Lx vs Dx: What's The Best Rx For Endometriosis?
Grade 9
Presentation
Problem
This systematic literature review and analysis compared the effectiveness of LNG-IUS and DNG in reducing pelvic pain, dysmenorrhea, dyspareunia, and adverse events in patients with endometriosis. By comparing these two treatments, this analysis aimed to provide a clearer understanding of the benefits and risks associated with these therapies.
Method
Methods
Search Strategy
A systematic search of three electronic databases (PubMed, Medline, Embase) was conducted using the following queries: “endometriosis” AND “dienogest”, “endometriosis” AND “levonorgestrel”, as well as “endometriosis” AND “levonorgestrel-releasing”. All English publications of single, independent studies on the treatment of endometriosis by the LNG-IUS or DNG from January 2001 to August 2024 were identified. PRISMA guidelines were followed by one independent researcher.
Inclusion criteria
All included studies met the following criteria: they specifically evaluated endometriosis, investigated either DNG (2 mg) or LNG-IUS (52 mg), provided data for a treatment period of 24 weeks, and assessed at least one of the selected outcomes with the specified measures (VAS for pelvic pain, dysmenorrhea, and dyspareunia, or the number of adverse events). Additionally, studies were required to have clear outcome reporting, be written in English, have full-text versions available, and be classified as randomized controlled trials (RCTs), prospective studies, or retrospective studies.
Exclusion criteria
Studies were excluded if they studied patients with adenomyosis or other non-endometriosis conditions, investigated combination therapies of DNG or LNG-IUS with other medications, used incorrect dosages (2 mg DNG, 52 mg LNG-IUS), failed to report 24-week outcomes, focused on specific “special” groups (e.g., seniors, adolescents, or those considered obese), had unclear data (e.g., graphs without numerical values), did not include any of the selected outcomes, were not written in English, lacked full-text access, or were reviews, conference papers or case studies.
Data Extraction
Data was extracted from all selected studies and entered into a Google spreadsheet. Extracted data included study characteristics (author, year published, study design, setting, and sample size), patient characteristics (mean age, mean BMI, severity of condition (based on revised American Society of Reproductive Medicine (r-ASRM) stages), and study inclusion/exclusion criteria), baseline pain characteristics (mean VAS scores for pelvic pain, dysmenorrhea, and dyspareunia), 6-month pain scores (mean VAS scores for pelvic pain, dysmenorrhea, and dyspareunia), and 6-month adverse event counts. All VAS scores were measured in millimetres on a 1-100 mm scale.
Outcomes
The primary outcomes for this systematic review were 6-month reduction of pelvic pain scores (VAS, mm), dysmenorrhea (VAS, mm), dyspareunia pain scores (VAS, mm), and adverse event (incidence ratio) outcomes.
Statistical Analysis
Analyses were performed using inverse variance methods to calculate the weighted mean differences (WMDs) for pelvic pain, dysmenorrhea, and dyspareunia outcomes. A random-effects model was used to account for variability across studies. Incidence rates (IRs) for adverse events were calculated by pooling the number of adverse events and the total person-time (person-years) for each study.
Individual statistical analyses were conducted separately for each treatment (LNG-IUS and Dienogest) to calculate pooled weighted mean differences (WMDs) for pain-related outcomes and incidence rates (IRs) for adverse events. Rather than conducting a direct statistical comparison between treatments, a narrative synthesis of the pooled results was performed to avoid the risk of misleading conclusions due to a naïve direct comparison. P-values were considered to be significant when p < 0.05. The heterogeneity among studies was evaluated using the I² statistic, with values above 50% indicating heterogeneity.
All statistical analyses, including the calculation of WMDs, IRs, p-values, heterogeneity, and generation of forest plots, were conducted using the online R software with the ‘meta’ package.
Research
Introduction
Endometriosis is a chronic condition that affects an estimated 10% of reproductive-aged women and girls globally and accounts for up to 80% of women with pelvic pain. It is characterized by the presence of endometrial-type tissue outside of the uterus, leading to symptoms such as infertility and pain. For many patients, these symptoms are extremely severe, significantly impacting fertility, work efficiency, and quality of life. While there is no cure for endometriosis; therefore, treatment options aim to reduce pain, enhance quality of life, and preserve fertility. Aside from surgical interventions, hormone therapies, such as progestins and gonadotropin-releasing agonists, are often used to manage symptoms.
