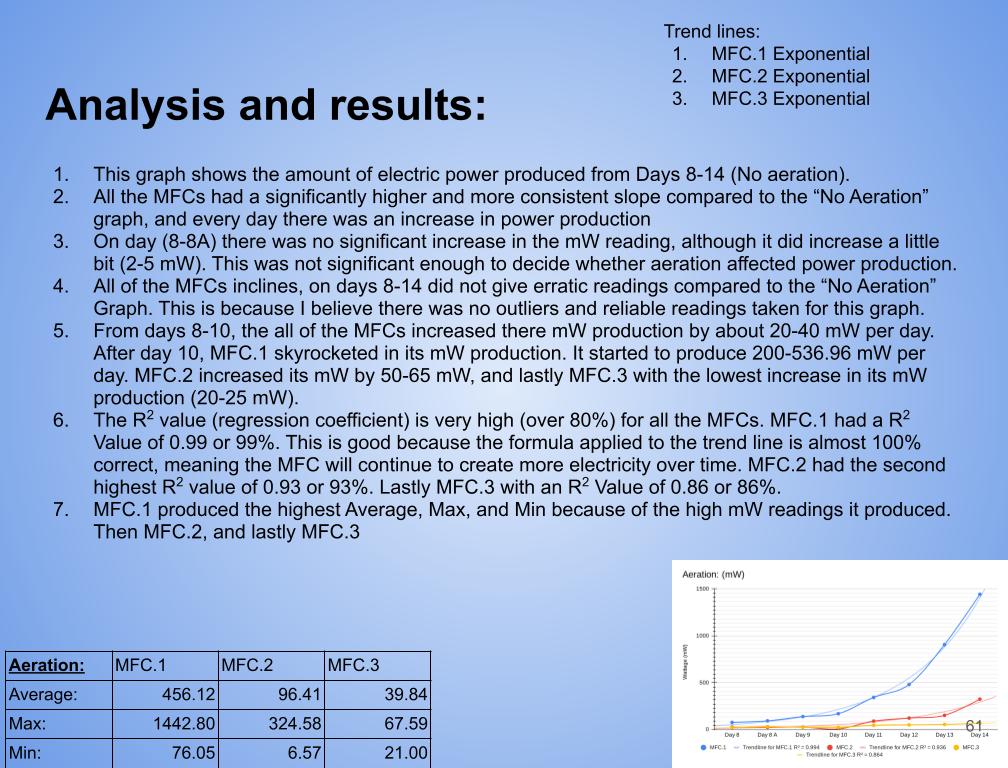
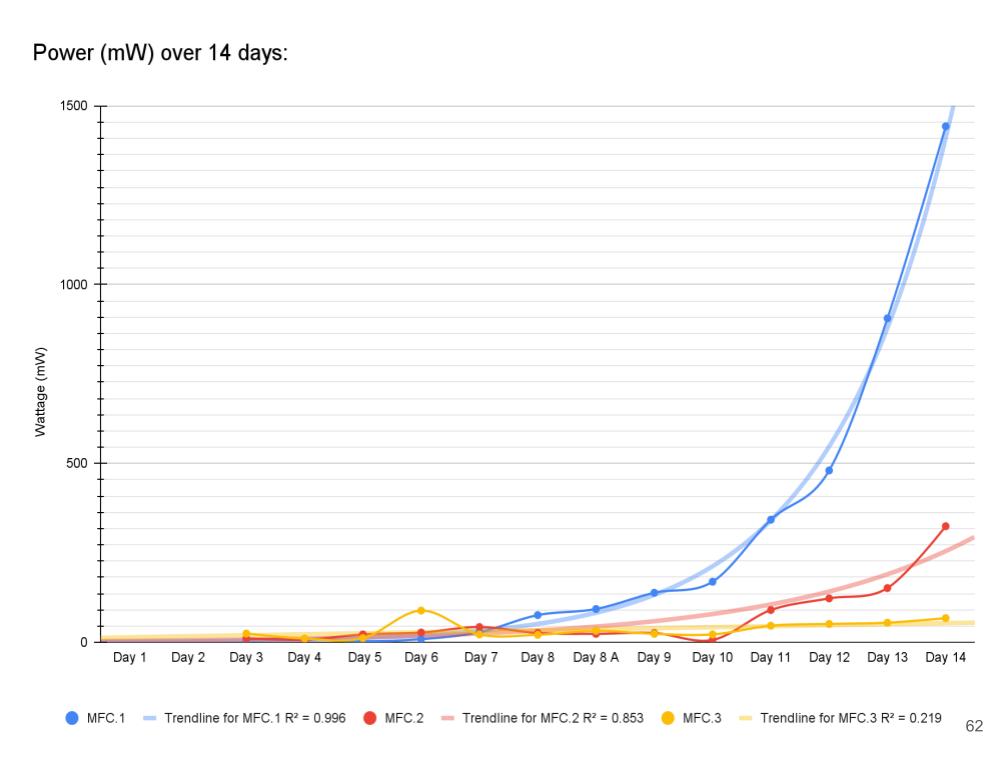
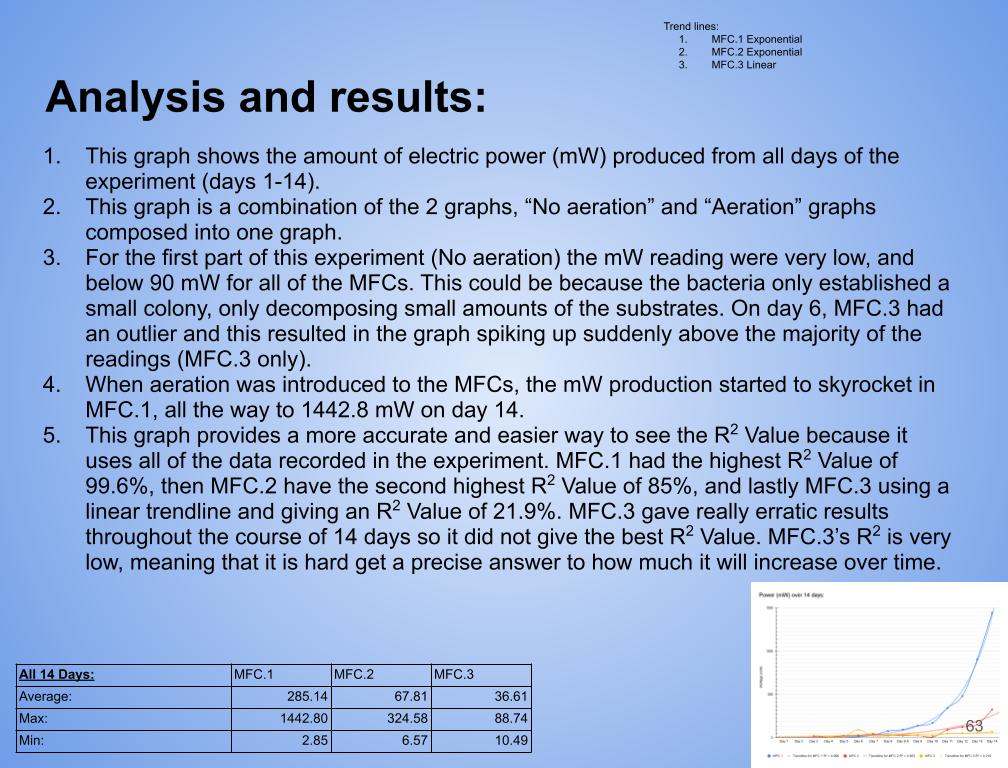
- Created 3 graphs: No aeration (Day 1-7 Vs Time), Aeration (Day 8-14 Vs Time), and Wattage over 14 days (Power Vs Time)
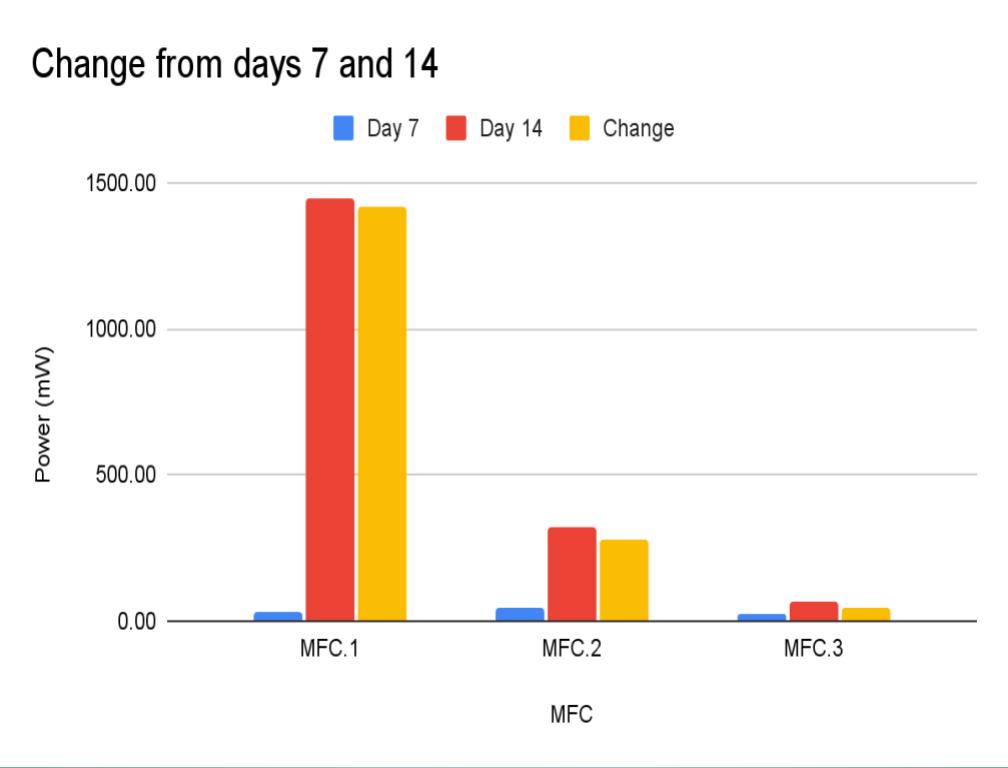
Change:
- First it was need to calculate the change and percent change between day 7 and day 14 (No aeration, and aeration)
- (This is calculated by Day 14 - Day 7) ← Change
