How Does Sea Acidification Affect Shellfish?
Grade 8
Presentation
No video provided
Hypothesis
Our hypothesis is that the shells in the water with higher acidification levels will show greater signs of deterioration (softening, dissolving, losing mass) as opposed to those in the neutral water, because acidic water will break down calcium carbonate faster, the primary component of shells. With this theory, we believe that the water representing the future of the Gulf of St. Lawrence, will likely detoriate the shells the fastest.
Research
What is sea acidification?
- Sea acidification, also known as ocean acidification, is the decrease in PH of the ocean over an extended period of time. The primary reason for this sea acidification is because of carbon dioxide( CO2) emissions being absorbed into the ocean. The ocean takes in about thirty percent of CO2 through the atmosphere. Because of global warming and the carbon dioxide created from burning fossil fuels, the amount of CO2 is increasing which results in the ocean slowly becoming more, and more acidic. When CO2 is absorbed by seawater, chemical reactions occur causing an increase in hydrogen ions, decreasing the amount of carbon ions, and making the water acidic.
- The ocean's PH level falls around 8. Since the industrial revolution, the oceans PH level has dropped from 8.20 to 8.05, which may sound like a small change but has an extensive reach. It equates to an increase in acidity of about 40%. Scientists predict that at this rate, the ocean will be 150% more acidic by the end of this century.
What is pH?
pH is a measure of how base or acidic a solution is. The way people determine pH is by calculating the concentration of hydrogen-ions. However since the physical presence of hydrogen- ions is hard to measure, we instead measure the electromotive force( energy, per unit), between electrodes to determine pH. Acid molecules typically have protons which can join an electron, or form a covalent bond to the electron. Base molecules can bond with protons, and consist of Hydroxide ions.
What is a shellfish?
A shellfish is an invertebrate animal that lives in water and has a shell, including many species of crustaceans, mollusks and echinoderms.
How does sea acidification affect shellfish in the world?
Since sea acidification lowers calcium carbonate ( CaCO3) , shellfishe's ability to form shells is put at risk. This is because of the fact that shellfish use calcite and aragonite to form their shells, both of which are primary components in CaCO3. Since hydrogen ions are combined with carbonate ions, they make bicarbonate ions. This shift causes less calcium carbonate, making it harder to form shells. In serious cases, there may even be corrosive damage to already existing shells.
How are shellfish a way of telling sea acidification?
Although sea acidification poses a threat to all of the ocean ecosystem, we chose shellfish as they show the visual effects of sea acidification very prominently. We also chose shellfish as eggshells are made up of close components ( CaCO3) of shellfish. That way we can actually do the experiment.
Why is sea acidification dangerous?
Sea acidification is incredibly dangerous because it makes it harder for marine organisms to form shells and skeletons, potentially leading to ecosystem disruption, food security issues, and economic impacts.
How can we model our experiment after the Gulf of St. Lawrence?
Since the ppt ( parts per thousand) of the St. Lawrence river is 30- 32. We can use 30 divided by a thousand times 600. This works as dividing 30 by a thousand calculates the ppt needed, and times it by 600 to get 18- 19 grams of salt per 600 grams of water. We will be using tap water as it resembles sea water better than distilled water. Calgary water typically has a PH of around 8.2- 8.5, while the Gulf of Saint Lawrence is currently at a PH of 8.1.
Why the Gulf of St. Lawrence?
Many marine ecosystems across the world are at risk due to ocean acidification, and we wanted to focus on one just to show how terrible the effects are. Here’s where the gulf of St. Lawrence comes in. As one of the largest and most productive marine ecosystems in Canada, along with historical and cultural significance, we couldn’t decide on a more perfect body of water to perform the experiment on. To elaborate, the Gulf is home to many shellfish, including oysters, mussels, and snow crabs, which depend on calcium carbonate to build their shells. Increased acidity dissolves calcium carbonate, threatening these species and the industries that rely on them. This in turn has some serious consequences for humans, especially for communities that depend on the ocean for food, jobs, and economic stability. By choosing the Gulf of St Lawrence, our project highlights why protecting the Gulf is not just about saving marine life, it’s also about preserving human livelihoods and the economy.
