How to make things transparent?
Grade 9
Presentation
Hypothesis
Question: How is it possible to make organic material transparent?
It is possible to make things transparent by removing pigments that could make things more opaque and by matching the refractive index of the light passing through, essentially reducing the scattering of light, making light that goes through the material more unified.
Research
Imagine there was a way to make a human transparent! Well... there is a method that is close to it at least and I first discovered this by reading a science article which blew me away. After all, the secret ingredient to make this solution is found in Doritos.....?
To make organic materials transparent, the scattering of light must be reduced by matching the refractive index as well as the removal of any pigments that can absorb light. There will be two methods, one using tartrazine (an orange dye found in Sour Patch, Doritos, and more) and the other using fructose. According to a group of scientists (Ou, Z., Duh, Y.-S., Rommelfanger, N. J., Carl, Jiang, S., Brinson, K., Zhao, S., Schmidt, E. L., Wu, X., Yang, F., Cai, B., Cui, H., Qi, W., Wu, S., Adarsh Tantry, Roth, R., Ding, J., Chen, X., Kaltschmidt, J. A., & Brongersma, M. L., 2024), using tartrazine at a 0.6g/M concentration and taking pictures under UV light will result in the organic material becoming transparent. This is because the tatrazine absorbs the UV light, changing the original refractive index. By using fructose, it is possible to clear organic materials to make them more translucent (Hildebrand, S., Schueth, A., Klaus von Wangenheim, Mattheyer, C., Pampaloni, F., Hansjürgen Bratzke, Roebroeck, A. F., & Ralf A. W. Galuske., 2020), this is done by matching the refractive index to around 1.5 which is about as transparent as glass is. The way the fructose works is by reducing the scattering of light causing the waves to align properly. The way fructose typically works is that it takes out some water and replaces with fructose, hence giving it the name clearing. There will also be several experiments that are dedicated to demonstrating the removement of pigments within an organic material (Smith, R. A. W., Garrett, B., Naqvi, K. R., Fülöp, A., Godfrey, S. P., Marsh, J. M., & Chechik, V., 2017) to prevent the absorbtion and scattering of the light.
Variables
Independant: Organic material being tested, the method of making organic material transparent
Dependant: The transparency of the organic material
Controlled: The amount of light being inputted, method of testing the transparency
Procedure
I will be using two methods to try and prove transparency. The first method will be using a process known as clearing and the second will be using a method using tartrazine discovered by a group of scientists. The first method requires the organic material to be soaked thoroughly in a 70% fructose concentration dissolved in water. This will match the RI and prevent the scattering of light which is one of the major causes of opaqueness or translucency as stated in the research section. The absorption of light is also a contributing factor but it is miniscule in comparison to the refraction of light (scattering). Tartrazine requires a concentration of 0.6g/M (formula is n=m/M or moles = mass ÷ molar mass) and the organic material must be soaked thoroughly, letting it reach everywhere. It has to be put in a UV chamber and photographed. In order to test the transparency I will need to set a metric standard of measuring the light. This metric will use a quantitative measurement (numbers) and will be done by making the image black and white, then using Image J (software) to take the mean value of the intensity. Another side experiment done will solve the issue of making organic material that is opaque transparent, and it will use hairs and hydrogen peroxide.
Experiment 1 (Egg)
Steps needed prior to the experiment:
The egg must lose the shell that it has in as the shell itself contains pigments that's opacity cannot be altered. This can be done by using the reaction between the egg and vinegar. What this does is that the vinegar's acetic acid will react with the calcium carbonate that makes up the eggshell, dissolving and making carbon dioxide, water, and Calcium ions. This will leave with a shell-less egg, however, it will still retain its membrane.
1) Measure what 70% fructose in 50mL of water, make several copies, make sure thoroughly dissolved
2) Prep 3 eggs and put them all in Vinegar to activate chemical reaction that dissolves the shell and leaves the egg without the outer membrane while having inner membrane attached
3)Soak one in the 70% fructose solution, another in air, and last one in water, make sure to refrigerate
4) Measure weight after a few days, test transparency, record and repeat
Experiment 2 (Tartrazine)
1) Make several tubes of 50mL of 0.6M/g concentration of Tartrazine
2) Prepare some raw chicken sliced in a few pieces
3) Soak raw chicken in Tartrazine in from a tube in step 1 for a day or so
4) Create a metric system (grid by using sharpie to write on the glass) that will be used to measure the translucency of what is being tested, make three of them
5) Take a sample of Tartrazine and mix it with milk, the base will be a coverslip with the metrics on it and then the solution will be poured on top
6) Prepare a Tartrazine sample by pouring some of the contents of the tube onto the base which is the coverslip with the metrics, four variations will be tested, one without coverslip on top, one with cover slip on top, one with glass rod between the two coverslips, and one with a metal rod between the two coverslips.
7) Put the raw chicken that is soaked with Tartrazine onto a cover slip thats in a UV light imaging chamber with the previously mentioned metric system set up behind it and capture pictures
8) Repeat step 7 except swap out the raw chicken with tartrazine that was tested with the milk and tartrazine solution and a tartrazine only solution
9) Adjust the height of both the tartrazine and tartrazine milk solution by adding a glass rod (view below) between the cover slip