- (Change ÷ Day 7 = Percent change)
- Then It was needed to put it into a table and then one chart/graph
Percent Change (Compared to control)
- First it was needed to calculate the percent change from each MFC compared to the control.
- This is calculated by ↓
- (MFC_ - MFC.3) ÷ MFC.3 = Percent change.
- Then it was needed to put the data into a table and put it into one graph.
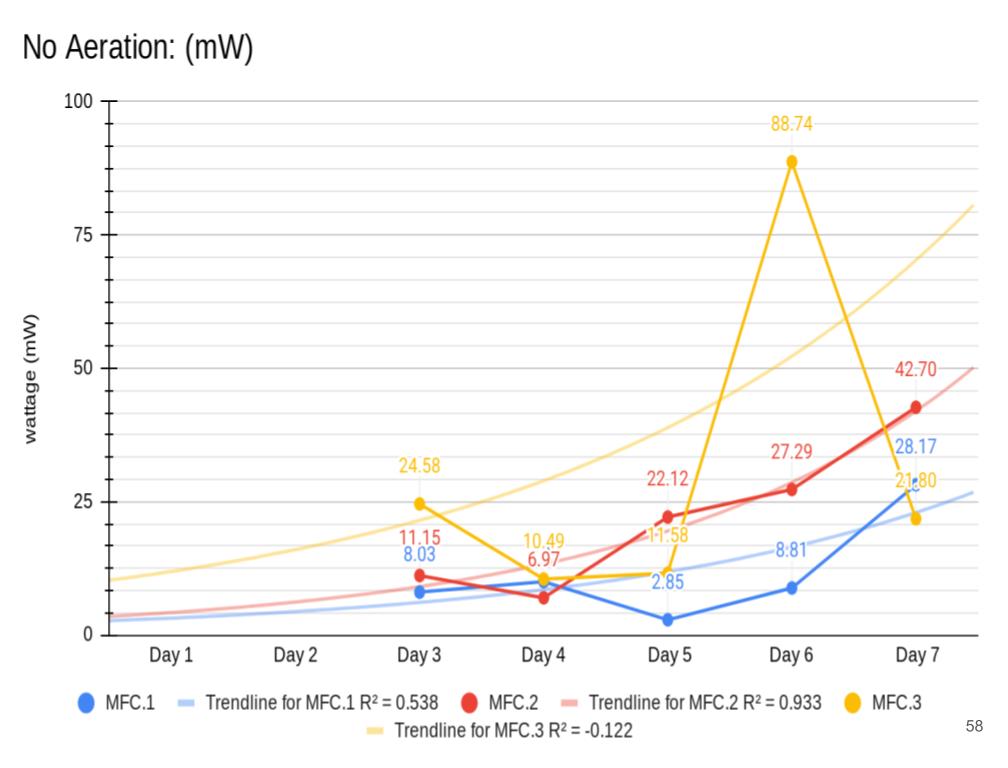
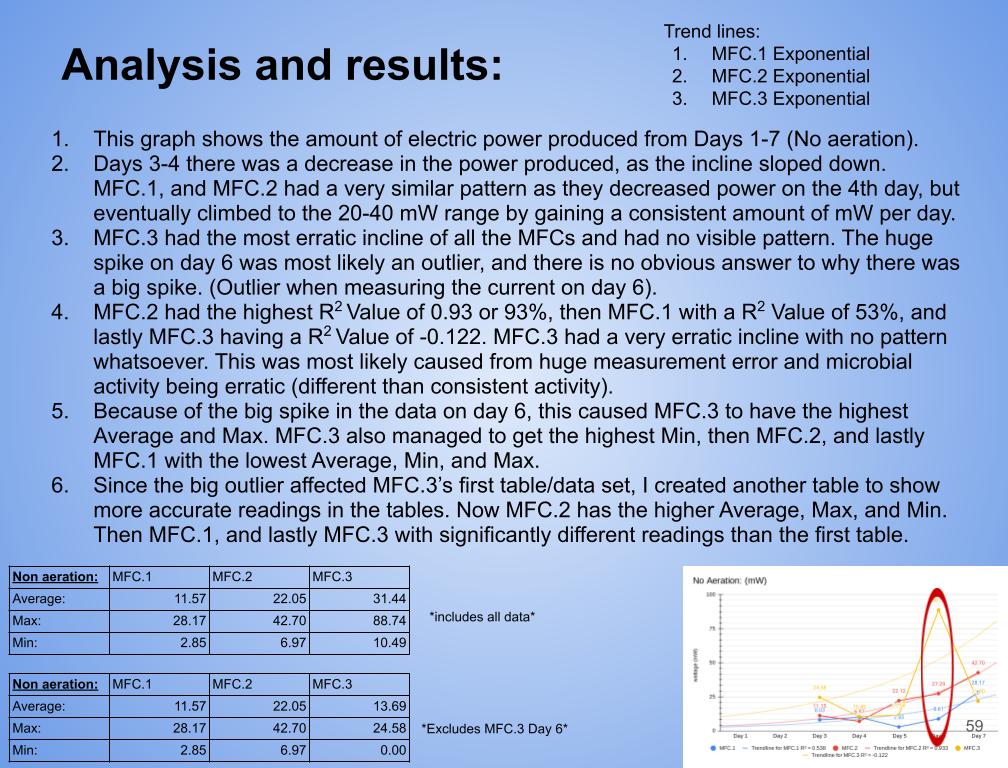
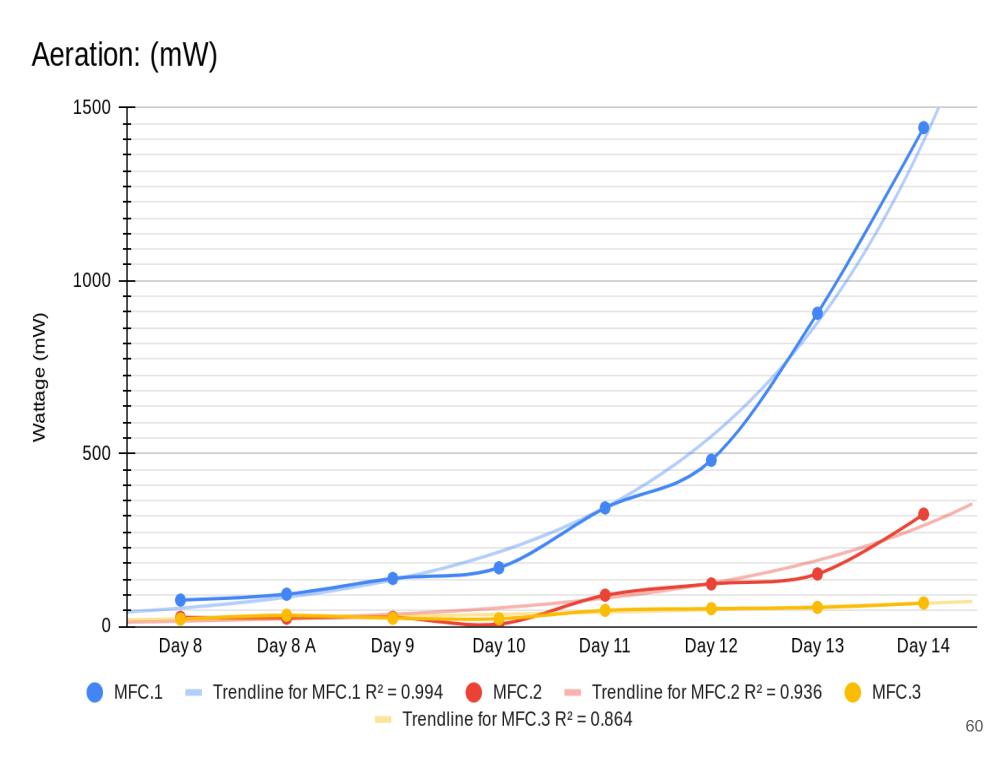
