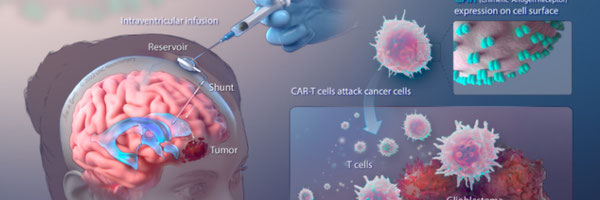
Design of Chimeric Antigen Receptor (CAR) towards EGFRVlll as a GBM therapeutic target
Grade 10
Presentation
Problem
What factors contribute to the lack of effective treatments that achieve complete regression without recurrences in glioblastoma?
- Glioblastoma, the most prevalent and deadly brain tumor, remains a challenge with no effective treatment achieving complete regression and preventing recurrences. Despite conventional treatments such as surgery, radiotherapy, and chemotherapy, the prognosis remains bleak, with an average survival period of 14.6 months and frequent recurrences. Nonetheless, emerging immunotherapeutic strategies, including oncolytic viruses, immune checkpoint inhibitors, and modified T cells like CAR-T therapy, offer a new promise.
What are the most effective structural components of chimeric antigen receptors (CARs) targeting EGFR for glioblastoma multiforme (GBM) therapy?
- EGFR-Targeting Domain: Specifically designed to recognize EGFR on GBM cells.
- Signaling Domains: CD3ζ and co-stimulatory domains like CD28 or 4-1BB for CAR-T cell activation.
- Spacer and Transmembrane Domains: Provide structural support and facilitate signaling.
- Co-stimulatory Molecules: Enhance CAR-T cell proliferation and anti-tumor activity.
- Persistence Enhancers: Features like cytokine receptors or suicide genes improve CAR-T cell survival in the tumor microenvironment.
What is the impact of incorporating co-stimulatory molecules into EGFR-targeting CARs on the persistence and anti-tumor activity of CAR-T cells in GBM models?
- Incorporating co-stimulatory molecules like CD28 or 4-1BB into EGFR-targeting CARs boosts CAR-T cell activation and proliferation, leading to enhanced persistence and anti-tumor activity in GBM models. These signals not only prolong CAR-T cell survival within the tumor but also amplify their cytotoxic effects, resulting in more effective tumor cell eradication.
Method
1.Prepare the 3D structures of the CAR-T cell receptor and the EGFRvIII mutant peptide antigen
2.Define the active sites and parameters for the docking simulation, specifying the binding pocket on the CAR-T cell receptor and the EGFRvIII mutant peptide antigen.
3. Perform molecular docking simulations allowing the CAR-T cell receptor to interact with the EGFRvIII mutant peptide antigen.
4. Analyze the docking results focusing on hydrogen bond formation and other key atomic interactions that contribute to stable binding.
Research
Glioblatoma Multiforme (GBM):
- Glioblastoma multiforme (GBM) stands out as an aggressive primary brain malignancy, affecting more than 4 individuals per 100,000 annually in Canada. The disease initiates from uncontrolled proliferation of astrocytes, essential glial cells supporting brain function. GBMs typically exhibit rapid growth and invasive behavior, primarily localized within the cerebral hemispheres but potentially spreading to less common brain areas, including the cerebellum, brainstem, or spinal cord. Despite conventional treatments such as surgery, radiotherapy, and chemotherapy, the prognosis remains bleak, with an average survival period of 14.6 months and frequent recurrences. Nonetheless, emerging immunotherapeutic strategies involving tumour neo-antigen vaccines, modified T cells, oncolytic viruses, and immune checkpoint inhibitors offer promising avenues for addressing this formidable disease.
Symptoms
- The symptoms produced by a brain tumour largely depend on its location rather than its specific pathological properties. While some tumours can rapidly cause symptoms, others may remain asymptomatic until they grow to a larger size. Gliomas, in general, lead to symptoms due to both their physical mass affecting the surrounding brain and their direct infiltration of brain tissue. Common signs include persistent headaches, nausea, vomiting, and seizures, resulting from the tumours compression and damage to healthy brain tissue. Additionally, specific neurological deficits arise depending on the tumours location. For example, if it affects the cerebellum lobe, it may cause weakness to muscles, coordination and balance; involvement near the sensory cortex could lead to numbness or difficulty identifying objects through touch. Tumours in the frontal lobe may trigger changes in personality, behavior and memory; while those impacting temporal lobe may hinder speech, emotion, and hearing. Occipital lobe involvement might result in partial or complete vision loss, creating a range of symptoms that vary based on the tumours location and extent of spread.
Figure 1. Illustrates the GBM tumour exerting pressure on the frontal lobe, resulting in the manifestation of headaches and associated symptoms
Admin. (2021, March 19). Glioblastoma multiforme | Altair Health. Altair Health. https://altairhealth.com/glasser-center/glioblastoma-multiforme/
Dignosis
- Diagnosing glioblastoma entails a multimodal approach to thoroughly evaluate the state of the brain. One approach begins with imaging examinations such as Computed Tomography (CT) scans or Magnetic Resonance Imaging (MRI), which are essential for identifying the location, size, and features of tumours. Another important phase in the process is the biopsy, which involves looking under a microscope at a small sample of tumour tissue to identify the kind, grade, and particular molecular characteristics that affect prognosis and treatment strategy. Furthermore, neurologic exams evaluate overall neurological functions, detecting abnormalities or deficiencies brought on by GBM tumours. Genomic analysis delves into genetic testing of tumour tissue, uncovering genetic mutations that inform personalized treatment strategies. These diagnostic approaches, inclusive of advanced imaging and invasive procedures like biopsies, provide clinicians with comprehensive insights into glioblastoma, guiding tailored treatment approaches based on the tumours characteristics. Definitive diagnosis relies on advanced imaging like MRI, while the essential tissue biopsy confirms the tumours presence and guides personalized therapeutic interventions based on its unique molecular markers.
Figure 2. MRI spectroscopy of normal brain. The NAA peak is the most prominent (If the amount of NAA is more than choline, that would suggest a normal brain, the opposite raises suspicion of a tumour).
P Thakkar, J. P. T., Paolo Peruzzi, P., & C Prabhu, V. (n.d.). Glioblastoma multiforme – symptoms, diagnosis and treatment options. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme#:~:text=Glioblastoma%20(GBM)%2C%20also%20referred,not%20spread%20to%20distant%20organs
Figure 3. Glioblastoma as visualized through Magnetic Resonance Imaging (MRI) scans.
Lobera, A., MD. (n.d.). Glioblastoma (Multiforme) imaging: practice essentials, computed tomography, magnetic resonance imaging. https://emedicine.medscape.com/article/340870-overview
Glioblastoma (GB) - Brain Tumour Foundation of Canada. (2019, November 19). Brain Tumour Foundation of Canada. https://www.braintumour.ca/brain_tumour_types/glioblastoma-gb/#:~:text=The%20incidence%20of%20glioblastoma%20(GB,Brain%20Tumour%20Registry%20of%20Canada
Osmosis from Elsevier. (2023, September 24). Glioblastoma (Year of the Zebra) [Video]. YouTube. https://www.youtube.com/watch?v=waliaz_0-54
Major Current Therapeutic Options For This Grade IV Tumour:
Surgery:
-
Glioblastomas (GBMs) are treated mostly through surgery, which attempts to remove as much tumour tissue as possible while protecting critical brain regions that are essential for proper neurological function. The problem, however, is that GBMs are infiltrative, meaning that the tumour cannot be completely removed since the tumour cells migrate and invade other brain regions.
