Genetic Differences Between Endangered and Non-Endangered Pine Species and How They Affect Plant Responses to Climate Change
Grade 11
Presentation
Problem
This study will explore how genetic differences between endangered and non-endangered pine species influence their reaction to climate change and how this impacts biodiversity loss. The aim is to discover the genetic differences between an endangered and a non-endangered pine species by comparing the genomes of the Whitebark(endangered) and Lodgepole Pine(non-endangered) to both a control genome, the Loblolly Pine, and each other. Along with discovering the differences, habitat projections and climate models for the endangered and non-endangered pine species will be made to show the future ramifications that these differences have on a pine tree’s ability to survive in an environment that is constantly changing. In the long term, this research aims to raise awareness of the importance of synthetic biology to biodiversity preservation and add insight into the gene sequences that may be options to alter in pine trees to increase their tolerance to environmental stressors.
Method
This study utilized the sequenced genomes of the Whitebark Pine (endangered) and the Lodgepole Pine (non-endangered) to compare similarities and differences between the genomes regarding environmental stressors.
The initial steps of the comparison process used comparative biology. This process helps uncover patterns and processes in diversity, to determine the phenotypes and reactions that are a result of the genetic differences that had been found by determining which physical characteristics were responsible for the differences in gene function in the pine trees. Research was done to determine which physical characteristics play a role in mitigating the effects of the environmental stressors found. These were then contrasted to see any major differences between the phenotypes of the Whitebark and Lodgepole Pine that could be influencing their ability to survive in the harsher habitats created by climate change.
The coding program R (Ripley & Others, 2001)was used to find and select the environmental stressors that were most prevalent for each species. This was achieved using the package biomod2 (Ensemble Platform for Species Distribution Modeling, n.d.), which uses data in maps containing information about a region's climate, temperature, precipitation, and soil nutrients (Abatzoglou et al., 2018). These maps were then compiled with another map showing the occurrences (GBIF, n.d.), points in space where the species has been recorded, in the area of study to show which habitat the pine species prefers. These were analyzed to see which regions held the majority of the species and were limited to British Columbia and Western Alberta. Compiled maps were created for the Lodgepole and Whitebark pine and compared against each other. This showed which climatic factors and environmental stressors were the most influential for both plants as well as the differences present in their environments.
In order to find the function of the similar gene sequences found between the endangered plant species and the non-endangered species. This study included a control plant, the Loblolly Pine. This was because, while the Lodgepole cDNA (Falk et al., 2018) genome had annotations as to the functions of some gene sequences the sequenced Whitebark genome (Neale et al., 2023) used was a scaffold, which does not contain annotations regarding the function of genes. The annotated Loblolly cDNA (Falk et al., 2018) genome was primarily used to verify the function of the similar gene sequences found in the Whitebark Pine as well as any sequence within the Lodgepole genome whose function was unknown. This was because it is well studied and there is more information on the genes present in this species.
The full genomes of the Whitebark and Lodgepole Pine were compared against the full Loblolly genome to get a general similarity using rBLAST (Hahsler, n.d.). This provided information about percent similarity, where the alignment happens in the genome, as well as any gaps in the alignment. Specific gene sequences from the Loblolly cDNA were then selected based on the data collected in the habitat models as to which environmental factors have the greatest impact on each pine species. The alignments were then filtered through to have over 95% similarity, as this is the standard, and by making the chosen sequences from the Loblolly cDNA genome the query sequences, and the pine genomes the search set the gene copy number for the sequences were found for both the Whitebark and the Lodgepole Pine. The final analysis of whether or not there were genetic differences was done by comparing the copy number of each sequence for both the endangered and non-endangered species. If the gene copy number was similar it was determined that the gene sequence was present in both species.
Research
Human activity is the main source of climate change, especially concerning atmospheric composition which is known as anthropogenic climate change or human-induced climate change (Karl & Trenberth, 2003). It is also not simply a regional issue as air from one part of the world can travel halfway across the earth in a week spreading contaminants. Due to the large increase in carbon dioxide that is present in the atmosphere, the natural greenhouse effect has been enhanced (Kiehl & Trenberth, 1997). Along with the anthropogenic emissions of gases from burning fossil fuels, methane, and nitrous oxide, all of which have long lifetimes and therefore accumulate, the atmospheric composition has begun to change with a nearly 31% increase in carbon dioxide (Karl & Trenberth, 2003). Sulphate aerosols also greatly impact the climate by reflecting solar radiation on Earth (Karl & Trenberth, 2003). The effects these gases have will only rise as the amount of greenhouse gas emissions and the concentration of aerosols is steadily increasing (Crutzen et al., 1979).