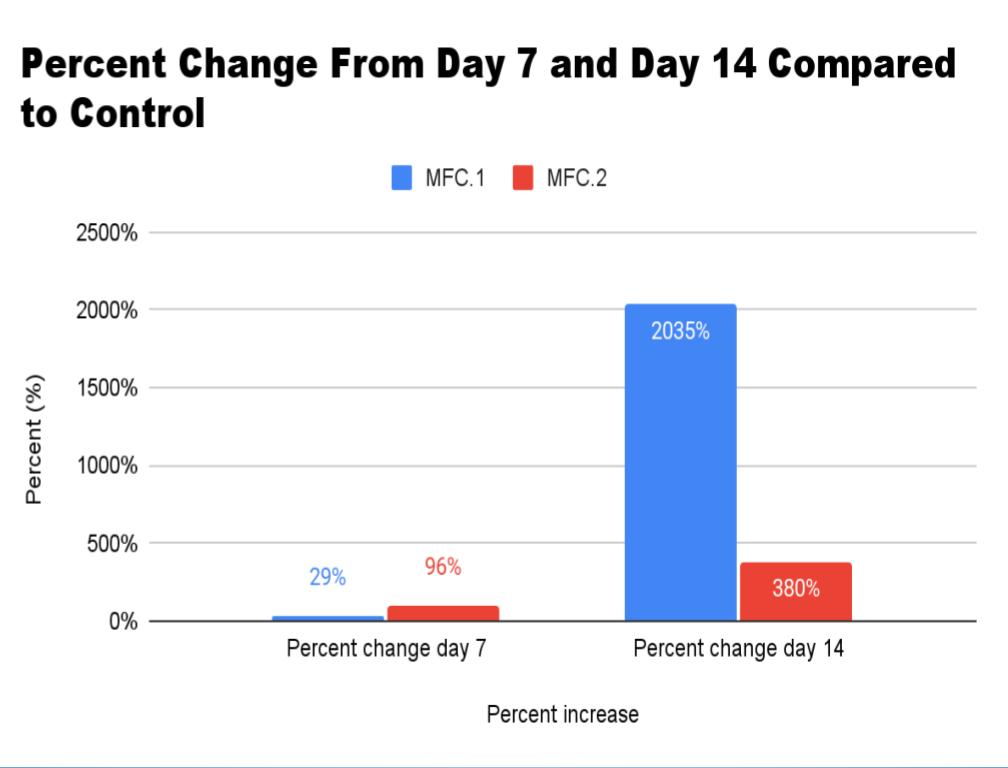
Conclusion
Conclusions
The following are conclusions based off of my analysis, results, and observations:
- In conclusion, all 3 MFCs produced electricity. This means they were built precise enough to sustain all the necessary reactions required for electricity production. (MFC.1-3)
- In conclusion my hypothesis 1.0 was wrong and MFC.1 (benthic mud) produced the most electricity. It produced 1442.8 mW on the last day of experimentation (day 14). MFC.1 produced about 4x more power than MFC.2 and 21x more power than MFC.3. This is because benthic mud has the most rich soil, meaning that it will have better nutrients and more abundance of organic matter for the bacteria/microbes to populate and reproduce. Benthic mud has a higher abundance of easily accessible nutrients because, the water/river where the benthic mud is collected from hits the mud constantly, eroding the nutrients and and making it finer for bacteria to decompose the mud. Also the bacteria found in benthic zone are ideally suited for decomposing tough organic matter found in the benthic zone, thus making it more efficient and faster for decomposing substrates. MFC.1 also had other decomposers in the substrate such as worms, algae, fungus, snails, (E.T.C) The nutrients was broken down into smaller components, making it easier for MFC.1s bacteria to decompose its substrate.