10) Place both of the solutions mentioned in step 9 into the UV light imaging chamber and take pictures
11) Adjust the height once again of both the tartrazine and tartrazine milk solution by removing the glass rod and adding a more thinner metal rod between the cover slip
12) Place both of the solutions mentioned in step 11 into the UV light imaging chamber and take pictures
13) Take cover slip and metal rods off the solutions
14) Place both of the solutions mentioned in step 13 into the UV light imaging chamber and take pictures
15) Analyze and compare pictures
Experiment 3 (Hair)
1) Take some hair and place it within some Hydrogen Peroxide 10% (20mL).
2) Wait around a month or so
3) Take pictures of result of hair
Experiment 4 (Meat)
1) Cut two 5g of raw chicken breast
2) Put two pieces of chicken breast in two separate 25mL of 100% alcohol
3) After a few days, take one piece and put it into a 25mL 70% fructose solution
Experiment 5 (Leaf)
1) Harvest 4 water jasmine tree leaves
2) Place half into Hydrogen Peroxide
3) Wait a month or so for the decolourization to take effect
4) Take 1 of the other leaf remaining and put it in 70% fructose (50mL)
5) Take the last leaf remaining and put it in water (50mL)
6) Wait a few days
7) Take photos and compare and contrast
Experiment 6 (Egg membrane)
1) Crack an egg
2) Take the membrane that is aligned with the egg and divide into similar pieces
3) Put one of the piece in 70% concentration of fructose
4) Wait a few days
5) Compare by placing it on a plate with distinct features and capture pictures for further analysis
Experiment 7 (Milk)
1) Place some 20% of 2% milk in a tube with water with radius 0.5 cm
2) Place a piece of black paper with white letters behind
3) Place a tube with radius 0.5 cm with water and air in front of the black paper and take pictures
4) Put the tube with the milk in front of the black paper and see if the letters behind are visible, if letters aren't visible proceed with step 5
5) Dump half the milk out add water, shake thoroughly to make sure it is mixed well, retry step 4
6) Count the numbers of times step 5 was executed, then take 20/ (2)^number of times step 5 was executed, that is the percentage of concentration of milk assuming that the 2% milk was full concentration
7) Compare and analyze the pictures
Observations
During the removal of the eggshell, the eggs are shown in Figure 1 (ignore the paint on the side of the glass), there is Carbon Dioxide forming on the side of the egg shell.

Figure 1
Afterwards in figure 2, it can be seen that the egg shell is coming off.