Why use baking soda and vinegar to change pH?
We used baking vinegar (an acid) and baking soda (a base) to change pH because they react chemically to neutralize each other, with baking soda raising the pH of acidic solutions and vinegar lowering the pH of basic solutions.
What is molarity?
Molarity in the concentration ( solvent divided by solute) of a solution. It is equivalent to the amount of millimoles present in 1 ml of water.
What is log?
Log, or Logarithm, is a math equation that gives you the exponent you need to raise the base to a certain number. There are two types of logarithms: common, and natural. Common logarithmic equations with bases of 10. The natural logarithmic equation uses the basic Euler constant, to calculate how many times we multiply it to get a desired number. Using the logarithmic pH scale allows us to more easily compare and contrast differences of hydrogen ion concentrations.
Variables
Independent - Amount of vinegar/ baking soda in cups.
Dependent- Disintegration of eggshells.
Controlled- Amount of time eggshells are in vinegar, shells of the same type of eggs, amount of water.
Procedure
Procedure
Materials: Eggshells, 750 ml of water, 81g of salt, 3 cups, vinegar with a 7%/ 4L acetic acid, baking soda, pipet.
1. Prepare 3 cups of tap water, adding 8 grams of salt to all three cups( This is to mimic the salinity of the Gulf of St. Lawrence) .
2. Crack 3 eggs, wash shells carefully remove any protective coating, as that way, acetic acid can directly reach eggshell
3. Follow calculations below.
4. For the first cup, it will represent the Gulf of St. Lawrence Pre- Industrial revolution ( before the 1800s). Although there were no specific tests, it is estimated that the PH of the ocean was 7.8 to 8.3. For the sake of our calculations, I will be using a pH of 8.
Firstly, we calculate PH
(pH = -log[H+])
The way this formula works is as follows:
- (-log) is simply changing the molarity to be accurate on the pH scale/ changing [H+] into the logarithmic scale. We use negative to guarantee a positive number at the end of the calculations, as that is the nature of the pH scale is logarithmic.
- [H+] is the molarity/ moles per unit of the hydrogen ion concentration, which we need to determine the pH level.
Simplifying this, we are essentially trying to determine how much acetic acid we need to neutralize the hydrogen ions, therefore, making the pH more base. However since we are trying to find out the hydrogen ion concentration(H+) We will rearrange the formula.
(H+)= 10^(-pH)
- 10 is the basis of logarithmic scale
- The negative exponent is to guarantee that as pH increases, the concentration of hydrogen ions decreases. It makes sure we deal with a number that is not too big nor small. As [ H+] is a very small number.
So the hydrogen concentration of the pH’s would be.
pH of 7.4/ tap water: (H+)= 10^(-7.4)
pH of 8.0/ tap water with baking soda: (H+)= 10^(-8.0)
Subtracting, we get;
10^(-7.4) - 10^( -8.0)= 3 x 10^(-8)
The reason we subtract is to calculate the difference between the two concentrations. That way, we can figure out how much baking soda we need. This is because sodium reacts to [H+] with a 1:1 ratio.
So finally, we need to calculate the volume of baking soda needed to change the pH.
Volume of Baking soda= 3.0 x 10^(-8) x 1M approx. 30 microliters
1M is the baking soda's 1:1 ratio to [ H+] when the water is at 1L, the baking soda will have a molecular level of 1 M/ 1L of water, the molarity of baking soda, as we need that to neutralize the hydrogen ions.
So to change the pH of 7.4 worth of tap water into a pH of 8.0, with 1L we will need 30 microliters of baking soda. Since we are only using 250ml, I will have a quarter mount of vinegar, so in total we only need 7.5 microliters of baking soda.
5. For the second cup, it will represent the present ( 2022). I cannot find the exact pH of the ocean right now, but in 2022 the Gulf of St. Lawrence was currently/ approx. at a pH of 7.5.