The levonorgestrel-releasing intrauterine system (LNG-IUS) and dienogest (DNG) have emerged as promising treatments for endometriosis. The LNG-IUS works locally on the uterus, releasing approximately 20 µg of levonorgestrel daily for up to 8 years. The levonorgestrel thins the endometrial lining to prevent it from thickening and forming painful endometrial lesions. It also lowers the synthesis of estradiol, one of the primary hormones involved in the menstrual cycle, to further lessen pelvic pain, dysmenorrhea, and other endometriosis symptoms. Finally, its local effects may help lower systematic hormone exposure, which may reduce side effects.
In comparison, DNG is a synthetic progestin with potent progestational effects and mild anti-androgenic properties. DNG works primarily by suppressing ovulation and reducing estrogen production, which helps decrease the growth and activity of endometrial tissue. Similar to levonorgestrel, DNG thins the endometrial lining, reducing endometrial lesions and pelvic pain.
Both DNG and LNG-IUS are often preferred because they reduce endometrial tissue growth and relieve pain without causing menopausal symptoms that are commonly linked to other hormone therapies, such as gonadotropin-releasing hormone agonists. Additionally, other hormonal therapies, such as patches, vaginal rings, or injections, can be less convenient for patients due to the need for frequent replacements or to visit a physician. In contrast, DNG is delivered orally via a pill, and the LNG-IUS only requires a single insertion, offering greater convenience. These options also provide different mechanisms of action, allowing for personalized treatment approaches based on patient preferences and clinical needs. However, despite the widespread use of both LNG-IUS and DNG, there are very few direct comparisons between the two.
Data
Results
Study Characteristics and Selection
After the search was conducted, 822 articles were identified. Of these, 89 duplicates were automatically removed using Endnote and an additional 111 duplicates were manually removed. 467, 105, and 34 articles were removed in title, abstract, and full-text screening, respectively, as they did not meet the inclusion criteria. 16 articles were included in the analysis — 10 for the DNG group and 7 for the LNG group. (Figure 1). One study was used for both groups, as they provided data for both groups (Porto, 2024). The selected studies included a total of 4,659 patients with 4,439 in the DNG group and 220 in the LNG-IUS group.
The characteristics of the studies, including the name of the first author, year published, setting, sample size, study design, mean age, mean BMI, and stage of disease studied are shown in Table 1.
Synthesis of Results
Pelvic Pain
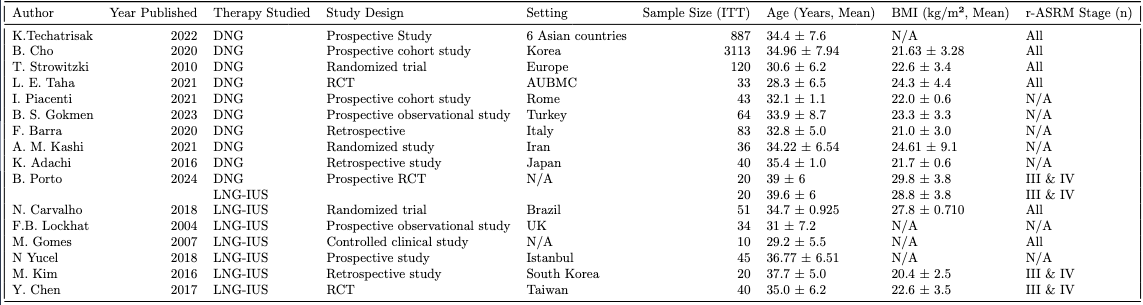
Eight out of ten studies assessed pelvic pain for DNG. The WMD of VAS scores from baseline to 6 months was -36.51 (95% CI, -48.26 to -24.77, p < 0.0001) mm, indicating that DNG significantly reduced pain scores (Fig. 2) . For LNG-IUS, three studies that investigated pelvic pain, with a WMD from baseline to 6 months of -47.69 (95% CI, -64.4795 to -30.9074, p < 0.0001) mm (Figure 3). This result also demonstrated a significant reduction in pelvic pain scores. In comparison, LNG-IUS showed a greater average reduction in pelvic pain scores than DNG. The I² statistics for DNG and LNG-IUS, respectively, were 99.4% and 91.5%, indicating significant heterogeneity in both groups.