A global temperature increase is not the only thing to worry about. While the evaporation of surface moisture does help to cool things down it comes with the consequences of sped-up land drying and increased water vapour, which increases both the risk of droughts and heavy precipitation (Pochanart et al., 2002). Action must be taken sooner rather than later because while the length, size, and effects of anthropogenic climate change are largely unknown the magnitude is still higher than that of natural changes (Karl & Trenberth, 2003). If there is no interference made there is a 90% chance that the global temperature will increase 1.7-2.9 degrees between 1990 and 2100 (Wigley & Raper, 2001).
Climate change and biodiversity have a bidirectional relationship, meaning if one changes then the other will be altered as well (Meena & Jha, 2023), which creates a vicious cycle where the loss of biodiversity could threaten food security and diversity (Muluneh, 2021) This means that to prevent damage to the biosphere we need to predict exactly how climate change alters biodiversity (Urban et al., 2016).
Some sudden changes in the climate that play a large role in biodiversity are the frequency of natural disasters, the amount of precipitation, and temperature changes (Meena & Jha, 2023). An increase in any one of these factors increases biodiversity loss. With anthropogenic climate change being a large influence on the collapse of biodiversity, it may become the main driver in the decrease of biodiversity in coming years (Mace et al., 2012). The claim is made that the impacts of biodiversity loss may rival other driving factors of climate change (Cardinale et al., 2012).
Biodiversity is an incredibly important part of the earth’s ecosystem and its loss could be detrimental not only to the environment but to human quality of life and health. Several studies have shown urban environments to be harmful to human health due to asphalts' tendency to both emit and store heat in higher amounts than natural (Marselle et al., 2021). Vegetation has been shown to reduce the effects of these ‘heat islands’ via evapotranspiration and shading (Marselle et al., 2021). More plant life has also been shown to decrease the effects of urban stressors like environmental noise (Markevych et al., 2017). Excessive exposure to environmental noise can impact the respiratory and cardiovascular systems as well as metabolic functions in humans (Recio et al., 2016). The structural components of plants can buffer noise and with a high enough quantity of plant life can create a noise barrier (Marselle et al., 2021).
The loss of biodiversity has also been shown to reduce human exposure to important symbiotic microbes. Diverse microbes are key to both the immune and digestive systems of humans. The biodiversity of microbiomes is decreased due to the loss of plant biodiversity, having adverse effects on human health (Renz et al., 2017). Both the World Health Organization and the Convention of Biological Diversity agree with the potential that lies in aligning health and climate goals with biodiversity goals (Marselle et al., 2021). Marselle and colleagues argue that biodiverse environments should be the foundation for public health and that biodiversity is essential for survival (Marselle et al., 2021).
Even though biodiversity’s effects on humans are incredibly important, its effects on the environment as a whole should not be understated. Many scientists have come together to create a few consensus statements about the importance of biodiversity to the environment (Cardinale et al., 2012). One of these points is that diverse communities are more productive (Huston, 1997). The productivity of diverse ecosystems is further enforced by the knowledge that biodiversity loss reduces the efficiency of resource collection, biomass production, and decomposition or recycling of nutrients (Cardinale et al., 2012). There is also the idea that functional traits, characteristics that are relevant to an organism's response to its environment and/or environmental changes, impact the magnitude of ecosystem functions and therefore can create numerous possible impacts of extinction on ecosystem functions. The biological foundation of ecosystem services can be influenced by both ecological and social factors, and depending on the traits lost there can be a vast difference in the impact on ecosystem functions (McGill et al., 2006). However many of the experiments that support this idea are being questioned due to the duration of the experiment, as that can affect the results (Cardinale et al., 2007).