So the hydrogen concentration of the pH’s would be.
pH of 7.4/ tap water: (H+)= 10^(-7.4),
pH of 7.5/ tap water with baking soda: (H+)= 10^(-7.5)
Subtracting, we get;
10^(-7.4) - 10^( -7.5)= 8.19 x 10^(-9)
The reason we subtract is to calculate the difference between the two concentrations. That way, we can figure out how much baking soda we need. This is because sodium reacts to [H+] with a 1:1 ratio.
So finally, we need to calculate the volume of baking soda needed to change the pH.
Volume of Baking soda= 8.19 x 10^(-9) times 1M approx. 8.19 nano liters
So to change the pH of 7.4 worth of tap water into a pH of 8.0, with 1L we will need 8.19 nanoliters of baking soda. Since we are only using 250ml, I will use the amount of vinegar, so in total we only need 2.0475 nanoliters of baking soda.
6. For the third, and last cup, it will represent the Gulf of St. Lawrence in the future. Scientists are already predicting an 150% increase of acidity in the next 100 years. If we assume that the pH of 7.5 will have a 300% increase in acidity in the following 200 years, we can assume that the pH will roughly be around 6.9.
Firstly, we calculate PH.
(pH = -log[H+])
So [ H+]= 10^(-7.5)
Now to calculate a 300% increase, we need to add the initial 300% to the 100% of the convertration, therefore, we need to multiply it 4 times.
So [ H+]= 4 x 10^(-7.5)
We separate the -7.5 into -7, and - 0.5.
So, it would also be known as [ H+]= 4 x 10^(-0.5) x 10^(-7)/ 4 x 0.3162 x 10^(-7)
[ H+] = 4 ×10−7.5 equal approx. 1.2648 x 10^(-7)
Finally, The new pH = -log( 1.2648 x 10^(-7)) approx. a pH of 6.9.
To get to the pH of 6.9, we will be using 1.75% acetic/ litre acetic acid, in 250ml of 7.4 tap water. Now we need to calculate how much acetic acid we need.
Firstly, the hydrogen concentration of the tap water's pH would be.
[ H+]= pH of 7.4/ tap water: (H+)= 10^(-7.4)
[ H+]= pH of 6.9/ tap water with vinegar: (H+)= 10^(-6.9)
Subtracting, we get
10^(-7.4) - 10^(-6.9) = −8.6×10(-8)
As we already know, our vinegar is 1.75% acetic/ 1L, so we must now calculate its molarity.
Molarity of [ H+]= Molarity x volume.
Molarity of acetic acid=100mL/ 1.75g × 60 g/ 1 mol= 0.2917mol/L
We get 60g/ 1 mol as that is the standard molar mass of acetic acid.
Now we need to calculate the amount of [ H+] increase to get our desired pH.
Amount of H+= 8.6× 10−8M × 0.250L= 2.15 × 10(−8)mol
We will not need to calculate on a 1:1 ratio as before, as acetic acid has a different standard of molar mass to hydrogen ions.
Finally, we divide the molarity of our vinegar by the hydrogen ion concentration of the difference of the two waters, to find the needed amount of vinegar to change pH.
Volume of acetic acid= 2.15 × 10(−8)mol/ 0.2917mol= 0.0737 microliters.
We will need 0.0737 microliters of vinegar with a molarity of 0.2917mol/L to change the pH of 7.4 to 6.9
7. Add all amounts specified into cups. Stir well to dissolve properly if needed.
8. Submerge eggshells within solution.
Observations
Dimensions of Eggshells.
| Height |
Diameter
|
|
| Egg1 (barely cracked) | 4cm | 3.9cm |
| Egg 2 (somewhat cracked) | 3.5cm | 45cm |
| Egg 3 (most cracked) | 3.9cm | 3.9cm |
| Starting weight ( Day 1) Feb, 23, 2025 | Ending weight ( Day 22) March, 15, 2025 | |
| Egg 1 ( Past) | 5g | 6g |
| Egg 2 (Present) | 5g | 5g |
| Egg 3 ( Future) | 5g | 5g |
- The weight of the past and future egg did not change throughout the entire experiment.