Dysmenorrhea
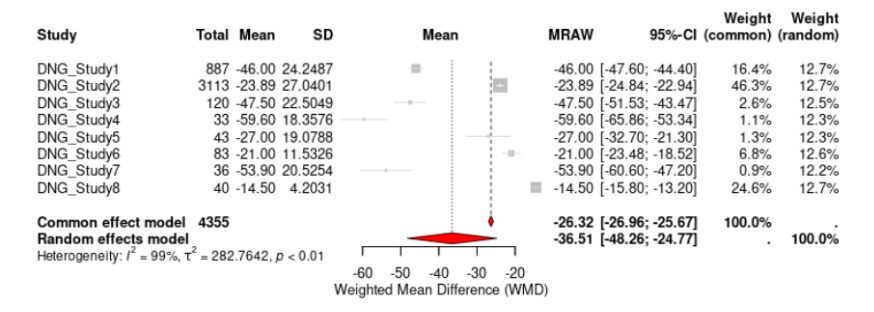
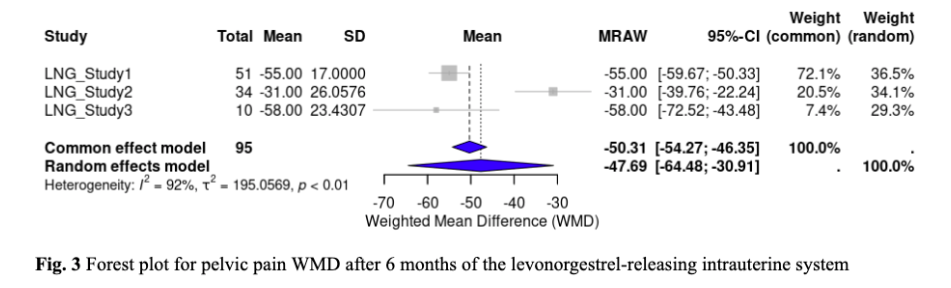
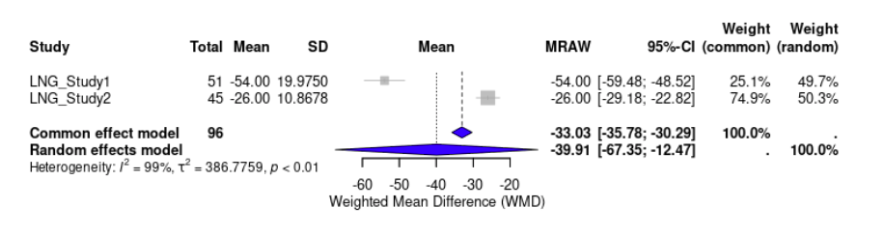
Two out of ten studies evaluated the effectiveness of DNG in reducing dysmenorrhea. The WMD of VAS scores from baseline to 6 months was -22.72 (95% CI, -38.39 to -7.05, p < 0.0001) mm, indicating that DNG had significantly reduced dysmenorrhea pain scores (Fig. 4). The effect of LNG-IUS on dysmenorrhea scores was examined by two studies. LNG-IUS demonstrated a significant reduction of dysmenorrhea pain scores with a WMD of -39.91 (95% CI, -67.35 to -12.47, p < 0.0001) mm (Fig. 5). Overall, the LNG-IUS showed a greater reduction in dysmenorrhea scores compared to DNG. For heterogeneity, the I² for DNG and LNG-IUS were 94.9% and 98.7%. respectively.
Dyspareunia
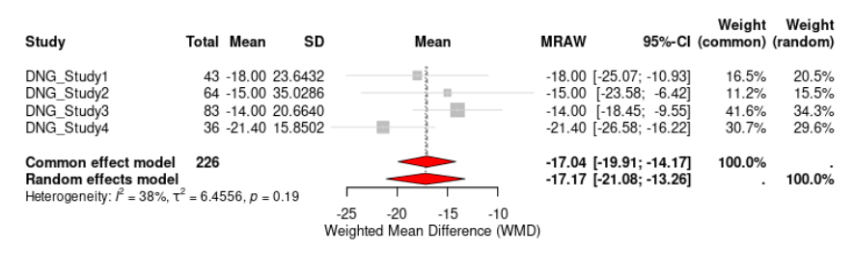
Four out of ten studies examined the effectiveness of DNG in reducing dyspareunia. The WMD of VAS scores from baseline to 6 months was -17.17 (95% CI, -21.08 to -13.26, p < 0.1864) mm (Fig. 6). As such, DNG reduced dyspareunia pain scores, but the result was not statistically significant. For LNG-IUS, only one study evaluated the effectiveness of dyspareunia and therefore a WMD could not be calculated. This study reported a statistically significant reduction of VAS scores of -29.30 (95% CI, -32.0757 to -26.5243, p<0.0001) mm. While it appears that LNG-IUS had a greater reduction of dyspareunia compared to DNG, there is an imbalance of studies for each group and so conclusions cannot be made. The I² for DNG was 37.6%.