Even with these concerns, it is important to understand extinction risks and how they influence function to know the consequences of extinction. This inconclusiveness warrants more research in this area to find out how extinction risks influence function. Biodiversity loss must also be addressed relatively quickly as the effects of a lack of diversity increase over time, and current research suggests that the minimum levels of biodiversity needed for an ecosystem to function have been vastly underestimated (Cardinale et al., 2012).
Plants are integral to slowing down the effects of climate change as they can regulate carbon dioxide emissions via photosynthesis, however rising temperatures have the adverse effect of reducing photosynthesis as the high temperature indicates to the guard cells that the stomata should be closed reducing the amount of carbon dioxide the plants can consume (Dusenge et al., 2019). There is evidence that rising carbon dioxide levels could lower photorespiration, though this is not entirely a negative phenomenon as the elimination of photorespiration could increase photosynthesis rates by 12-55% (Walker et al., 2016), and therefore aiding in slowing down the negative effects of climate change. However, this is only true if the carbon is present in recalcitrant pools or the ocean which is not the case (Dusenge et al., 2019). The net effect of this change in carbon dynamics largely depends on plant responses to the carbon flux, the rate of transfer between carbon pools. A lack of water and nutrients also decreases the positive responses to rising carbon dioxide levels which is shown by the fact that there are negative impacts on photorespiration and photosynthesis during a drought (Duan et al., 2013). This is accompanied by high carbon dioxide levels affecting