-
Surgeons use advanced techniques such as intraoperative mapping and neuronavigation, with computer-assisted guiding to identify critical brain regions and tumour boundaries. Furthermore, cutting-edge methods have gained popularity, such as fluorescence-guided surgery employing agents like 5-aminolevulinic acid (5-ALA). This method helps surgeons remove tumours more precisely by illuminating the tumour cells and making them more visible under particular light wavelengths than normal brain tissue.
- Surgery offers essential benefits by diminishing solid tumour volume, targeting resilient cells at the tumours core that might resist radiation or chemotherapy, and alleviating intracranial pressure. Surgery has a reducing effect that may help patients have a greater life span and have a higher quality of life overall.
Figure 4. Glioblastoma microenvironment and development of ideal fluorescent probes for fluorescence-guided surgery. Created with BioRender.com.
Chirizzi, C., Pellegatta, S., Gori, A., Falco, J., Rubiu, E., Acerbi, F., & Bombelli, F. B. (2023). Next‐generation agents for fluorescence‐guided glioblastoma surgery. Bioengineering & Translational Medicine. https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/btm2.10608
Radiation:
-
A crucial stage in the treatment of glioblastoma is radiation therapy, which comes after surgery and wound healing. The main goal is to specifically target and eliminate any remaining tumour cells that have been embedded in the nearby healthy brain tissue. Traditional external beam radiation therapy targets the infiltrating tumour cells while inevitably damaging the surrounding normal tissue. It consists of several sessions of standard-dose "fractions" of radiation administered to the tumour location and its surrounding tissue.
-
Although the targeting is non-specific, radiation treatment takes advantage of the varying capacities of cells to mend itself. Because healthy cells have effective mechanisms for repair, they usually recover between radiation treatments, while tumour tissue is less able to repair itself and so becomes more damaged over the course of multiple sessions. Depending on the features of the tumour, this approach is carried out over the course of 10 to 30 treatment sessions, often once day, five days a week. When radiation therapy is used in place of only surgery or supportive care, many patients results are improved.
- Newer methods of radiation therapy, such proton therapy or intensity-modulated radiation therapy (IMRT), are intended to improve the way radiation is delivered. With the help of IMRT, radiation dose modulation can be adjusted more precisely, improving tumour conformance and reducing radiation exposure to nearby healthy tissues. By concentrating energy at the tumour site, proton therapy reduces damage to neighbouring healthy tissue and provides a more focused kind of radiation therapy. These developments could potentially improve radiation therapy's efficiency in treating glioblastoma.
Figure 5. Glioblastoma stem cells (GSCs) possess the unique ability to self-renew, trigger tumour initiation, and exhibit regression when subjected to radiotherapy.
Yousuf Ali, M., R. Oliva, C., & M. Noman, A. S. (2020, September 3). Radioresistance in Glioblastoma and the Development of Radiosensitizers. https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.mdpi.com%2F2072-6694%2F12%2F9%2F2511&psig=AOvVaw0TgUj9lVvHF729FxtQLqsw&ust=1704591430693000&source=images&cd=vfe&opi=89978449&ved=0CBMQjRxqGAoTCIjFvpfQx4MDFQAAAAAdAAAAABCmAQ
Radiosurgery:
-
In order to minimize radiation exposure to nearby healthy brain tissue, radiosurgery, a precision radiation treatment, uses specialized devices to target tumour areas with high accuracy. Radiosurgery is an essential treatment for glioblastoma in many cases, especially when there are recurrent tumours even if it is not as prevalent as it formerly was. Advanced imaging modalities like as Positron Emission Tomography (PET) scans and Magnetic Resonance Spectroscopy (MRS) also support radiosurgery by offering comprehensive information about the metabolic activity and features of the tumour, which helps with target delineation and treatment planning
-
Because GBM is an invasive and diffuse illness, it can be difficult to efficiently target every tumour cell, which contributes to the restricted use of radiosurgery in initial treatment. Its importance in recurrent tumours frequently stems from its capacity to target exact regions with radiation, successfully treating localized recurrences while avoiding needless radiation exposure to adjacent healthy brain tissue. To manage this aggressive brain cancer, complete multimodal therapy are necessary, as the overall effectiveness of radiosurgery as a stand-alone treatment for GBM remains restricted. Optimizing therapeutic advantages and improving outcomes may be possible by incorporating radiosurgery into a multimodal therapy plan that is customized for each patient.
Chemotherapy:
-
Patients undergoing chemotherapy are given specialized medications designed to combat malignant cells. The current acknowledged standard of therapy for glioblastoma, involves the use of temozolomide as one of the primary drugs. This drug is often taken every day while receiving radiation therapy. During the maintenance phase, it is continued for six cycles, each lasting 28 days. Every cycle starts with the five days of temozolomide treatment, followed by a 23-day rest period.
-
Temozolomide is a member of the alkylating agent class of drugs and is an essential component of the chemotherapy treatment. It functions by causing damage to the DNA of rapidly dividing cells (cancer cells), which eventually results in their death or inhibits them from proliferating. This drug is a standard treatment for newly/early diagnosed GBM. Temozolomide is effective because it can cross the blood brain barrier, allowing it to reach and target the tumour cells, where the drug methylates (adding a chemical group called
methyl) the DNA strand.
Figure 6. Treatment with TMZ causes DNA methylation, leading to cell arrest. If DNA damage repair is successful, the cell recovers to the proliferating pool, or undergoes apoptosis otherwise.
Sorribes, I. C., Handelman, S. K., & Jain, H. (2020). Mitigating temozolomide resistance in glioblastoma via DNA damage-repair inhibition. Journal of the Royal Society Interface, 17(162), 20190722. https://royalsocietypublishing.org/doi/10.1098/rsif.2019.0722
Immunotherapies:
-
By using the immune system's capacity, developments in immunotherapy have demonstrated a strong potential to improve outcomes for patients with brain tumours Numerous strategies have demonstrated noteworthy bioactivity against different forms of cancer. These strategies include immune checkpoint inhibitors, modified T cells, and tumour neo-antigen vaccines.
- Chimeric antigen receptor (CAR) T cell therapy is one of these approaches that stands out as a cutting-edge GBM treatment plan. This novel treatment modifies a patient's T cells to express specific receptors that bind to particular antigens on tumour cells. Preclinical tests have shown promising results, but the transition to clinical trials has not yet produced clear advantages. To efficiently target GBM antigens, T cells are extracted and modified as part of this therapy before being reintroduced into the patient's system.
P Thakkar, J. P. T., Paolo Peruzzi, P., & C Prabhu, V. (n.d.). Glioblastoma multiforme – symptoms, diagnosis and treatment options. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme#:~:text=Glioblastoma%20(GBM)%2C%20also%20referred,not%20spread%20to%20distant%20organs
Fernandes, C., Costa, A., Osório, L., Lago, R. C., Linhares, P., Carvalho, B., & Caeiro, C. (2017). Current standards of care in glioblastoma therapy. In Codon Publications eBooks (pp. 197–241). https://www.ncbi.nlm.nih.gov/books/NBK469987/
Targeted Immune Therapy For GBM:
Potential Targets:
1. Human Epidermal Growth Factor Receptor-2 (HER2)
-
In about 80% of cases of Glioblastoma (GBM), human epidermal growth factor receptor 2 (HER2) is significantly overexpressed; showing promise for targeted therapies, and being linked to tumour growth and aggressiveness. However, the challenge in targeting HER2 specifically in GBM lies in its dual role, not only promoting tumour cell growth but also its wide expression in healthy tissues such as epithelial cells of the gastrointestinal tract, lungs, and ovaries, among others. Therapeutic strategies aimed at HER2 must balance effectively targeting the tumour while minimizing harm to healthy tissues, mitigating potential side effects such as on-target-off-tumour effects.