- MFC.1 and 2 had a consistent bacterial growth pattern compared to MFC.3. This is because there is more nutrients in benthic mud and compost soil than topsoil. When bacteria are exposed to lots of accessible nutrients, bacteria can reproduce more to decompose a substrate. MFC.3 did not have lots of accessible nutrients for the bacteria to colonize, thus leading to erratic and slow bacterial growth.
- MFC.2 and 3 did not produce as much power as MFC.1. This is because these MFCs did not have other organisms to help break down organic matter. MFC.2 and 3s bacteria had to decompose large organic mass, this was a big task for the bacteria and therefore it took longer for the bacteria to grow and reproduce. This is reflected in the power graphs that shows a lower incline compared to MFC.1.
In conclusion, my hypothesis 2.0 was correct, the MFCs power production spiked up immediately after aeration was introduced. Aeration had little to no effect on the voltage readings because the potential energy only comes from the anode chamber which is controlled by the bacterial growth.
Aeration is designed to affect current because when the cathodes are exposed to lots of oxygen, this allows reactions (Positive charges bonding with oxygen) to happen faster. When the reactions happen faster, this allows the electrons to move faster, thus increasing the current flow.
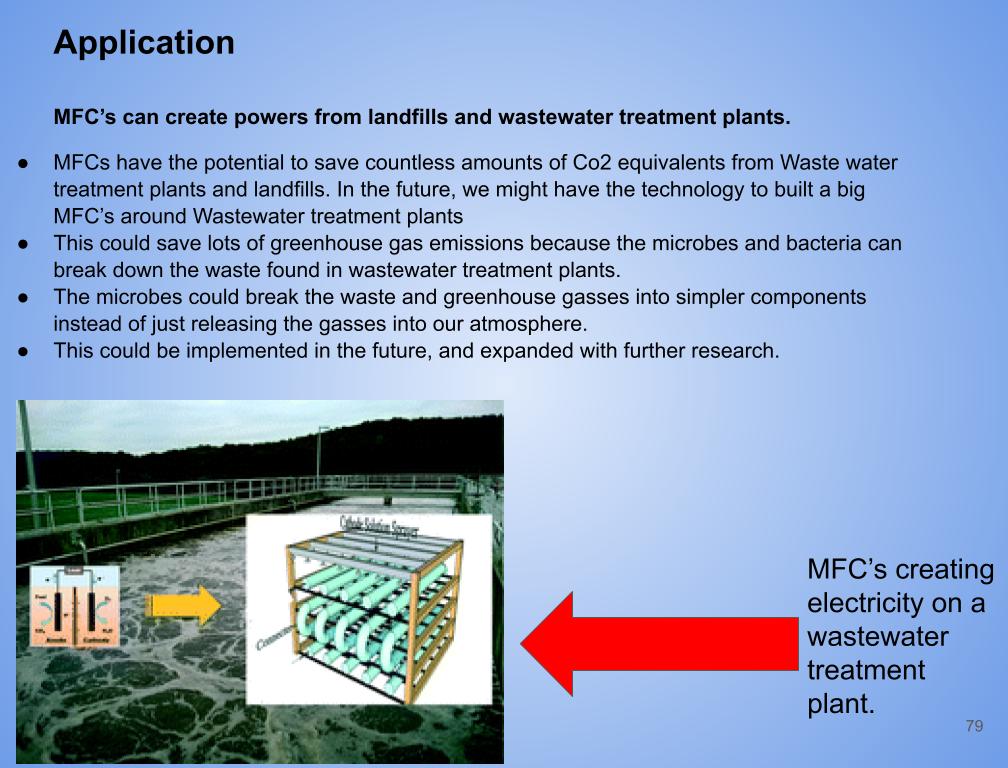
Application











Sources Of Error
Sources of Error
- Measurement error: In taking the reading of voltage and current, the digital multimeter may have read the readings incorrectly because the alligator clips where loosely connected to the copper wire. Instead of applying an insulator, (like electrical tape) to the alligator clips, the current could have varied the current or voltage readings, depending of the position the two metal sides (Alligator clips, and copper wire). Thus this could affect the way the digital multimeter read the readings.