plant responses to drought by decreasing Ci levels faster (Gray et al., 2016). Water stress and drought also enhance the effects of warming on plant carbon metabolism as warm and dry conditions during the spring lead to an expedited bud burst which depletes the already low soil moisture faster due to the early leaf development (Dusenge et al., 2019). This could be a large underlying factor in drought's influence on how a plant can respond to temperature changes.
Solutions to the decrease in biodiversity due to climate change must be found. In recent years synthetic biology has started being looked into as an option to aid in biodiversity preservation (Synthetic Biology and Its Implications for Biodiversity Conservation, n.d.). This process uses engineering to alter certain plant functions by altering gene sequences to allow for increased plant survival in new and harsher conditions (Synthetic Biology and Its Implications for Biodiversity Conservation, n.d.). However, due to the lack of research into this field, some scientists are uncertain as to which parts of the plant should be targeted to best improve plant responses to climate change.
Data
I got my data regarding the tree occurrences from the site GBIF and my Lodgepole and Loblolly genomes from treegenesdb.org and the Whitebark genomes came from NCBI.
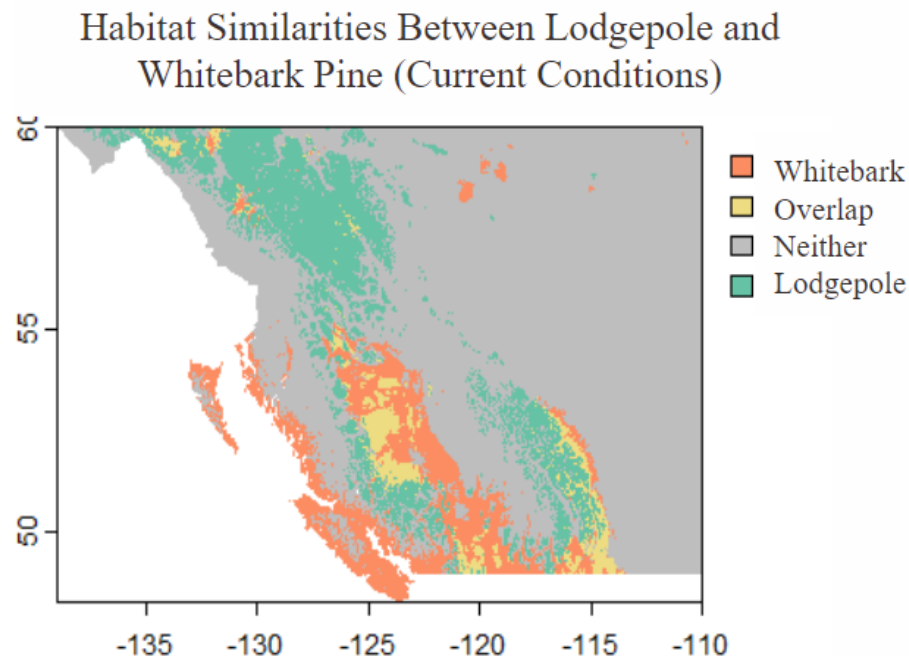
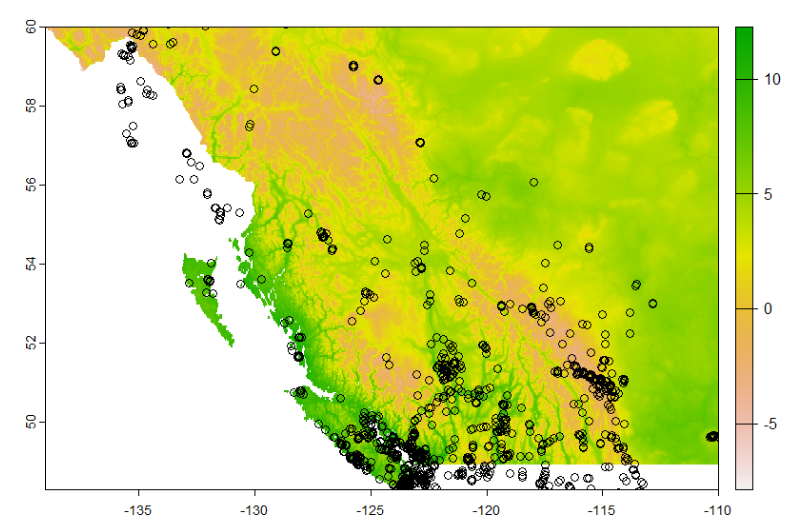
Here we can simply see the habitat maps for both the whitebark and the lodgepole and compare them to see that not only does the whitebark have a smaller suitable habitat but there is very little overlap between the habitat of each species.
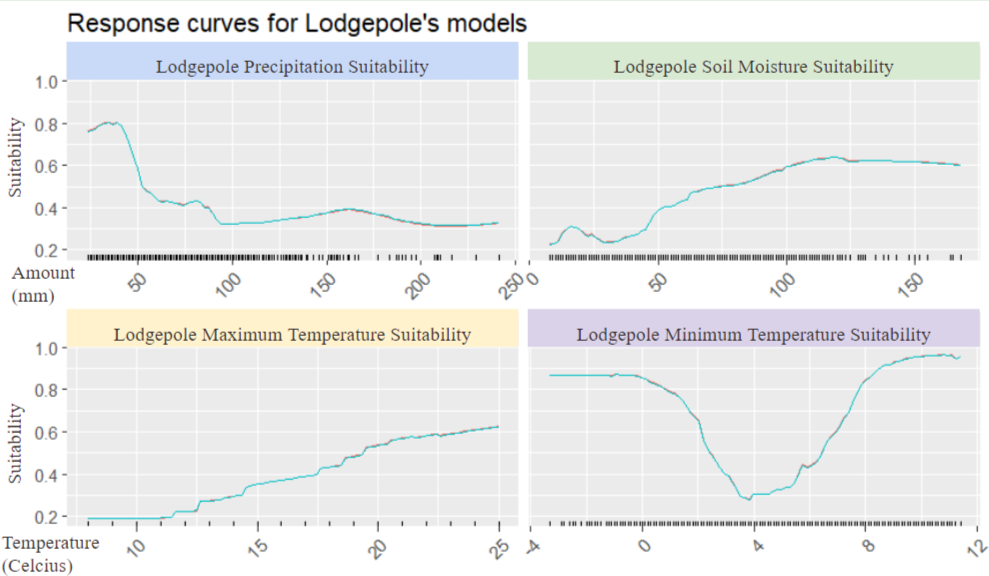