- Encouraging safety profiles have been observed in clinical trials examining the effectiveness of HER2-targeted therapies in patients with GBM; adverse events are mainly linked to lymphodepletion rather than specific HER2-related toxicity. Some patients demonstrated potential benefits despite the concerns associated with off-target effects. Notably, a subset of patients showed improvements, with a median survival of about 24.5 months following diagnosis and cases of apparent non-progression.
-
Recent studies show promising outcomes with third-generation HER2-specific CAR-T cells efficiently eliminating GBM cells in vitro and exhibiting enhanced activity when combined with PD-1 blockade. Clinical trials demonstrated successful administration of HER2-specific CAR-T cells, providing clinical benefits in treated GBM patients. While challenges persist in balancing effective tumor targeting and minimizing harm to healthy tissues, these trials offer hope for the potential effectiveness of HER2-targeted CAR-T therapy in GBM.
Luksik AS, Yazigi E, Shah P, Jackson CM. (2023 Feb 23). CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10000604
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
2. Interleukin-13 Receptor Subunit Alpha-2 (IL13Ra2)
-
One prominent target for CAR-T cell therapy is the unique variant receptor known as interleukin-13 receptor subunit alpha-2 (IL13Rα2), which is primarily present on 75% of glioblastoma (GBM) tumour cells. This receptor variant contributes significantly to the progression of brain tumours because it lacks a downstream signalling pathway that is typically associated with the normal receptor. Although IL13Rα2 is expressed in the majority of both adult and pediatric GBM tumors, it is not significantly present in normal brain tissues, except for the testis. This selective expression pattern makes it an appealing target for CAR-T cells, which can be engineered with a mutated form of IL-13 in the CAR construct.
-
Research examining the effectiveness and safety of this treatment in individuals with recurrent GBM has yielded conflicting results. IL13Rα2-specific CAR-T cells have demonstrated feasibility and safety in a first-in-human pilot study, with encouraging clinical responses reported in patients with recurrent GBM. However, the outcomes remain variable among individuals undergoing this therapy, emphasizing the need for further research to optimize its effectiveness and address conflicting results in clinical settings. Despite one patient showing an initial positive response, most patients overall outcomes did not show significant improvements. Even though there were some patients who showed decreased tumour recurrence in peripheral areas, the treatment did not have a conclusive effect. These results illustrate the range of reactions among patients receiving this particular treatment.
Luksik AS, Yazigi E, Shah P, Jackson CM. (2023 Feb 23). CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10000604
- Chimeric antigen receptor (CAR) T cells were administered to a patient with recurrent multifocal glioblastoma; CAR-T cells were engineered to target interleukin-13 receptor alpha 2 (IL13Rα2), a specific protein associated with GBM. Over the course of 220 days, multiple infusions of these CAR-T cells directly into the brain via two separate routes: infusions into the resected tumour cavity followed by infusions into the ventricular system. Following this treatment, a remarkable outcome was observed, a complete regress of all brain and spine tumours as well as an increase in certain immune cells and cytokine levels in the fluid surrounding the brain and spinal cord.
Figure 7. Regression of Recurrent Multifocal Glioblastoma, Including Spinal Metastases, after Intraventricular Delivery of IL13Rα2-Targeted CAR-T Cells.
Brown, C. E., Alizadeh, D., Starr, R., Weng, L., Wagner, V., Naranjo, A., Ostberg, Ms, B., Kilpatrick, J., Simpson, J., Kurien, A., Sj, P., Wang, X., Tl, H., D’Apuzzo, M., Ja, R., Mc, J., Me, B., Chen, M., . . . Badie, B. (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. The New England Journal of Medicine, 375(26), 2561–2569. https://doi.org/10.1056/nejmoa1610497 https://www.nejm.org/doi/full/10.1056/nejmoa1610497
3. Disialoganglioside GD2
- Gangliosides, a type of glycosphingolipids crucial for cellular processes, are potential targets for cancer immunotherapy. Disialoganglioside GD2, elevated in certain tumours like melanoma, retinoblastoma, and neuroblastoma, has been identified in glioblastoma (GBM) as a relevant target antigen. GD2-specific CAR-T cells show promise in preclinical studies, exhibiting potent cytotoxicity against neuroblastoma and GBM cells. Innovative approaches, like incorporating a truncated GD2-specific CAR into mesenchymal stromal/stem cells (MSCs), aim to enhance therapy precision. Ongoing trials, including those targeting high-grade gliomas like GBM, aim to optimize GD2-specific CAR-T therapy with strategies like expressing a constitutively active IL-7 cytokine receptor in CAR-T cells. GD2, actively explored in CAR-T cell therapy, holds potential for more effective and targeted treatment strategies in GBM and other cancers.
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
4. CD70
- CD70, a protein found in certain cancers, including glioblastoma (GBM), contributes to immune evasion by promoting T cell death. Its presence on GBM cells serves as an immune escape mechanism, allowing the tumour to avoid recognition and attack by the immune system. This evasion strategy is achieved through the constitutive expression of CD70 on GBM, creating an environment that hampers the immune response, particularly by inducing T cell death. To counter this immune evasion, studies have investigated CD70-specific CAR-T cells, which have shown the potential to recognize and eliminate CD70+ GBM tumours in preclinical models without inducing toxicity. This targeted immunotherapeutic approach aims to disrupt the immune evasion tactics employed by GBM.
5. Epidermal Growth Factor Receptor Variant lll (EGFRvlll)
-
40% of all glioblastomas (GBMs) have been shown to have increased levels of the epidermal growth factor receptor (EGFR), and in about 50% of cases, this receptor has a variant known as EGFRvIII. EGFRvIII, a deletion-mutation form of the epidermal growth factor receptor (EGFR), alters the structure of the extracellular domain, providing unique epitopes for targeted therapy. EGFRvIII expression is observed predominantly in GBM tumors, limiting its presence in healthy tissues and minimizing on-target/off-tumor toxicity risks. With its tumor-specific presence and restricted expression in normal tissues, EGFRvIII is an appealing target for CAR-T therapy, reducing the risk of toxicities.
-
Targeting EGFRvIII with third-generation CAR-T cells in clinical trials has resulted in unfavourable events linked to lymphodepleting chemotherapy, including severe neurological side effects. Trial outcomes have indicated a median progression-free duration of just one month post-treatment initiation, with an average survival period of 6.9 months following therapy. Despite clinical trial challenges, targeting EGFRvIII for GBM treatment holds promise, prompting ongoing research to refine strategies, enhance CAR-T cell efficacy, and reduce side effects. While preclinical models demonstrate effective tumor control with EGFRvIII-specific CAR-T cells, clinical trials face hurdles like antigen loss, adaptive resistance, and potential dose-dependent adverse events. The heterogeneous expression within gliomas poses a limitation, leading to the generation of escape variants resistant to CAR-T cell therapy and diminishing enthusiasm for EGFRvIII as a consistent and reliable CAR-T cell target.
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Luksik AS, Yazigi E, Shah P, Jackson CM. (2023 Feb 23). CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10000604
Combination Therapy with JQ1 and EGFR CAR-T:
-
Antigens such as EGFRvIII have been the subject of clinical trials that have provided insight into their safety and efficiency in treating GBM. On the other hand, the ability of CAR-T cells to identify tumour cells, endure in the tumour microenvironment (TME), and maintain their functional efficiency is necessary for the treatment of GBM. Specifically, after EGFRvIII CAR-T infusion, there is increased expression of immunosuppressive molecules like PD-L1 and IDO1 in the GBM TME, raising concerns about compromised immune responses triggered by CAR-T cells.