- The weight of the past egg, however, reached 6g at the fifteenth day mark.
- This record in the final weigh in, as weight stayed generally the same ( Except Present egg.)
General observations:
- "Future" egg had the most diesinegration, with the cloudiest water.
- " Future" egg had a dotted cracked like pattern on the surface, that was scattered all over, and particulary at the bottom of the egg.
- " Future" egg was the most fragile. ( Most cracking, breaking, etc.)
- No visible changes in "Present" egg.
- Slight cloudieness in "Past" egg. Although the slight cloudieness suggests disintegration, the weight actually increased.
- Rewinding footage, Water levels seem to decrease slightly each day. As there was a significant decline from day 1 to day 9, That was how long I recorded.)
Visual changes.
Days 1-2
- Only change was the slight cloudiness in the " Future" cup
Day 2-7
- More cloudiness in " Future" cup + Start of cracking pattern along sides of egg.
- Strat of cloudiness in " Past" cup. ( Faintly white)
Day 7- 12
- More cloudiness in " Future" cup.
- More cloudieness in " Past" cup+ blackish cracking pattern on shell/ increase in cracking.
Day 12- 17
- More cloudiness in " Future" cup+ Dot like pattern increasing.
- " Future" cup egg membrane starts seperating from shell.
- More cloudiness in " Present" cup.
Day 17- 22
- Slightly, black cracks in " Past" egg.
- Crystal- like substance on " Future" egg forming along edges.
- More cloudiness in " Future" cup
- More cloudiness in " Present" cup
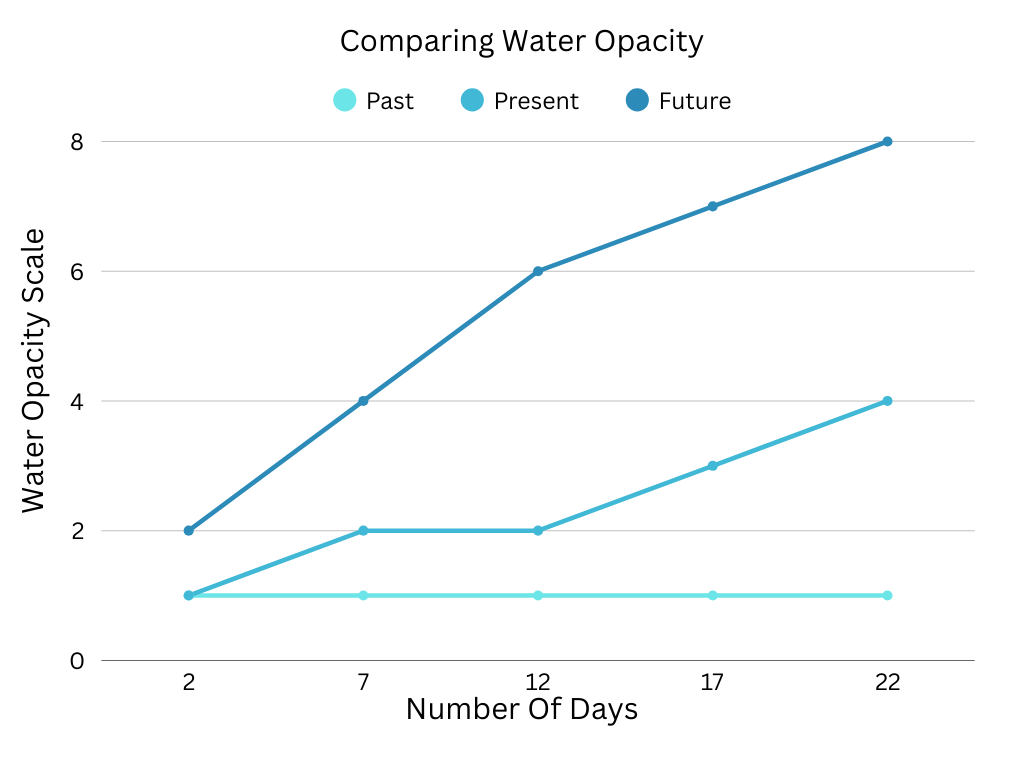
Legend
1- Crystal clear. 0% Opacity
2- Slight cloudiness, but barely noticable. 5- 10% Opacity
3- Cloudy enough to notice, but not by much. 15- 20% Opacity
4- Cloudy water. What people would consider " slightly cloudy" 25- 30% Opacity
5- Just below the halfway threshold. Where water is less opaque than egg. 35- 40%
6- Halfway mark. 45- 50% Opacity
7- Water is more visible than egg. Egg is " shrouded" in water 55- 60% Opacity.