Here we can see the response curves of the Lodgepole pine to varying environmental conditions. These were selected by doing research as to which conditions the lodgepole is the most susceptible to. As we can see the lodgepole has less of a population in areas of high precipitation and does well is regions of high soil (what soil part is measured). Interestingl the lodgepole does better in regions with higher maximum temperatures as well as minimum temperatures but can still thrive in colder areas as can be seen with the large amount in lower minimum temperatures.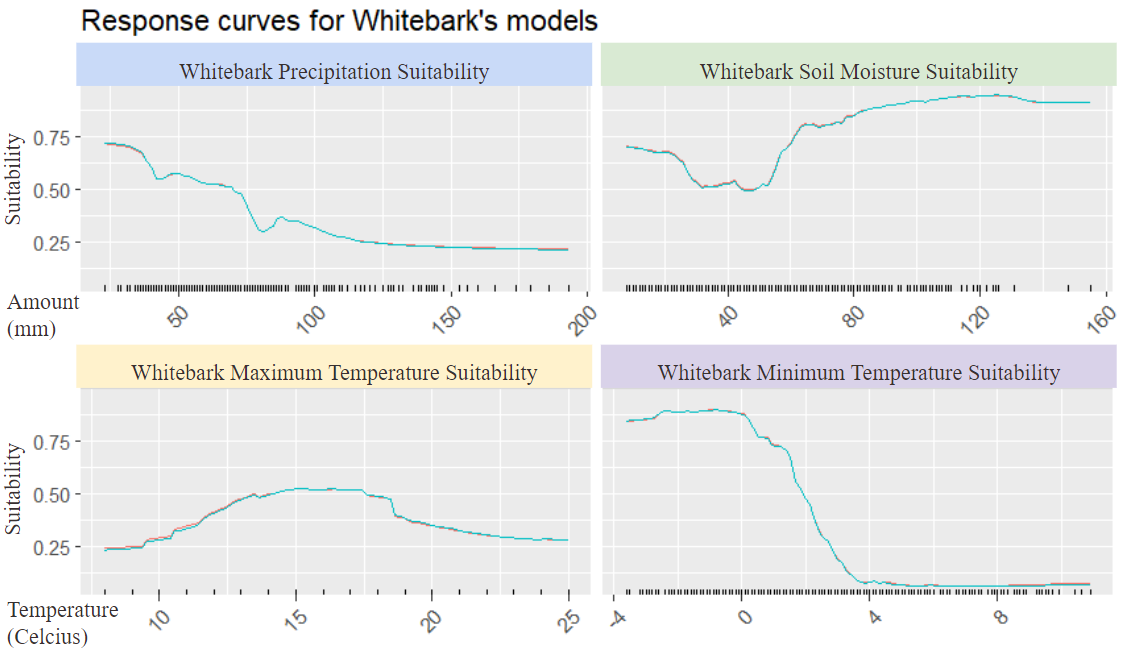
The whitebark is fairly similar in terms of population in regions with higher precipitation not fairing as well as when there is less amount of precipitation. However as compared to the lodgepole while they do have similar preferences for higher amounts of soil (whatever) the whitebark does seem to prefer higher amounts as seen by the scale. The whitebark also does not fare as well in areas with a higher maximum temperature and prefers colder regions as seen with the graph displaying the minimum temperatures of the higher population. With this we can see that temperature is an important environmental factor and the change that some contribute to climate change could be an area of stress. We can also see that increasing precipitation and less soil (whatever) are also areas of environmental stress for both species of pine
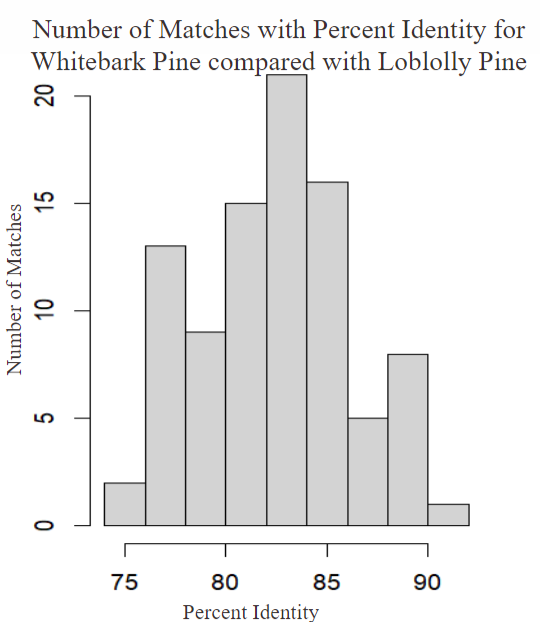
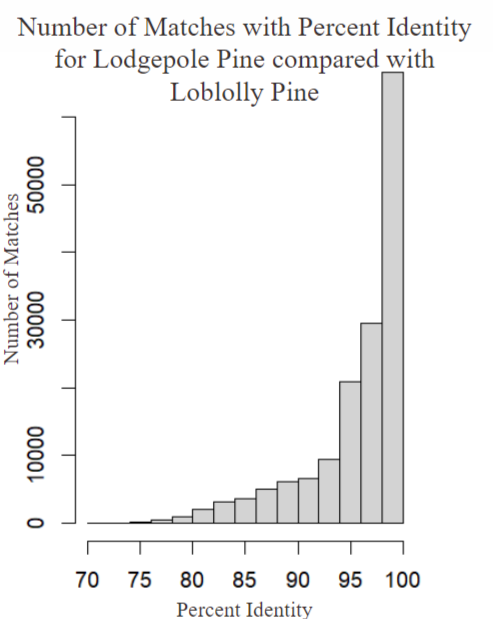
Here we can simply see that the Lodgepole is far more similar to the Loblolly Pine than the Whitebark is as the general percent identity is higher using this we can assume that in general there is a fairly distinct difference between the genomes of the Whitebark Pine and the Lodgepole pine so what exactly are the differences

Lodgepole has more copy matches for heat shock while whitebark has none.