-
Furthermore, the study shows the significance of super-enhancers—clusters of active enhancers that regulate key genes linked to cancer. Here, BRD4 is a member of bromodomain and extraterminal (BET) protein family, it contributes to gene expression by binding to particular DNA regions. It specifically binds to DNA regions referred to as super-enhancers, which stimulates the transcription of certain cancer genes. Because BRD4 is susceptible to inhibition, compounds such as JQ1 can obstruct its activity. JQ1 influences the expression of genes connected to the development of cancer and the regulation of the immune system by preventing BRD4 from binding to DNA, suggesting possible therapeutic implications.
-
The function of active enhancers triggered by CAR-T cells was interfered with when BRD4 was inhibited by JQ1. The disruption had a major impact on the expression of genes that inhibit the immune response in the microenvironment of GBM. In xenograft models, JQ1 and EGFR CAR-T cells reduced immunosuppression, which in turn inhibited tumour growth and metastasis. The findings suggest that to overcome immunosuppression in treating GBM, a combination approach of EGFR CAR-T cells and BRD4 inhibition using JQ1 could significantly enhance the long-term effectiveness of CAR-T therapy. This proposition arises from the observed exhaustion signature in the T cells infiltrating GBM, indicating an adaptive resistance. However, agents that stimulate the anti-tumour functions of CAR-T or endogenous T cells might now help overcome this resistance and extend the efficiency of treatment beyond the initially limited success.
Figure 8. A working model for combination therapy with EGFR CAR-T cells and JQ1 in GBM
Lin Xia, Jun-yi Liu, Zao-zao Zheng, Guo-sheng Hu, Ning-shao Xia, Wen Liu. (2021, May 21). BRD4 Inhibition Boosts the Therapeutic Effects of Epidermal Growth Factor Receptor-targeted Chimeric Antigen Receptor T Cells in Glioblastoma.. https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(21)00301-4
Challenges faced in immune therapy/ limitions:
1. Blood-Brain Barrier (BBB):
-
A protective barrier that controls the flow of substances from the bloodstream into the brain is called the blood-brain barrier (BBB). One of the biggest obstacles to getting therapeutic agents—such as CAR-T cells to the brain is the blood-brain barrier (BBB). While the BBB is essential for maintaining a stable brain environment, its selective permeability makes it difficult to administer treatments effectively. To overcome this obstacle, novel approaches must be investigated, such as the modification of CAR-T cells or the use of cutting-edge delivery techniques like nanoparticles to aid in their entry into the central nervous system.
Figure 9. Illustrating the challenges posed by the Blood-Brain Barrier in primary brain tumours, including glioblastoma.
Dubois, L. G., Campanati, L., Righy, C., D’Andrea-Meira, I., De Sampaio E Spohr, T. C. L., Porto-Carreiro, I., Pereira, C. M., Balça-Silva, J., Kahn, S. A., DosSantos, M. F., De Almeida Rabello Oliveira, M., Ximenes-Da-Silva, A., Lopes, M. C., Faveret, E., Gasparetto, E. L., & Moura‐Neto, V. (2014). Gliomas and the vascular fragility of the blood brain barrier. Frontiers in Cellular Neuroscience, 8. https://www.frontiersin.org/articles/10.3389/fncel.2014.00418/full
2. Tumour Heterogeneity:
-
Primary brain tumours, particularly glioblastomas, exhibit heterogeneity in the expression of tumour-associated antigens (TAAs). Different cells within the tumour have different protein, phenotype, and genetic material profiles, making it challenging to identify a single antigen that can be targeted by CAR-T cells to treat the entire tumour. This can lead to antigen escape because the cancer cells may mutate and change the antigens on their surface or some cells may not have the antigen to begin with, making them unrecognizable to the CAR-T cells that were programmed into fighting the original cancer targets.
3. Immunosuppressive microenvironment:
-
Primary brain tumours often establish an immunosuppressive microenvironment, creating a challenge for effective CAR-T cell therapy infiltration. This microenvironment involves various immune cells and signaling molecules such as Inhibitory cytokines such as transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10), collectively hinder the body's immune response against the tumour. Within this setting, immune-suppressive factors released by the tumour cells contribute to an environment that restricts the activity of immune cells, making it difficult for them to mount a robust anti-tumour response.
- Within the immunosuppressive microenvironment of primary brain tumours, tumour-associated macrophages (TAMs) play a critical role by acting as the "first responders" in the body's immune system. Attracted to the tumour site by signals released from tumour cells, TAMs possess the ability to detect specific molecules or markers on the surface of tumour cells, facilitating the recognition of abnormal cells. This recognition typically triggers an immune response, activating other immune cells to join the fight against the tumour. However, TAMs can adopt a dual role, becoming either pro-inflammatory or immunosuppressive. In their immunosuppressive state, TAMs send signals to other immune cells that discourage immune cell activity often involving immunosuppressive molecules such as TGF-beta or IL-10; instructing them to stay away from the tumour site or dampen their activity. This immunosuppressive behavior of TAMs presents a significant challenge, as it hinders the CAR-T cells ability.
4. CAR-T Cell Exhaustion:
-
CART cell exhaustion presents a substantial challenge in maintaining effective and enduring anti-tumour responses. This state, marked by a gradual decline in cytotoxic functions, is notably influenced by prolonged exposure to tumour antigens and the suppressive microenvironment within tumours The design of CAR-T cells is crucial, weak structures may induce ligand-independent tonic signaling, contributing to exhaustion. Additionally, the cytokine composition during in vitro expansion and the milieu of the tumour microenvironment play pivotal roles in shaping CAR-T cell exhaustion. CAR-T cell exhaustion involve multifaceted challenges; ligand-independent tonic signaling refers to the activation of CAR-T cells without the presence of specific antigens, potentially leading to premature exhaustion. The composition of cytokines, essential for the expansion of CAR-T cells in vitro, needs meticulous consideration as imbalances or prolonged exposure can cause exhaustion. The tumour microenvironment, enriched with immunosuppressive factors like inhibitory cytokines, regulatory T cells, and myeloid-derived suppressor cells, further compounds the exhaustion challenge.
5. Acquired resistance:
- Acquired resistance in glioblastoma multiforme (GBM) poses a significant challenge to the effectiveness of chimeric antigen receptor (CAR) T cell therapies. Gliomas, including GBM, are characterized by substantial genetic, epigenetic, and environmental intratumoral heterogeneity. This intratumoral heterogeneity, coupled with the dynamic nature of the tumour microenvironment, contributes to the development of acquired resistance. One notable aspect is the loss or downregulation of the targeted tumour-associated antigens (TA) following treatment. For example, in the case of EGFRvIII, a common target in GBM, previous studies have observed escape mechanisms where a considerable proportion of patients experiencing disease recurrence had lost the expression of EGFRvIII. This phenomenon has been documented both in response to EGFRvIII-targeted peptide vaccines and EGFRvIII-specific CAR-T cell administration. The emergence of cancer cells that no longer express the targeted TA poses a significant hurdle as these cells can evade CAR-T cell-mediated killing, thereby allowing the disease to progress with an altered phenotype.
Figure 10. CAR-T cell therapy faces several limitations, encompassing challenges such as the immunosuppressive tumour microenvironment (TME), constrained access through the blood-brain barrier (BBB), on-target off-tumour toxicity, cytokine release syndrome, tumour lysis syndrome, and the potential for selective antigen loss.