Figure 2
The completely deshelled eggs are shown in Figure 3

Figure 3
The eggs in figure 3 look very yellow and are bouncy and a little squishy from what was observed. The yolk inside is pretty indistinguishable from the egg white.
Three eggs were tested and are shown in the images below. All were refrigerated. After a few days they were taken out and examined
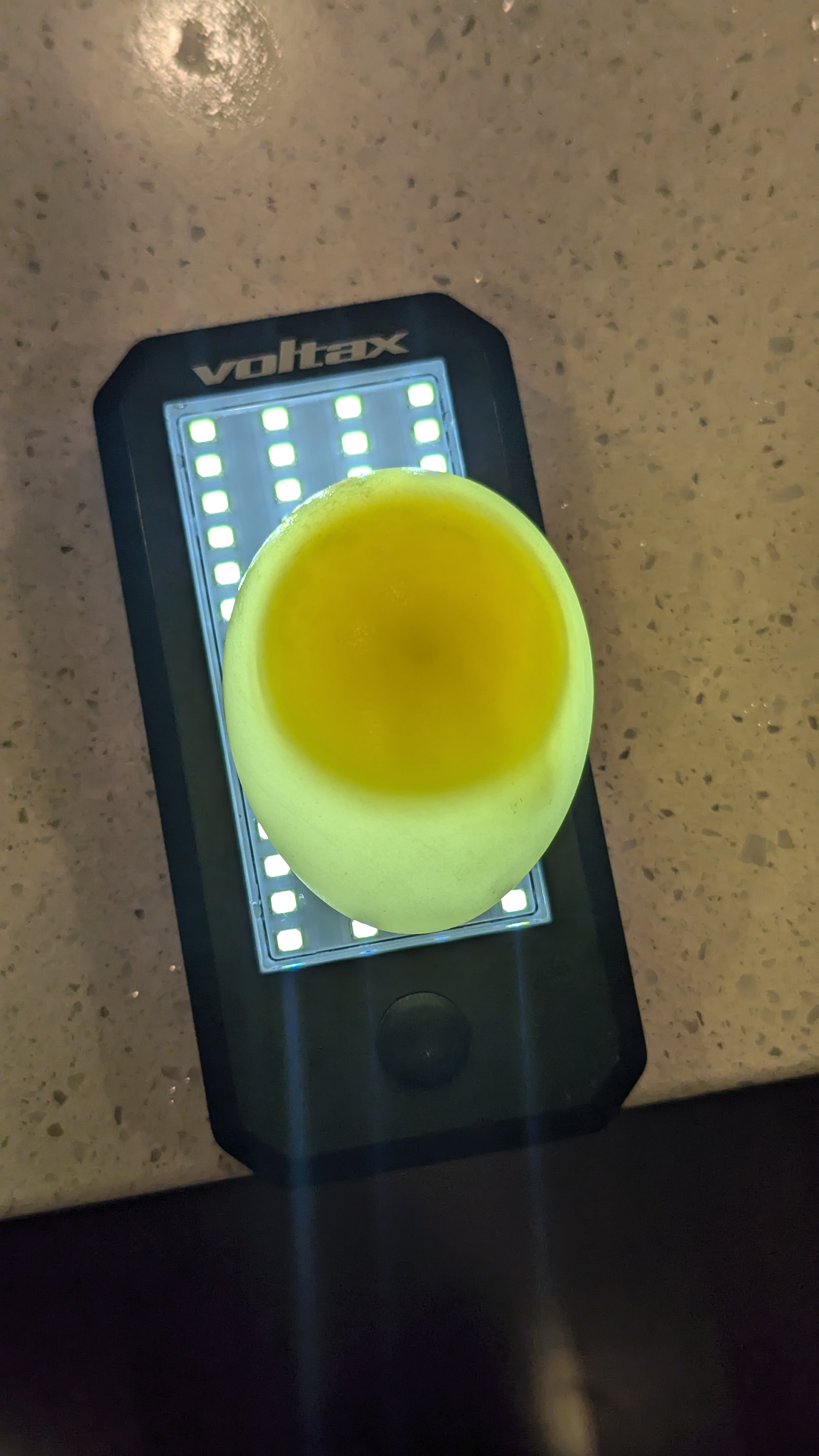
| Egg in Fructose Solution (figure 4) | Egg in Water (figure 5) | Egg in Air (figure 6) |
 |
 |
 |
Figure 4's egg is shown to be much more clearer visually (this serves as a way of using visualization to discern the transparency).
The egg in water and the egg in air (figure 5 and 6) is seen to be much more blurry and it is harder to discern the egg yolk.
The egg in fructose mass increased significantly, going from 68 grams to start and ending at around 83 grams. The egg in air had relatively no mass change and was about 85 grams while the egg in the water stayed around 86 grams.