- Difference in genetics in regard to response to heat shock
Lodgepole has matches for harpin but whitebark has none
- Difference in response to pests
Lodgepole has matches for temperature
- Difference in response to changes in temperature
White bark is the endangered one so it makes sense that it doesn’t have as many/ any gene copies for the sequences that code for response to environmental stressors as it most likely doesn’t have as good of a response, but the most interesting one in the harpin induced protein as pests are an active threat to the whitebark. Lodgepole prefers colder and damper environments from this we can gather that the lodgepole is better genetically equipped to handle changes in environment that lead to plant stress
This is just showing the similarity between the lodgepole gene sequence and loblolly gene sequence
Conclusion
I was able to some to the conclusion that there are genetic differences between an endangered a non endangered pine species and that these differences do have a correlation to why the whitebark is endangered and not the lodgepole. Firstly as the percent identity graphs show the lodgepole is very similar to the lodgepole while the whitebark is fairly different meaning that there are some substantial genetic differences between the Whitebark and Lodgepole Pine. We were also able to determine that at least some of these difference were in regard to gene sequencing in response to the environmental stressors of drought, temperature, and pests. The lodgepole has many gene copies for gene sequences expressed by the loblolly that code for responses to varying environmental stressors with the main ones meaning heat and drought stress. The whitebark has no copies for the selected gene sequences meaning that in terms of this project it does not have as many safeguards to protect the plant from the changes that are happening to its environment due to climate change. This has led to the whitebark to have a smaller available habitat and ‘worse’ responses to environmental stressors which has led it to become endangered instead of the lodgepole pine. However, I did overlook one key aspect of why the Whitebark is going endangered and that is pests (more specifically White Pine Blister Rust) While I did look into this somewhat with the Harpin Induced Protein I did not give it the attention it deserved leaving a gap in my research. I also could have looked for a more well recorded endangered species with more of its genome sequenced as while I was able to determine what sequences due to environmental stressors the whitebark didn’t have I was unable to find out the responses it did have. In future projects I aim to remedy these errors. The next steps I hope to take in regards to this project would be to hopefully explore pest genetic responses more but more so begin actual experimental work to see if it is possible to alter or add some of the responsive genetic sequences to the genome of the whitebark and see if that helps its response to environmental stressors.