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Strategies to Enhance Efficiency of Car-Based Immunotherapy Against GBM:
1. Locoregional administration:
-
Locoregional administration methods, such as intratumoural or intracavitary injection are targeted delivery method for therapeutic agents, enhances the precision and efficiency of CAR-T cell therapy. In this context, CAR-T cells administered directly into or in close proximity to the tumour, offers distinct advantages over systemic delivery. The approach aims to concentrate therapeutic cells precisely where needed, minimizing systemic circulation and potential off-target effects. This precision is crucial for engaging with heterogeneous tumours, ensuring CAR-T cells effectively target cancer cells expressing specific antigens. Additionally, locoregional administration optimizes cytokine release, such as IL2, within the tumour microenvironment. This localized cytokine production fosters sustained CAR-T cell activity, addressing challenges associated with maintaining T cell function in the immunosuppressive tumour microenvironment. The strategy exemplifies a significant advancement in refining the delivery and performance of CAR-T therapy.
2. Combination Therapies:
-
Specifically tailored for glioblastoma, a novel approach involves combining CAR-T cells with checkpoint inhibitors, showcasing promising outcomes in combatting this aggressive brain tumour and its immunosuppressive cells. Additionally, scientists are exploring the integration of CAR-T cells with glioblastoma-targeted oncolytic viruses. These engineered viruses not only selectively infect and eliminate glioblastoma cells but also contribute to an inflammatory response, rallying immune cells for a concerted assault on the tumour. To enhance CAR-T cell infiltration into the glioblastoma microenvironment, enzymes are utilized, clearing a path for therapeutic impact. Moreover, antibodies also aid in the treatment by neutralizing and blocking immunosuppressive cells, amplifying the potency of CAR-T cells in tackling glioblastoma.
-
Nanoparticles enhance CAR-T cell delivery through their ability to navigate the blood-brain barrier (BBB) and improve overall therapeutic efficiency. Their small size and surface modifications facilitate BBB penetration, ensuring CAR-T cells reach the central nervous system. Surface engineering enables targeted delivery to specific receptors on tumour cells or the BBB, enhancing precision. Nanoparticles also offer protective encapsulation, shielding CAR-T cells from degradation and providing sustained release, optimizing therapeutic impact. This approach minimizes off-target effects, concentrating treatment at the tumour site.
- A primary tactic involves utilizing PD1 and CTLA4 inhibitors to counteract the inhibitory signals contributing to CAR-T cell exhaustion. This approach aligns with current clinical practices, although the application of immune checkpoint inhibitors with CAR-T cells remains a relatively uncharted territory. Furthermore, understanding the distinctive characteristics of exhausted CAR-T cells, such as their epigenetic changes and transcriptomic abnormalities, introduces layers of complexity to intervention strategies. Additionally, targeting specific factors within the tumour microenvironment (TME) responsible for driving exhaustion are being explored. The TME, enriched with immunosuppressive elements like inhibitory cytokines, regulatory T cells, and myeloid-derived suppressor cells, poses a formidable challenge to CAR-T cell function. Researchers are strategically developing interventions to modulate this intricate environment, seeking to create a more conducive milieu for CAR-T cell activity.
3. Multi-targeting:
-
In glioblastoma, multi-targeting within CAR-T cell therapy strategically addresses challenges associated with this aggressive brain tumour. This approach involves targeting specific antigens on glioblastoma cells, aiming to enhance overall therapeutic effectiveness. By selecting multiple antigens as targets, the strategy diminishes the likelihood of glioblastoma cells developing resistance and mutated variants, creating a barrier against immune cells. This complexity also solves potential issues such as antigen escape and tumour heterogeneity, characteristic challenges in glioblastoma treatment. The multi-targeting approach, by concurrently recognizing and targeting multiple antigens, has the potential to amplify the number of glioblastoma cells identified and eliminated by CAR-T cells.
Figure 11. Antigen-specific CAR-T cells can be administered to patients to selectively recognize and eliminate tumour antigen-expressing glioblastoma cells.
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
4. Engineering CAR Constructs to Induce or Secrete Active Cytokines:
- One approach to enhance the antitumor efficiency of CAR-T cells involves engineering the CAR constructs to induce or secrete active cytokines. Cytokines, such as interleukins, play crucial roles in regulating immune responses. In this context, CAR-T cells, which are designed to express additional cytokines, can have improved activity and persistence within the tumour microenvironment. For instance, a study showed IL13Rα2 CAR-T cells engineered to produce interleukin-15 (IL-15) demonstrated greater anti-glioma activity in preclinical models. This modification not only enhances the direct cytotoxic effects of CAR-T cells on cancer cells but also promotes their prolonged survival and persistence, contributing to a more sustained antitumour response.
5. Disrupting Immunosuppressive Immune Checkpoint Molecules:
- Immune checkpoint molecules often hinder the effectiveness of CAR-T cells by weakening their activity within the tumour microenvironment. To overcome this challenge, strategies have been developed to disrupt the signaling pathways associated with immunosuppressive molecules. For example, CAR-T cells can be engineered to secrete antibodies against programmed cell death ligand 1 (PD-L1), a molecule involved in suppressing immune responses. Additionally, genetic modifications, such as CRISPR/Cas9-mediated knockout of genes associated with checkpoint molecules like PD-1 and Lag3, have been explored. Furthermore, innovative designs, like incorporating a PD-1 ectodomain linked to the transmembrane and cytoplasmic domains of CD28, aim to convert immunosuppressive signals into co-stimulatory ones.
Maity, R., Benaoudia, S., Zemp, F. J., Lee, H., Barakat, E., Leblay, N., Ahn, S., Mahoney, D. J., Neri, P., & Bahlis, N. J. (2021). A BCL2L1 armoured BCMA targeting CAR T cell to overcome exhaustion and enhance persistence in multiple myeloma. Blood, 138(Supplement 1), 327. https://ashpublications.org/blood/article/138/Supplement%201/327/478084/A-BCL2L1-Armoured-BCMA-Targeting-CAR-T-Cell-to
Lin, Y., Mashouf, L. A., & Lim, M. (2022). CAR T cell therapy in primary brain tumors: current investigations and the future. Frontiers in Immunology, 13. https://www.frontiersin.org/articles/10.3389/fimmu.2022.817296/full
Mo, F., Pellerino, A., Soffietti, R., & Rudà, R. (2021). Blood–Brain barrier in brain tumors: Biology and clinical relevance. International Journal of Molecular Sciences, 22(23), 12654. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8657947/
Zhu, X., Li, Q., & Zhu, X. (2022). Mechanisms of CAR T cell exhaustion and current counteraction strategies. Frontiers in cell and developmental biology, 10, 1034257. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9773844/
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Potential Side Effects/risks:
1. Cytokine Release Syndrome (CRS):
-
Cytokine Release Syndrome (CRS) results from the activation of immune cells, particularly T cells, post CAR-T cell infusion. When CAR-T cells engage with cancer cells, they release excessive cytokines such as interleukins (IL-6, IL-2, etc.) into the bloodstream, leading to a rapid immune system activation, and causing flu-like symptoms, fever, and potentially severe organ damage. Plus, in Cytokine Release Syndrome (CRS), there is a dysregulation of the normal balance between pro-inflammatory and anti-inflammatory cytokines; causing an imbalance between the pro-inflammatory and anti-inflammatory signals in the body. Excessive pro-inflammatory cytokines contribute to the inflammatory response characteristic of CRS, leading to symptoms such as fever, hypotension, and organ damage. On the other hand, anti-inflammatory signals may be released in an attempt to counteract the overwhelming inflammation. Tocilizumab, an antibody blocking interleukin-6 (IL-6) receptors, shows promise in alleviating CRS.