- Temperature: In this experiment, the temperature was not regulated at one specific temperature through the 14 days of testing. This could have impacted the results because the bacteria may have catalysed the reactions faster according to the temperature they like best. The majority of bacteria have a specific temperature they like to thrive, and reproduce in. Because it is not certain which type bacteria where in my experiment, when the temperature went up/down, the bacteria might have had a surge of activity.
- Leakage: MFC.2 and 3 had 1-2 leaks, this let numerous amounts of salt to drain out of the cathode chamber, this may of lowered the voltage/current reading because there was a low amount of salt to regulate positive/negative charges in the MFCs, thus slowing the reactions down.
- Acidity: The PH levels could have varied in the MFCs, this affected the results because, bacteria can grow better in less/more acidic environments, as other microorganisms, and byproducts in the anode chamber (produced by bacteria) could have changed the acidity around all of the MFCs
Citations
Sources and Cites
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biologhttps://www.sciencedirect.com/topics/biochemistry-geneticsobial-fuel-cel
- https://www.youtube.com/watch?v=bECIaInLmRw&t=15s
- https://www.britannica.com/science/catalyst
- https://climate-woodlands.extension.org/basic-soil-components/
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Voltaic_Cells#:~:text=The%20purpose%20of%20the%20salt,causing%20the%20reaction%20to%20stop.
- https://www.merriam-webster.com/dictionary/electrode
- https://www.gov/publications/dictionaries/cancer-terms/def/oxidation
- https://www.youtube.com/watch?v=a2v7ph3kLXo
- https://byjus.com/chemistry/electrochemical-cell/#:~:text=An%20electrochemical%20cell%20is%20a,energy%20to%20cause%20chemical%20reactions
- https://pubchem.ncbi.nlm.nih.gov/compound/Agar
- Agar - an overview | ScienceDirect Topics
- https://www.britannica.com/science/ion-exchange-reaction
- https://link.springer.com/article/10.1007/s11581-016-1876-x
- https://study.com/academy/lesson/polysaccharide-definition-examples-quiz.html#
- https://www.sciencedirect.com/topics/chemistry/agar#:~:text=and%20Gelidium
- https://www.degruyter.com/document/doi/10.1515/psr-2017-0018/html?lang=en
- .https://ginobiotech.com/agar-composition-overview/
- https://link.springer.com/article/10.1007/s13205-021-02802-y#Bib1
- https://pubs.acs.org/doi/10.1021/es0491026
- https://www.sciencedirect.com/science/article/abs/pii/S0378775319307438
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/agar
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/17%3A
- https://www.google.com/search q=what+%25+of+organic+matter+is+in+compost&sca_esv=a07c2ae409b09fe4&rlz=1CAHBFI_enCA1103CA110
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6620762/#:~:text=Activated%20charcoal%20adsorbs%20many%20noxious,8%2C%2015%2C%2016).
- 25. https://www.news-medical.net/health/What-is-Activated-Charcoal.aspx#:~:text=Activated%20charcoal%20is%20a%20special,and%20coconut%20shells%2C%20among%20others.
-
29. https://www.youtube.com/watch?v=3SdEo2Se-Ac
↑ Google sheets features I used:
30. https://www.okotoks.ca/sites/default/files/2020-12/Managing_Electricity_at_Home_2015_web_final.pdf
32. https://pubs.rsc.org/en/content/articlelanding/2018/ew/c7ew00293
Acknowledgement
- I Would like to thank all of my Science Fair teachers at my old school Prince Of Wales Elementery for teaching me all the steps of the experimental process, how to format my slides, and many more.
- Second, I would like to thank Miss Burkell for organizing my science fair and helping me with the science fair forms, and improving my science fair.
- Third, I would Like to thank my homeroom teacher for presenting the opportunity to do science fair.
- Lastly, I would like to thank my dad for supervising (helping be build the MFC's) , and paying for my science fair.