Citations
Ensemble platform for species distribution modeling. (n.d.). Retrieved February 10, 2024, from https://biomodhub.github.io/biomod2/
GBIF. (n.d.). Retrieved February 10, 2024, from https://www.gbif.org/
Hahsler, M. (n.d.). rBLAST: Interface for the Basic Local Alignment Search Tool (BLAST) - R-Package. Github. Retrieved February 9, 2024, from https://github.com/mhahsler/rBLAST
Neale, D. B., Zimin, A. V., Meltzer, A., Bhattarai, A., Amee, M., Corona, L. F., Allen, B. J., Puiu, D., Wright, J., Torre, A. R. D. L., McGuire, P. E., Timp, W., Salzberg, S. L., & Wegrzyn, J. L. (2023). A Genome Sequence for the Threatened Whitebark Pine. bioRxiv : The Preprint Server for Biology. https://doi.org/10.1101/2023.11.16.567420
Angilletta, M. J. (2009). Thermal Adaptation: A Theoretical and Empirical Synthesis. OUP Oxford. https://play.google.com/store/books/details?id=yrvqlaHdRZIC
Cahill, A. E., Aiello-Lammens, M. E., Fisher-Reid, M. C., Hua, X., Karanewsky, C. J., Ryu, H. Y., Sbeglia, G. C., Spagnolo, F., Waldron, J. B., Warsi, O., & Wiens, J. J. (2013). How does climate change cause extinction? Proceedings. Biological Sciences / The Royal Society, 280(1750), 20121890. https://doi.org/10.1098/rspb.2012.1890
Cardinale, B. J., Duffy, J. E., Gonzalez, A., Hooper, D. U., Perrings, C., Venail, P., Narwani, A., Mace, G. M., Tilman, D., Wardle, D. A., Kinzig, A. P., Daily, G. C., Loreau, M., Grace, J. B., Larigauderie, A., Srivastava, D. S., & Naeem, S. (2012). Biodiversity loss and its impact on humanity. Nature, 486(7401), 59–67. https://doi.org/10.1038/nature11148
Cardinale, B. J., Wright, J. P., Cadotte, M. W., Carroll, I. T., Hector, A., Srivastava, D. S., Loreau, M., & Weis, J. J. (2007). Impacts of plant diversity on biomass production increase through time because of species complementarity. Proceedings of the National Academy of Sciences of the United States of America, 104(46), 18123–18128. https://doi.org/10.1073/pnas.0709069104
Crozier, L., & Dwyer, G. (2006). Combining population-dynamic and ecophysiological models to predict climate-induced insect range shifts. The American Naturalist, 167(6), 853–866. https://doi.org/10.1086/504848
Crutzen, P. J., Heidt, L. E., Krasnec, J. P., Pollock, W. H., & Seiler, W. (1979). Biomass burning as a source of atmospheric gases CO, H2, N2O, NO, CH3Cl and COS. Nature, 282(5736), 253–256. https://doi.org/10.1038/282253a0
Duan, H., Amthor, J. S., Duursma, R. A., O’Grady, A. P., Choat, B., & Tissue, D. T. (2013). Carbon dynamics of eucalypt seedlings exposed to progressive drought in elevated [CO2] and elevated temperature. Tree Physiology, 33(8), 779–792. https://doi.org/10.1093/treephys/tpt061
Dusenge, M. E., Duarte, A. G., & Way, D. A. (2019). Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. The New Phytologist, 221(1), 32–49. https://doi.org/10.1111/nph.15283
Gilman, S. E., Urban, M. C., Tewksbury, J., Gilchrist, G. W., & Holt, R. D. (2010). A framework for community interactions under climate change. Trends in Ecology & Evolution, 25(6), 325–331. https://doi.org/10.1016/j.tree.2010.03.002
Gray, S. B., Dermody, O., Klein, S. P., Locke, A. M., McGrath, J. M., Paul, R. E., Rosenthal, D. M., Ruiz-Vera, U. M., Siebers, M. H., Strellner, R., Ainsworth, E. A., Bernacchi, C. J., Long, S. P., Ort, D. R., & Leakey, A. D. B. (2016). Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nature Plants, 2(9), 16132. https://doi.org/10.1038/nplants.2016.132
Hoffmann, A. A., & Sgrò, C. M. (2011). Climate change and evolutionary adaptation. Nature, 470(7335), 479–485. https://doi.org/10.1038/nature09670
Huston, M. A. (1997). Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia, 110(4), 449–460. https://doi.org/10.1007/s004420050180
Karl, T. R., & Trenberth, K. E. (2003). Modern global climate change. Science, 302(5651), 1719–1723. https://doi.org/10.1126/science.1090228