Figure 7
During the preparations of the tartrazine as shown in figure 7, dissolving it was very hard as the Tartrazine powder didn't dissolve completely and was very hard to completely dissolve with a small amount of water. Chunks of powdered Tartrazine would stick to the side of the tube.

Figure 8
The metric shown in Figure 8 is an similar example to what is behind the Tartrazine in Figure 9

Figure 9
The top (cover slip numbered 1) was filled completely with Tartrazine and the bottom (cover slip numbered 2) was Tartrazine that was very pasty (the concentration of the solution changed due to the addition of milk).
Below is the table for whether or not the grid could be seen through
(X means light couldn't pass through and C means it did pass through)
| Milk and Tartrazine | Tartrazine | |
| Without cover slip | C | C |
| Between two cover slips (lowest height) | C | C |
|
Between two cover slips with metal rods in between (medium height) |
X | X |
| Between two cover slips with glass rods in between (tallest) | X | X |
| No UV light (Figure 10) | With UV light (Figure 11) |
 |
 |
It can be identified that the grid is unable to be seen in figure 10 however it can be seen under the UV light in figure 11
The chicken soaked in Tartrazine wasn't transparent enough to see the grid behind it as shown in figure 12 and 13.
| No UV light (Figure 12) | With UV light (Figure 13) |
  |
 |
The closest chicken and Tartrazine that was close to transparency as it can see the partially of the meat inside but the grid is still not in sight if looked through the chicken (figure 15).
| No UV light (Figure 14) | With UV light Figure 15 |
 |
 |
In contrast to the fructose observations, most of the tartrazine UV images remained quite similar to the original and didn't have a huge difference after being shined by the UV light on.

Figure 16
The hair turned from black to a palish, light brown, white colour (figure 16 to figure 17).

Figure 17
The chicken didn't show a huge change between the one soaked in fructose compared to the one doused in alcohol (figure 18, left fructose, right alcohol). They also looked as if they were both cooked.

Figure 18

Figure 19
Shown under the light source (in figure 19) they both look pretty similar, with the alcohol on the right side looking more transparent (though it is probably due to the thickness of the meat).
The leaf soaked in hydrogen peroxide looked like those leaves that were used for bookmarks, it was very clear and the veins of the leaves were clearly shown (figure 20).

Figure 20
The two leaves (one in water, one in fructose) didn't have much difference, except that the fructose soaked leaf had crumpled and folded in on itself (figure 21).

Figure 21

Figure 22
The membranes were folded the same amount of times (refer to figure 22) and in comparison visually, the fructose (left) looks way more clearer than the normal one (right).
The glass filled with air was very visible (figure 23) and the glass filled with water (figure 24) was visible but had the effect of refraction
| Air, figure 23 | Water, figure 24 |
 |
|

Figure 25
The starting tube of milk was completely opaque as shown in figure 25.