8- Very opaque. 65- 70% Opacity.
Final observations.
- Water drainage of " Future" cup total approx. 74.88 ml
3.14r2h= Volume
3.12 x 4cm(2) x 1.5cm = 74.88 cm cubed/ 74.88 ml
50.24/ 5 approx. 14.976 ml per day
- Water drainage of " Present" cup total approx. 74.88 ml
3.14r2h= Volume
3.12 x 4cm(2) x 1cm = 50.24 cm cubed/ 50.24 ml
50.24/ 5 = 10.048 ml per day
- Water drainage of " Past" cup
3.14r2h= Volume
3.14 x 4cm(2) x 1cm = 50.24 cm cubed/ 50.24 ml
50.24/ 5 = 10.048 ml per day
- " Past" egg had a blackish crack pattern, mild cloudieness, and an increase in weight.
- No visible changes to " Present" egg, except water drainage. No change in weight.
- " Future egg had a greyish dot pattern, very cloudy water, and no change in weight.
Analysis
Through this experiment, we are discovering the way sea acidification has changed overtime. By modelling our experiment after the Gulf of St. Lawrence, we hope to draw out conclusions on how this issue impacts the ecosystem, now, in the past, and in the future.
Experiment ment recap.
- The experiment we have documented has lasted 22 days.
- We have set up three cups, representing three different time periods, with different pH's each.
- We are basing our experiment off of the Gulf of St. Lawrence.
Data recap.
| Pattern | Water Opacity | Water Drainage | Egg Structure | Other | |
| Future | Grey dots covering shell | Cloudy | 74.88 ml | Fragile | Crystallization of edges |
| Present | NA | Clear | 50. 24 ml | Strong | NA |
| Past | Black cracks on shell | Slightly cloudy | 50. 24 ml | Strong | NA |
Future egg analysis.
The most notable thing about this egg is its disintegration rate. This is likely because it is the only cup with vinegar in it, causing it to dissolve the most. Therefore, the egg was being broken down by the acidity, and therefore releasing calcium bicarbonate into the water. Although there is no weight change, that is likley because it has only been around twenty days of this experiment. This would also explain why it is so fragile.
For the pattern, it is formed because of the vinegar/ acetic acid reacting to the shell of the egg. The cause of this discoloration is because of mineral concentration of the egg, reacting to the acetic acid.
Water drainage in the future egg was the most. Although our environment for this experiment is not ideal, and external factors such as temperature or wind could have affected water drainage, it still had the most water loss, meaning that there has to be another reason why it lost so much more. The reason behind this is likely because this water had vinegar in it. Since the egg was interacting with the vinegar, this caused surface tension, as the egg released bicarbonate, it changed the composition. With this, that is the reason why it had the most water drainage.
Crystals were forming on the edges of the egg. It was very scarce, but still there. Again, this is likely due to the dissolving of calcium bicarbonate ions in, not only in the water, but also concetrating into crystal like formations.
Present egg analysis
Since it had a minimal change of pH ( 7.4 to 7.5) it makes sense that there is no visible change to the egg.
Water drainage likely came from external factors, ie. wind, causing evaporation.
Past egg analysis.
Although the slight cloudiness suggests disintegration, the weight actually increased, meaning more likley, a thin layer of bicarbonate has actually formed around the shell because of the baking soda, therefore increasing weight. This means that in the past, marine creatures were likely to be able to build shells quickly, as it has gained 1g of weight, in the course of only fifthteen days.