Kiehl, J. T., & Trenberth, K. E. (1997). Earth’s Annual Global Mean Energy Budget. Bulletin of the American Meteorological Society, 78(2), 197–208. https://doi.org/10.1175/1520-0477(1997)078<0197:EAGMEB>2.0.CO;2
Mace, G. M., Norris, K., & Fitter, A. H. (2012). Biodiversity and ecosystem services: a multilayered relationship. Trends in Ecology & Evolution, 27(1), 19–26. https://doi.org/10.1016/j.tree.2011.08.006
Markevych, I., Schoierer, J., Hartig, T., Chudnovsky, A., Hystad, P., Dzhambov, A. M., de Vries, S., Triguero-Mas, M., Brauer, M., Nieuwenhuijsen, M. J., Lupp, G., Richardson, E. A., Astell-Burt, T., Dimitrova, D., Feng, X., Sadeh, M., Standl, M., Heinrich, J., & Fuertes, E. (2017). Exploring pathways linking greenspace to health: Theoretical and methodological guidance. Environmental Research, 158, 301–317. https://doi.org/10.1016/j.envres.2017.06.028
Marselle, M. R., Lindley, S. J., Cook, P. A., & Bonn, A. (2021). Biodiversity and Health in the Urban Environment. Current Environmental Health Reports, 8(2), 146–156. https://doi.org/10.1007/s40572-021-00313-9
McGill, B. J., Enquist, B. J., Weiher, E., & Westoby, M. (2006). Rebuilding community ecology from functional traits. Trends in Ecology & Evolution, 21(4), 178–185. https://doi.org/10.1016/j.tree.2006.02.002
Meena, P., & Jha, V. (2023). Environmental Change, Changing Biodiversity, and Infections-Lessons for Kidney Health Community. Kidney International Reports, 8(9), 1714–1729. https://doi.org/10.1016/j.ekir.2023.07.002
Muluneh, M. G. (2021). Impact of climate change on biodiversity and food security: a global perspective—a review article. Agriculture & Food Security, 10(1). https://doi.org/10.1186/s40066-021-00318-5
Pochanart, P., Akimoto, H., Kinjo, Y., & Tanimoto, H. (2002). Surface ozone at four remote island sites and the preliminary assessment of the exceedances of its critical level in Japan. Atmospheric Environment, 36(26), 4235–4250. https://doi.org/10.1016/S1352-2310(02)00339-4
Recio, A., Linares, C., Banegas, J. R., & Díaz, J. (2016). Road traffic noise effects on cardiovascular, respiratory, and metabolic health: An integrative model of biological mechanisms. Environmental Research, 146, 359–370. https://doi.org/10.1016/j.envres.2015.12.036
Renz, H., Holt, P. G., Inouye, M., Logan, A. C., Prescott, S. L., & Sly, P. D. (2017). An exposome perspective: Early-life events and immune development in a changing world. The Journal of Allergy and Clinical Immunology, 140(1), 24–40. https://doi.org/10.1016/j.jaci.2017.05.015
Urban, M. C., Bocedi, G., Hendry, A. P., Mihoub, J.-B., Pe’er, G., Singer, A., Bridle, J. R., Crozier, L. G., De Meester, L., Godsoe, W., Gonzalez, A., Hellmann, J. J., Holt, R. D., Huth, A., Johst, K., Krug, C. B., Leadley, P. W., Palmer, S. C. F., Pantel, J. H., … Travis, J. M. J. (2016). Improving the forecast for biodiversity under climate change. Science, 353(6304). https://doi.org/10.1126/science.aad8466
Walker, B. J., VanLoocke, A., Bernacchi, C. J., & Ort, D. R. (2016). The Costs of Photorespiration to Food Production Now and in the Future. Annual Review of Plant Biology, 67, 107–129. https://doi.org/10.1146/annurev-arplant-043015-111709
Wang, D., Heckathorn, S. A., Wang, X., & Philpott, S. M. (2012). A meta-analysis of plant physiological and growth responses to temperature and elevated CO(2). Oecologia, 169(1), 1–13. https://doi.org/10.1007/s00442-011-2172-0
Wigley, T. M., & Raper, S. C. (2001). Interpretation of high projections for global-mean warming. Science, 293(5529), 451–454. https://doi.org/10.1126/science.1061604
Acknowledgement
I received assistance from my teacher Dr. Garcia in terms of presenting and coming up with my project. I also worked with a mentor from the University of Calgary in the form of a Graduate Student who helped me with the coding aspect of the project as well as understanding and interpreting my results.