Figure 26
The starting concentration of 20% of 2% milk had to be diluted all the way to 0.625% of 2% milk in order for it to be somewhat translucent (figure 26).
Results of Transparent experiments below
| Sample | Pigment Removal | Method of Transparency | Transparency Result |
| Green Leaf | H₂O₂ oxidation | Fructose immersion | Successfully transparent |
| Human Hair | H₂O₂ oxidation | Fructose immersion | Didn't have a high enough resolution microscope to test the transparency |
| Chicken egg membrane | Not required | Fructose immersion | Successfully transparent |
| Whole Chicken Egg (deshelled) | Shell removal (vinegar) | Fructose immersion | Successfully transparent (yolk highly visible) |
| Chicken meat | Not required | Fructose immersion | Unsuccessful |
| Chicken meat (Tartrazine/UV) | Not required | Internal refractive index modification (UV light) |
Partially successful, few parts of experiment worked |
Analysis
Milk Opacity Experiment (scattering of light), physics and biology:
This experiment is a demonstration of the effects of the scattering of light which is more commonly known as the Tyndall effect. Milk is considered a "colloid" (it has many fat particles floating in it) and therefore falls under the Tyndall effect, causing the scattering of light, which in turn results in it being opaque.
Higher percentage of milk within the solution meant that there would be more scattering of light while the smaller concentration of milk, which in turn means that there are less particles that could absorb or scatter the light, would be much clearer.
Fructose experiment (chicken meat, egg shell membrane, egg without the shell), physics and chemistry:
Within the organic material, there are many different parts that make it up, and each part most likely has a varying degree of a refractive index. This will likely cause the light to scatter around, making it opaque. By adding fructose (which has a refractive index of 1.5 meaning light travels 1.5 times slower in fructose then the air), it "corrects" the refractive index, making them all unionized.
Understanding the physics:
In order to get the fructose to enter the organic material, it isn't just possible to grab a syringe and inject the organic material with it. Instead, this is where our good friend diffusion comes in. Diffusion occurs when a solution with a high concentration moves into a solution with a low concentration. In this case, the high concentration solution is fructose. The fructose which is at 70% concentration moves through to the organic material, which doesn't have any fructose at all. Once the organic material is soaked with fructose, it will become much more transparent.
Breakdown of egg shell membrane and egg without the shell:
In order to test the egg, the shell must be removed as it is opaque from the start and cannot be turned transparent using the fructose method. This was performed by using the vinegar and calcium carbonate reaction which dissolved the egg shell into carbon dioxide, water, and calcium ions as previously mentioned in the procedure. The chemical formula is listed below:
CaCO3+2H+→Ca2++H2O+CO2
The egg without the shell experiment demonstrated that when put in fructose, it became much clearer and translucent. However, the mass did go up by around 15 grams, meaning that water got in. The water that got in got in by using osmosis, where the water traveled from the fructose into the egg. The egg white is known to be transparent while still raw and shouldn't have been influenced significantly by the fructose. In order to prove this, the egg shell membrane experiment was performed, using only the membrane as a test. As shown in observation the fructose soaked membrane did way better than the regular membrane. Confirming that most of the success in the egg without the shell experiment was dictated by the influence of the membrane.
For the egg without the shell, since the grid metric wasn't used, quantitative proof is needed and by using image J, the stats can be seen. In order to measure, the first step of action is to turn the images of the egg into black and white versions (8-bit), and use a mean to calculate the intensity of the light. This was done three times in different areas to make sure one of them wasn't just a fluke. The most leftist image has the first data set and is the fructose picture, the middle image has the second data set and is the egg left in air picture, and the right image has the third data set and is the egg in water picture, this will apply for all three figures. The results are shown below:

Figure 27

Figure 28

Figure 29
As it can be seen in all of the three samples, the leftist one had the lowest average, meaning that the light was being blocked out by the yolk the best. Scattering will typically cause the light to spread out evenly (same reason why there aren't very defined and well outlined shadows during cloudy days). So it can be concluded
Chicken meat breakdown:
The reason why the chicken meat experiment didn't provide positive results is most likely due to the fact that the alcohol denatured the meat, affecting the protein which turned its chemical structure completely different making it opaque. The meat might have also been different sizes leading to incorrect/flawed results. What could also be taken into consideration is that fructose itself is considered a very good preservative, which might maintain some physical properties of the meat, including the opaqueness
Leaf Experiment and Hair Experiment (Pigment Removal), chemistry and biology:
This experiment demonstrates that even with the power of changing the refractive index with fructose, there is also most of the time another step that must be executed in order for transparency or translucency to be reached. These experiments use Hydrogen Peroxide (H2O2) to try and "bleach the materials", removing every pigment that isn't transparent or translucent. It should be kept in mind that the fructose experiment attempts to change the refractive indexes of materials to match that similar to glass (RI 1.5). However, molecules (or in this cas pigments) from the beginning that are already opaque don't have a refractive index as it doesn't let light pass through, it only allows absorption and reflection to occur. For both the hair and leaf, they were successfully "bleached" and lost most of their colour.
Understanding the Chemistry:
In both of the cases the pigments that were removed were the chlorophyll which were green coloured and made up most of the leaves pigments (as well as the carotene which has an orange colour, the ones you see in the fall), and the melanin which made up most of the hair pigments.
When the H2O2 reacts with the pigments the H2O2 goes through a reduction reaction and the pigment goes through an oxidation reaction. The H2O2 gains electrons and loses oxygen making it become water or simply H2O while the pigment loses hydrogen ions and electrons, the latter makes it colourless. The pigments will also gain oxygen molecules, which is where the term oxidized comes in. To sum it up
Pigment(colored)+H2O2→Pigment+(colorless)+2H2O
In the end this chemical reaction helped remove pigment-induced opacity as shown by how the leaf and hair were much more transparent to the eye.
Tartrazine Meat Experiment, chemistry and physics:
Understanding the key principle:
The tartrazine experiment works due to one significant relation, the Kramers-Kronig relation. The Kramers-Kronig relation is when there is a stronger absorption of the UV light at shorter wavelengths (UV-blue) indirectly increases the refractive index at a tiny bit longer wavelengths (red or near-infrared). This relation affects Tartrazine because Tartrazine absorbs light within the range 400–500 nm. Therefore, Tartrazine doesn't absorb the red or near-infrared light, which makes the refractive index increase as the only light available that we can see is red and its refractive index is within a certain range (around 1.45). This greatly reduces the scattering of light and makes it much more transparent. The reason that the meat is able to be seen clearly, is also thanks to the fact that Tartrazine transmits light that is above 550 nm, causing it to be seen and recorded by the camera.
Analysis of the Tartrazine experiment:
The Tartrazine experiment utilizes the power of diffusion (just like fructose) in order to transfer to the organic material. The chicken in the Tartrazine didn't yield the result that was wanted as the grid behind it was unable to be seen. This result is most likely caused by the fact that the chicken was too thick, which didn't allow the Tartrazine to soak thoroughly. Tartrazine is able to make most organic material transparent and this is due to the reduction of light scattering, this is why the milk that was with the Tartrazine was able to be seen through. When using the glass and metal rods to add height, the experiment gave negative results which makes it seem as if the UV light isn't strong enough (the imaging machine was very old) and therefore couldn't send the required amount of light for the effects to be seen.
Conclusion
The goal of this project is to introduce the concept of transparency through multidisciplinary approaches. Where biology, chemistry, and physic crossover to make organic material turned transparent achievable.
The biology part of this project is that everything tested is an organic material. Organic materials are made up of many types of biochemical materials such as pigments, proteins, and lipids/fats. They interact with light by absorption, reflection, and transmission. Such materials absorb and scatter light making it opaque. In this project, I investigated the method of making opaque organic material transparent through chemistry and physics.