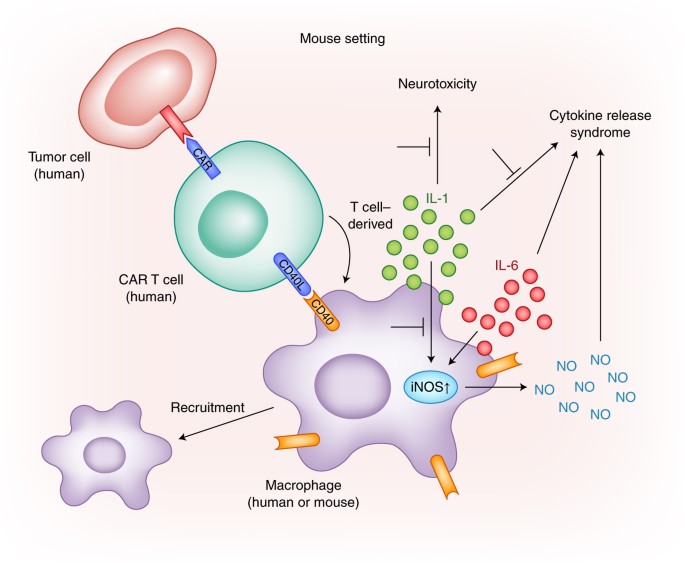
Figure 12. The activation of CAR-T cells in the presence of tumour-specific antigen (TSA) leading to the production of pro-inflammatory cytokines, including IL-6, and induce neurotoxicity through IL-1.
CRS Research Models - Creative Biolabs. (n.d.). https://www.creative-biolabs.com/car-t/crs-research-models.htm
2. Neurotoxicity in Brain-Targeted CAR-T Therapy:
- Administering CAR-T cells to the brain amplifies the risk of neurotoxicity, often concurrent with CRS. The exact mechanism remains unclear, but CNS endothelial cell activation is implicated. This activation increases blood-brain barrier permeability, leading to cytokine influx, seizures, and cerebral edema. While rare, a GBM CAR-T cell therapy trial reported a fatality, emphasizing careful consideration of CRS and neurotoxicity risks. While CAR-T therapy may also induce Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), marked by confusion and seizures. Anakinra, an IL-1 receptor-blocking antibody, could potentially address ICANS symptoms.
3. On-Target Off-Tumour/ Off-Target Off- Tumour:
- Beyond CRS and neurotoxicity, CAR-T cell therapy poses on-target off-tumour and off-target off-tumour effects. The on-target off-tumour effects occurs when CAR-T cells attack both cancer and healthy cells expressing the same antigen, potentially harming normal tissues. An example is CAR-T therapy specific to the target antigen HER2; its overexpressed in 80% of GBM cases, hovever, it also has a wide expression on the healthy cells of the gastrointestinal tract, lungs and ovaries, among others. The off-target off-tumour effects involves unintended attacks on antigens, causing damage to healthy tissues. Manufacturing CAR-T cells introduces risks, using viruses to modify T cells. Despite stringent designs, there's a risk of inadvertent disruptions in these mechanisms.
4. Safety Measures and Product Contamination:
- Ensuring CAR-T cell therapy safety demands meticulous adherence to protocols. Preventing product contamination is critical, averting complications like infections and sepsis. The CAR-T cell product, potentially carrying blood-borne pathogens, underscores the importance of strict adherence to handling and preparation guidelines. Balancing therapeutic benefits and mitigating risks is crucial for advancing CAR-T cell therapy in glioblastoma treatment.
Santomasso, B., Bachier, C., Westin, J. R., Rezvani, K., & Shpall, E. J. (2019). The other side of CAR T-Cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. American Society of Clinical Oncology Educational Book, 39, 433–444. https://ascopubs.org/doi/10.1200/EDBK_238691
CAR T-cell therapy and its side effects. (n.d.). American Cancer Society. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/car-t-cell1.html
Molecular Profile of GBM:
- Multigeneration EGFRvIII-specific CARs. CARs directed against EGFRvIII are produced by combining the humoral specificity of an EGFRvIII-specific antibody with the intracellular signaling domains of a T-cell receptor (TCR). In general, CARs are composed of the variable heavy and light chains of a mAb fused (via a transmembrane hinge) to CD3 z . More recently, CAR design has evolved to include additional costimulatory moieties—namely CD28 and/or 4-1BB—to improve OS, proliferation, and antitumor activity. The third-generation EGFRvIII-specific CAR incorporates the CD28, 4-1BB, and CD3 z signaling constructs. These same CAR designs can be used to target wild-type EGFR.
Molecular Docking Experiments:
- Molecular docking is a informatics technique used in the field of drug design that predicts the preferred/ optimal orientation of a molecule when bound to a target protein to form a stable complex, typically a protein-ligand complex. This technique helps in understanding the interactions between small molecules (ligands) and target proteins (receptors) at the atomic level. In this project, my focus lies on investigating the diverse binding sites present on the Epidermal Growth Factor Receptor (EGFR) and their relevance to Glioblastoma (GBM) pathogenicity. Specifically, I'll aim to identify potential targets for Chimeric Antigen Receptor (CAR) design within the EGFR structure, considering specific variants such as EGFRVlll, associated with GBM. Through molecular interaction studies, I'll assess the binding affinity between these EGFR sites and the designed CAR constructs, utilizing molecular docking techniques to simulate their interactions. Subsequently, experimental validation will be conducted to confirm the therapeutic efficacy of the designed CARs in targeting EGFR-positive GBM cells, thereby bridging computational predictions with practical applications in GBM treatment.
Figure 13. 3D silicon model of EGFR Protein.
UniProt. (n.d.). https://www.uniprot.org/uniprotkb/P00533/entry#sequences
Figure 14. 3D silicon model of Chimeric Antigen Receptor (CAR) scFv domain peptide.
Bank, R. P. D. (n.d.). 3D View: 7JO8. https://www.rcsb.org/3d-view/7JO8
STRUCTURE OF DSFV MR1 IN COMPLEX WITH THE PEPTIDE ANTIGEN OF THE MUTANT EPIDERMAL GROWTH FACTOR RECEPTOR, EGFRVIII
- The structure of DSFV MR1 in complex with the peptide antigen of the mutant Epidermal Growth Factor Receptor (EGFRvIII) provides insight into the mutated residues correspondence. Utilizing this information, I aim to modify the chimeric arm to enhance interactions with the mutated EGFR, thereby stabilizing their binding. My objective is to design a chimeric model capable of recognizing any and all antigens, particularly those associated with EGFR mutations. This approach opens avenues for novel strategies, including multi-targeting, by simultaneously binding to different sites on EGFR, thereby potentially enhancing therapeutic efficacy against EGFR-driven malignancies.
Molecular docking at an atomic level:
- To enhance CAR-T cell therapy targeting the EGFRVIII mutant peptide antigen, I use employ molecular docking methodologies to scrutinize the intricate atomic interactions between these entities. Central to our investigation is the identification of hydrogen bond sites, pivotal regions where CAR-T cells can securely bind to the mutant EGFRVIII antigen. Through molecular modeling and simulation analyses, we discern these specific binding sites, optimizing the configuration for antigen recognition. This strategic approach enables the seamless integration of antigen-recognition modules into the CAR-T construct, ensuring precise and effective targeting of EGFRVIII-expressing tumor cells.
Landry, R., Klimowicz, A., Lavictoire, S. J., Borisova, S., Kottachchi, D. T., Lorimer, I. a. J., & Evans, S. V. (2001). Antibody recognition of a conformational epitope in a peptide antigen: Fv-peptide complex of an antibody fragment specific for the mutant EGF receptor, EGFRvIII. Journal of Molecular Biology, 308(5), 883–893. https://www.sciencedirect.com/science/article/abs/pii/S0022283601946285?via%3Dihub
Cheung, J., Wazir, S., Bell, D. R., Kochenderfer, J. N., Hendrickson, W. A., & Youkharibache, P. (2023). Crystal structure of a chimeric antigen receptor (CAR) SCFV domain rearrangement forming a VL-VL dimer. Crystals, 13(4), 710.https://doi.org/10.3390/cryst13040710
Data
Figure 1. Lillustrates the GBM tumor exerting pressure on the frontal lobe, resulting in the manifestation of headaches and associated symptoms
Admin. (2021, March 19). Glioblastoma multiforme | Altair Health. Altair Health. https://altairhealth.com/glasser-center/glioblastoma-multiforme/
Figure 2. MRI spectroscopy of normal brain. The NAA peak is the most prominent ( If the amount of NAA is more than choline, that would suggest a normal brain, the opposite raises suspicion of a tumour).