To explain the opacity of the water, I think it became cloudier as while the baking soda was forming with the shell, it became more opaque due to chemical reactions.
Trends
- Consistent distengration of Future egg.
- Consistent water drainage throughout experiment,
- Little to no weight change throughout experiment. ( Only weight change was in past egg, in the fifthteenth day.)
Inferences
- Future egg will dissolve the first.
- Past egg will have the strongest structure, and weight.
- Present egg will remain neutral.
- Little to no weight change will occur in the next month.
Conclusion
Through our project, we have been testing the effects of sea acidification by using real data from the Gulf of St. Lawrence's. Our experiment serves as a window to the past, present and future illustrating how acidification is not only affecting Canadian waters but oceans worldwide. By modeling these changes, we can better understand how ocean acidification has progressed over time and how it will continue to do so in the future if we don’t do something to stop it.
In our analysis, we have proven our hypothesis correct: the Gulf of. St Lawrence is at risk, and if its acidification rates continue to rise, its future along with ours may be in trouble. Our experiment proves the urgent need for action to protect marine life, and ocean ecosystems.
We can also see that increasing the water's alkalinity helps protect ocean marine life, as the eggshell in the past, gained weight as opposed to the ones in present and future who lost weight. However, altering the ocean’s pH is only a short term solution that we cannot maintain in our current status quo. A long term solution for ocean acidification is still necessary to effectively reduce ocean acidification.
The long term solution is obvious: reduce global warming. However, this is far easier said than done. Global warming is a worldwide issue, and unfortunately there is currently no single efficient method to halting sea acidification completely. Given this challenge, it may be worthwhile to explore alternative strategies to stop or heavily decrease ocean acidification while working toward broader climate solutions.
Application
Our project on ocean acidification and its effects on shellfish has significant real-world applications, particularly in environmental conservation, fisheries management, and policy-making. The most obvious application is protecting fisheries and the seafood industry. Many coastal communities rely on shellfish, such as oysters, mussels, and crabs, for their main source of food and income. Beyond fisheries, this issue also affects economic stability and food security. The decline of shellfish populations threatens both local economies and global seafood supplies. Understanding acidification can help policymakers create strategies to support affected industries and stabilize production. In addition, this project highlights the importance of addressing climate change by reducing carbon emissions, as CO₂ is the primary driver of acidification. Research like this supports advocacy for global warming, and stricter regulations on marine pollution. By connecting scientific research with real-world industries, policies, and conservation strategies, this experiment emphasizes the urgency of protecting marine ecosystems before irreversible damage occurs.
Sources Of Error
1. All our math is theoretical. We are assuming that the pH of the tap water is exactly 7.4.
2. Since we do not have a micropipette, we used a pipette instead which leads to less accurate measurements.
3. Possible contamination could have occured while weighing eggs.
4. Our experiment is not set up in ideal conditions. External factors could have affected experiment ie. wind causing evaporation, temperature fluctuations, etc.
5. We expected the eggshells to began dissolving very quickly, but even after 2 weeks it only had minor changes in weight. Given this, we should've started our experiment way ahead of time.
Citations
How Does Ocean Acidification Affect Shells? - The Environmental Literacy Council
https://www.youtube.com/watch?v=OVWZyDz--30
How to Calculate pH - Formula and Examples
Gulf of Saint Lawrence | Canada’s Largest Estuary, Marine Ecosystem, & Map | Britannica
Calculating pH - Calculations, Methods and Examples | CK-12 Foundation
The St. Lawrence River is warming and its oxygen levels are falling | Montreal Gazette
PH | Definition, Uses, & Facts | Britannica
pH of Acids and Bases - Definition, pH Chart, and Examples
11.3: Solution Concentration - Molarity - Chemistry LibreTexts
Acknowledgement
We would like to acknowledge Mrs. Friesen for helping us so much on project ideas, and giving us feedback, and Mrs. Paddock for giving us the pipettes we needed for this experiment. Finally, we would like to acknowledge our parents who supported us every step of the way.