The chemistry part of this project is when precise concentration is required to measure the quantity of fructose and Tartrazine (e.g. grams/ Moles). Furthermore, it is important to understand how the reactions such as oxidization and reduction take place to decolour. This is very essential when trying to make pigment rich organic material transparent. In short, these help build up the foundation before transparency can be achieved using light interaction (physics).
The physics part of this project is how the light interacts with molecules. Does it go through? Does it get absorbed? Or has its direction changed? The incredible amount of ways light interacts is astounding. Not to mention the complex relationship between the refractive index and the wavelength absorption. But in the end it all ends with transmission and matching refractive index, whether or not the light can pass through unified, this is why some things are transparent and others are not. It is also when osmosis or diffusion happens, when molecules move from one substance to another due to the differing concentration.
Application
Being able to make things transparent can greatly increase the research efficiency and level of biological and medical research. This will mean that no more dissections or surgeries will be needed to visualize what is inside. It will allow the visibility of organs and tissues to increase. This might allow the opportunity for the studying of the various effects of disease or certain conditions on different parts of the body.
It will be very beneficial if transparency can help serve as educational demonstrations, teaching students about the internal structures of multicellular living organisms.
Transparency will allow the monitoring of cellular growth, regeneration, and distinction methods, which will improve tissue engineering methodologies. This can serve to make great strides of improvements within this field.
Sources Of Error
This project had multiple sources of error and to categorize them I will be using the experiment that took place.
The milk experiment:
When diluting the milk, it was based on the human mind calculating where the halfway mark was.
Fructose Chicken experiment:
The chicken slices weren't particularly similar in terms of height, length, and width. The alcohol denatured the chicken, changing its composition up.
Fructose Egg experiment:
For the membrane only, the two slices didn't exactly have similar dimensions and the folding was very rough.
For the entire egg without the shell, the starting mass of the egg soaked in fructose changed quite drastically after being soaked.
Hair Experiment:
The dates weren't completely recorded down and the time it spent in the hydrogen peroxide is a rough estimate. There wasn't a microscope that could be used to see if the hairs turned truly "transparent".
Leaf Experiment:
The leaves were left in their respective solution for too long.
Tartrazine Experiment:
The equipment wasn't the best suited for shining the UV light onto the Tartrazine. The incubation of meat in the Tartrazine wasn't long enough, which may have prevented the dye for penetrating all the way through the meat. The meat might have been too thick as it didn't yield any positive results.
Citations
Hildebrand, S., Schueth, A., Klaus von Wangenheim, Mattheyer, C., Pampaloni, F., Hansjürgen Bratzke, Roebroeck, A. F., & Ralf A. W. Galuske. (2020). hFRUIT: An optimized agent for optical clearing of DiI-stained adult human brain tissue. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-66999-3
Ou, Z., Duh, Y.-S., Rommelfanger, N. J., Carl, Jiang, S., Brinson, K., Zhao, S., Schmidt, E. L., Wu, X., Yang, F., Cai, B., Cui, H., Qi, W., Wu, S., Adarsh Tantry, Roth, R., Ding, J., Chen, X., Kaltschmidt, J. A., & Brongersma, M. L. (2024). Achieving optical transparency in live animals with absorbing molecules. Science, 385(6713). https://doi.org/10.1126/science.adm6869
Smith, R. A. W., Garrett, B., Naqvi, K. R., Fülöp, A., Godfrey, S. P., Marsh, J. M., & Chechik, V. (2017). Mechanistic insights into the bleaching of melanin by alkaline hydrogen peroxide. Free Radical Biology and Medicine, 108(108), 110–117. https://doi.org/10.1016/j.freeradbiomed.2017.03.014
What is Scattering of Light? - Definition, Examples & Types. (2021, August 2). GeeksforGeeks. https://www.geeksforgeeks.org/what-is-scattering-of-light/
Acknowledgement
I would like to thank my father for supporting me through most of the project. He had access to his lab that had equipment which was necessary for the tartrazine experiment. He also taught me the g/M formula. My father also guided me through some parts that I was struggling on such as research. He would also help me hold the objects of interest and I would take pictures of them. He taught me how to use Image J in analysis.
I would also like to thank Chat GPT for giving a lot of insight on things I didn't know such as the simplified explanation of the Kramers-Kronig relation. It really made it easier to swallow down what seemed like the most complicated things in the world.
My mother also played a huge supporting role during my project, correcting my gramatical and helping me take several pictures.
Thank you to Mr. Ross Thompson for overseeing the Science Fair Club and supporting this project.