P Thakkar, J. P. T., Paolo Peruzzi, P., & C Prabhu, V. (n.d.). Glioblastoma multiforme – symptoms, diagnosis and treatment options. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme#:~:text=Glioblastoma%20(GBM)%2C%20also%20referred,not%20spread%20to%20distant%20organs
Figure 3. Glioblastoma as visualized through Magnetic Resonance Imaging (MRI) scans.
Lobera, A., MD. (n.d.). Glioblastoma (Multiforme) imaging: practice essentials, computed tomography, magnetic resonance imaging. https://emedicine.medscape.com/article/340870-overview
Figure 4. Glioblastoma microenvironment and development of ideal fluorescent probes for fluorescence-guided surgery. Created with BioRender.com.
Chirizzi, C., Pellegatta, S., Gori, A., Falco, J., Rubiu, E., Acerbi, F., & Bombelli, F. B. (2023). Next‐generation agents for fluorescence‐guided glioblastoma surgery. Bioengineering & Translational Medicine. https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/btm2.10608
Figure 5. Glioblastoma stem cells (GSCs) possess the unique ability to self-renew, trigger tumor initiation, and exhibit regression when subjected to radiotherapy.
Yousuf Ali, M., R. Oliva, C., & M. Noman, A. S. (2020, September 3). Radioresistance in Glioblastoma and the Development of Radiosensitizers. Retrieved January 6, 2024, from https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.mdpi.com%2F2072-6694%2F12%2F9%2F2511&psig=AOvVaw0TgUj9lVvHF729FxtQLqsw&ust=1704591430693000&source=images&cd=vfe&opi=89978449&ved=0CBMQjRxqGAoTCIjFvpfQx4MDFQAAAAAdAAAAABCmAQ
Figure 6. Treatment with TMZ causes DNA methylation, leading to cell arrest. If DNA damage repair is successful, the cell recovers to the proliferating pool, or undergoes apoptosis otherwise.
Sorribes, I. C., Handelman, S. K., & Jain, H. (2020). Mitigating temozolomide resistance in glioblastoma via DNA damage-repair inhibition. Journal of the Royal Society Interface, 17(162), 20190722. https://royalsocietypublishing.org/doi/10.1098/rsif.2019.0722
Figure 7. Regression of Recurrent Multifocal Glioblastoma, Including Spinal Metastases, after Intraventricular Delivery of IL13Rα2-Targeted CAR T Cells.
Brown, C. E., Alizadeh, D., Starr, R., Weng, L., Wagner, V., Naranjo, A., Ostberg, Ms, B., Kilpatrick, J., Simpson, J., Kurien, A., Sj, P., Wang, X., Tl, H., D’Apuzzo, M., Ja, R., Mc, J., Me, B., Chen, M., . . . Badie, B. (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. The New England Journal of Medicine, 375(26), 2561–2569. https://doi.org/10.1056/nejmoa1610497 https://www.nejm.org/doi/full/10.1056/nejmoa1610497
Figure 8. A working model for combination therapy with EGFR CAR-T cells and JQ1 in GBM
Lin Xia, Jun-yi Liu, Zao-zao Zheng, Guo-sheng Hu, Ning-shao Xia, Wen Liu. (2021, May 21). BRD4 Inhibition Boosts the Therapeutic Effects of Epidermal Growth Factor Receptor-targeted Chimeric Antigen Receptor T Cells in Glioblastoma.. https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(21)00301-4
Figure 9. Illustrating the challenges posed by the Blood-Brain Barrier in primary brain tumors, including glioblastoma.
Dubois, L. G., Campanati, L., Righy, C., D’Andrea-Meira, I., De Sampaio E Spohr, T. C. L., Porto-Carreiro, I., Pereira, C. M., Balça-Silva, J., Kahn, S. A., DosSantos, M. F., De Almeida Rabello Oliveira, M., Ximenes-Da-Silva, A., Lopes, M. C., Faveret, E., Gasparetto, E. L., & Moura‐Neto, V. (2014). Gliomas and the vascular fragility of the blood brain barrier. Frontiers in Cellular Neuroscience, 8. https://www.frontiersin.org/articles/10.3389/fncel.2014.00418/full
Figure 10. CAR-T cell therapy faces several limitations, encompassing challenges such as the immunosuppressive tumor microenvironment (TME), constrained access through the blood-brain barrier (BBB), on-target off-tumor toxicity, cytokine release syndrome, tumor lysis syndrome, and the potential for selective antigen loss.
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Figure 11. Antigen-specific CAR T cells can be administered to patients to selectively recognize and eliminate tumor antigen-expressing glioblastoma cells.
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full

Figure 12. The activation of CAR-T cells in the presence of tumour-specific antigen (TSA) leading to the production of pro-inflammatory cytokines, including IL-6, and induce neurotoxicity through IL-1.
CRS Research Models - Creative Biolabs. (n.d.). https://www.creative-biolabs.com/car-t/crs-research-models.htm
Figure 13. 3D silicon model of EGFR Protein.
UniProt. (n.d.). https://www.uniprot.org/uniprotkb/P00533/entry#sequences
Figure 14. 3D silicon model of Chimeric Antigen Receptor (CAR) scFv domain peptide.
Bank, R. P. D. (n.d.). 3D View: 7JO8. https://www.rcsb.org/3d-view/7JO8
STRUCTURE OF DSFV MR1 IN COMPLEX WITH THE PEPTIDE ANTIGEN OF THE MUTANT EPIDERMAL GROWTH FACTOR RECEPTOR, EGFRVIII
Figure 15. CAR binded to sites on EGFR
Figure 16. Molecular docking at an atomic level
Conclusion
In conclusion, the exploration of CAR-T cell therapy targeting the tumor-specific mutation of the epidermal growth factor receptor (CART-EGFRvIII) holds promising potential in accumulating and targeting EGFRvIII-expressing gliomas. Our proposed approach involves the modification of the CART-EGFRvIII interaction interface through sophisticated modeling strategies, utilizing virtual mutational studies on the epitope binding domains of the CAR. Furthermore, validation of these modeling studies will be conducted through molecular simulation studies to elucidate the intricate interactions between the epitope and the CAR. By incorporating antigen-recognition moieties into our simulation data, we aim to enhance the affinity between CART-EGFRvIII and its target, thereby optimizing therapeutic efficacy. This comprehensive strategy not only addresses the challenges and potential side effects associated with CART-EGFRvIII therapy but also lays the groundwork for targeting alternative antigen combinations, including Human Epidermal Growth Factor Receptor-2 (HER2), Interleukin-13 Receptor Subunit Alpha-2 (IL13Ra2), Disialoganglioside GD2, and CD70. Furthermore, combination therapies, locoregional administration, multi-targeting approaches, engineering CAR constructs to induce or secrete active cytokines, and disrupting immunosuppressive immune checkpoint molecules represent additional avenues for advancing CAR-T cell therapy in the treatment of glioblastoma and other solid tumors.