Adverse Events
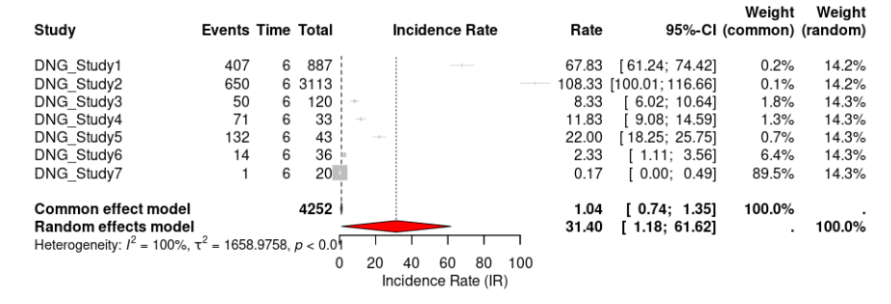
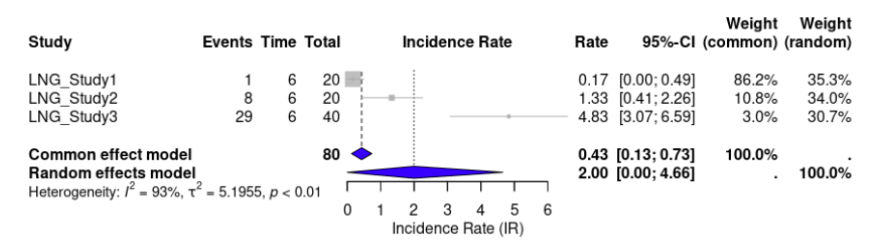
Seven out of ten studies reported adverse events for DNG. The incidence rate (IR) of DNG was 31.40 (95% CI, 1.1836 to 61.6248, p < 0.0001) events per 100 person-years (Fig 7). For LNG-IUS, three out of the seven studies reported adverse events. LNG-IUS had an IR of 2.00 (95% CI, 0.0000 to 4.6571, p < 0.0001) events per 100 person-years (Fig. 8). This suggests a much higher rate of adverse events for DNG than LNG-IUS. For heterogeneity, the I² for DNG and LNG-IUS, respectively, was 99.5% and 93.4% (Fig 5).
Overall, LNG-IUS was shown to be more effective and safer than DNG in all outcomes, though the high heterogeneity of studies should be taken into account when interpreting results.
Discussion
Overall, both DNG and LNG-IUS significantly reduced pelvic pain and dysmenorrhea VAS scores in individuals with endometriosis over 6 months. While LNG-IUS significantly reduced dyspareunia VAS score, this was not observed for DNG. Furthermore, LNG-IUS showed a greater pooled WMD for pain reduction in all outcomes. In addition, the LNG-IUS was associated with a lower incidence of adverse events compared to DNG.
This study is one of the first to synthesize and compare outcomes for DNG and LNG-IUS. Although some studies have studied outcomes of combination therapies involving these treatments with other medications, there is currently only one prospective study (Porto, 2024) directly comparing the two.
We found LNG-IUS to be more effective; this may be due to its localized action in the pelvic region. Its localized effects have a profound effect on the endometrium, causing decidualization of the stroma and subsequent atrophy of both the endometrium and ectopic endometrial tissue. This aligns with research by Nilsson et al., which demonstrated significantly higher concentrations of levonorgestrel in the endometrium with the LNG-IUS compared to oral LNG.
Systemic drug delivery, such as with DNG, can be subject to hepatic metabolism, renal clearance, and the variable pH environment of the gastrointestinal tract, all of which can modify potency. In contrast, the LNG-IUS operates as a zero-order release system, ensuring a consistent and localized delivery of levonorgestrel. This delivery method minimizes fluctuations in drug levels, which are often seen with oral treatments like DNG. With administration of one dose, drug levels rapidly rise beyond minimum effective levels but below toxicity; and over time the concentration decreases as the drug is metabolized or eliminated from the body, eventually falling below the effective threshold. At this point, a second dose is typically required, but without constant monitoring, it's difficult to pinpoint when this should be, and patients likely will not be able to do so on their own.