Citations
Brown, C. E., Alizadeh, D., Starr, R., Weng, L., Wagner, V., Naranjo, A., Ostberg, Ms, B., Kilpatrick, J., Simpson, J., Kurien, A., Sj, P., Wang, X., Tl, H., D’Apuzzo, M., Ja, R., Mc, J., Me, B., Chen, M., . . . Badie, B. (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. The New England Journal of Medicine, 375(26), 2561–2569. https://doi.org/10.1056/nejmoa1610497 https://www.nejm.org/doi/full/10.1056/nejmoa1610497
P Thakkar, J. P. T., Paolo Peruzzi, P., & C Prabhu, V. (n.d.). Glioblastoma multiforme – symptoms, diagnosis and treatment options. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme#:~:text=Glioblastoma%20(GBM)%2C%20also%20referred,not%20spread%20to%20distant%20organs
Lobera, A., MD. (n.d.). Glioblastoma (Multiforme) imaging: practice essentials, computed tomography, magnetic resonance imaging. https://emedicine.medscape.com/article/340870-overview
Glioblastoma (GB) - Brain Tumour Foundation of Canada. (2019, November 19). Brain Tumour Foundation of Canada. https://www.braintumour.ca/brain_tumour_types/glioblastoma-gb/#:~:text=The%20incidence%20of%20glioblastoma%20(GB,Brain%20Tumour%20Registry%20of%20Canada
Chirizzi, C., Pellegatta, S., Gori, A., Falco, J., Rubiu, E., Acerbi, F., & Bombelli, F. B. (2023). Next‐generation agents for fluorescence‐guided glioblastoma surgery. Bioengineering & Translational Medicine. https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/btm2.10608
Yousuf Ali, M., R. Oliva, C., & M. Noman, A. S. (2020, September 3). Radioresistance in Glioblastoma and the Development of Radiosensitizers. https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.mdpi.com%2F2072-6694%2F12%2F9%2F2511&psig=AOvVaw0TgUj9lVvHF729FxtQLqsw&ust=1704591430693000&source=images&cd=vfe&opi=89978449&ved=0CBMQjRxqGAoTCIjFvpfQx4MDFQAAAAAdAAAAABCmAQ
Sorribes, I. C., Handelman, S. K., & Jain, H. (2020). Mitigating temozolomide resistance in glioblastoma via DNA damage-repair inhibition. Journal of the Royal Society Interface, 17(162), 20190722. https://royalsocietypublishing.org/doi/10.1098/rsif.2019.0722
P Thakkar, J. P. T., Paolo Peruzzi, P., & C Prabhu, V. (n.d.). Glioblastoma multiforme – symptoms, diagnosis and treatment options. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme#:~:text=Glioblastoma%20(GBM)%2C%20also%20referred,not%20spread%20to%20distant%20organs
Fernandes, C., Costa, A., Osório, L., Lago, R. C., Linhares, P., Carvalho, B., & Caeiro, C. (2017). Current standards of care in glioblastoma therapy. In Codon Publications eBooks (pp. 197–241). https://www.ncbi.nlm.nih.gov/books/NBK469987/
Luksik AS, Yazigi E, Shah P, Jackson CM. (2023 Feb 23). CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10000604
Lin Xia, Jun-yi Liu, Zao-zao Zheng, Guo-sheng Hu, Ning-shao Xia, Wen Liu. (2021, May 21). BRD4 Inhibition Boosts the Therapeutic Effects of Epidermal Growth Factor Receptor-targeted Chimeric Antigen Receptor T Cells in Glioblastoma.. https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(21)00301-4
Janeway, C. A., Jr. (2001). The major histocompatibility complex and its functions. Immunobiology - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK27156/#:~:text=The%20major%20histocompatibility%20complex%20(MHC,to%20the%20T%2Dcell%20receptor.
Z. Song, E., Wang, X., I. Philipson, B., M. O’Rourke, D., Song, H., & C. Milone, M. (2022). The IAP antagonist birinapant enhances chimeric antigen receptor T cell therapy for glioblastoma by overcoming antigen heterogeneity. Molecular Therapy Oncology. https://www.cell.com/molecular-therapy-family/oncology/fulltext/S2372-7705(22)00139-5
Admin. (2021, March 19). Glioblastoma multiforme | Altair Health. Altair Health. https://altairhealth.com/glasser-center/glioblastoma-multiforme/
Osmosis from Elsevier. (2023, September 24). Glioblastoma (Year of the Zebra) [Video]. YouTube. https://www.youtube.com/watch?v=waliaz_0-54
Maity, R., Benaoudia, S., Zemp, F. J., Lee, H., Barakat, E., Leblay, N., Ahn, S., Mahoney, D. J., Neri, P., & Bahlis, N. J. (2021). A BCL2L1 armoured BCMA targeting CAR T cell to overcome exhaustion and enhance persistence in multiple myeloma. Blood, 138(Supplement 1), 327. https://ashpublications.org/blood/article/138/Supplement%201/327/478084/A-BCL2L1-Armoured-BCMA-Targeting-CAR-T-Cell-to
Lin, Y., Mashouf, L. A., & Lim, M. (2022). CAR T cell therapy in primary brain tumors: current investigations and the future. Frontiers in Immunology, 13. https://www.frontiersin.org/articles/10.3389/fimmu.2022.817296/full
Mo, F., Pellerino, A., Soffietti, R., & Rudà, R. (2021). Blood–Brain barrier in brain tumors: Biology and clinical relevance. International Journal of Molecular Sciences, 22(23), 12654. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8657947/
Zhu, X., Li, Q., & Zhu, X. (2022). Mechanisms of CAR T cell exhaustion and current counteraction strategies. Frontiers in cell and developmental biology, 10, 1034257. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9773844/
Dubois, L. G., Campanati, L., Righy, C., D’Andrea-Meira, I., De Sampaio E Spohr, T. C. L., Porto-Carreiro, I., Pereira, C. M., Balça-Silva, J., Kahn, S. A., DosSantos, M. F., De Almeida Rabello Oliveira, M., Ximenes-Da-Silva, A., Lopes, M. C., Faveret, E., Gasparetto, E. L., & Moura‐Neto, V. (2014). Gliomas and the vascular fragility of the blood brain barrier. Frontiers in Cellular Neuroscience, 8. https://www.frontiersin.org/articles/10.3389/fncel.2014.00418/full
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Santomasso, B., Bachier, C., Westin, J. R., Rezvani, K., & Shpall, E. J. (2019). The other side of CAR T-Cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. American Society of Clinical Oncology Educational Book, 39, 433–444. https://ascopubs.org/doi/10.1200/EDBK_238691
CAR T-cell therapy and its side effects. (n.d.). American Cancer Society. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/car-t-cell1.html
Landry, R., Klimowicz, A., Lavictoire, S. J., Borisova, S., Kottachchi, D. T., Lorimer, I. a. J., & Evans, S. V. (2001). Antibody recognition of a conformational epitope in a peptide antigen: Fv-peptide complex of an antibody fragment specific for the mutant EGF receptor, EGFRvIII. Journal of Molecular Biology, 308(5), 883–893. https://www.sciencedirect.com/science/article/abs/pii/S0022283601946285?via%3Dihub
Cheung, J., Wazir, S., Bell, D. R., Kochenderfer, J. N., Hendrickson, W. A., & Youkharibache, P. (2023). Crystal structure of a chimeric antigen receptor (CAR) SCFV domain rearrangement forming a VL-VL dimer. Crystals, 13(4), 710.https://doi.org/10.3390/cryst13040710
Acknowledgement
I am grateful for the invaluable support and guidance my mentors, Drs Kristopher Elistad (University of Calgary)and Manikandan (Mayoclinic). I also extend my appreciation to our science fair project team members at North trail Highs School in Calgary and my family.