The localized action of LNG-IUS likely also contributes to its lower incidence of adverse events. Localized drug delivery systems have consistently demonstrated reduced systemic side effects compared to systemic administration in various diseases. The findings of this study are consistent with this trend, underscoring the advantages of localized treatment approaches for endometriosis.
Clinically, both treatments are widely used for managing endometriosis. However, this study suggests that LNG-IUS may be a more favourable option over DNG, particularly for pain reduction and side effect considerations. The consistent pain reduction seen with the LNG-IUS, alongside its favourable safety profile, make it an excellent choice for patients who prioritize reducing the likelihood of adverse events. Conversely, patients who are unwilling to use an IUD may find oral DNG as a reasonable alternative. These findings provide valuable insights to clinicians for making evidence-based recommendations to patients considering hormone therapies, reinforcing LNG as a viable and effective choice for a range of endometriosis symptoms.
There are a few limitations to this study that may affect the quality of the review. Firstly, the sample sizes between the two treatments differed substantially, with the LNG-IUS group having one fewer study and approximately 4,200 fewer patients compared to the DNG group. This disparity was particularly evident in the dyspareunia outcome, where only one study with data was available for LNG-IUS, making it impossible to calculate a pooled WMD. This reduces the reliability of the comparisons made between the two treatments. Another limitation would be the high level of heterogeneity observed (particularly for adverse event incidence rates), which reflects underlying differences in study designs, populations, and/or reporting standards. The high heterogeneity limits the strength of the results. Finally, without more than a single direct head-to-head study in the current literature, a full meta-analysis could not be done. Thus, p-values could not be calculated when doing the actual comparison between the pooled groups.
Despite these limitations, this study has notable strengths. Firstly, it provides a comprehensive analysis of the effectiveness and safety of DNG and LNG-IUS for multiple outcomes, including pelvic pain, dysmenorrhea, dyspareunia, and adverse events. By considering a range of clinically relevant outcomes, our review provides a holistic understanding of how these treatments perform across different aspects of endometriosis management, enhancing its clinical applicability. Furthermore, the large patient population achieved through the pooling of data across multiple studies enhances the reliability of the findings. By integrating data from diverse study populations, this analysis provides a comprehensive overview that may be more representative of real-world clinical settings. Given the limited head-to-head comparisons in the current literature, the findings from this review are crucial for filling a significant gap in the evidence.
The results of this review also underscore the need for further research in this area. There is very little existing literature on the long-term outcomes of the two treatments, including pain recurrence or adverse event profiles beyond 6 months. Therefore, for future studies in the field, we would recommend that head-to-head RCTs be conducted to strengthen the current literature on both DNG and the LNG-IUS for endometriosis. Furthermore, standardizing outcome measures, such as scales used to measure pain reduction, would enhance the comparability and applicability of study results, making them more universally interpretable. Finally, studies assessing more outcomes, like fertility outcomes, quality of life, and cost-effectiveness could allow for a more holistic comparison between treatments.
Conclusion
Conclusion
Overall, my project demonstrates that while both DNG and LNG-IUS are effective treatments for endometriosis, the levonorgestrel-releasing intrauterine system has superior efficacy and safety outcomes. Further research with more rigorous methodologies and a broader scope of assessment is crucial to guide clinicians and patients in making informed decisions, and, ultimately, benefit every single patient living with endometriosis.
Citations
References
- World Health Organization. Endometriosis. World Health Organization. Published March 24, 2023. https://www.who.int/news-room/fact-sheets/detail/endometriosis
- Yale Medicine. Endometriosis. Yale Medicine. https://www.yalemedicine.org/conditions/endometriosis
- Fourquet J, Báez L, Figueroa M, Iriarte RI, Flores I. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertility and Sterility. 2011;96(1):107-112. doi:https://doi.org/10.1016/j.fertnstert.2011.04.095
- Cleveland Clinic. Endometriosis: Symptoms, Causes, Treatment & Tests. Cleveland Clinic. Published September 16, 2024. https://my.clevelandclinic.org/health/diseases/10857-endometriosis
- NICHD. What are the treatments for endometriosis? https://www.nichd.nih.gov/. Published January 31, 2017. https://www.nichd.nih.gov/health/topics/endometri/conditioninfo/treatment
- Viganò P, Somigliana E, Vercellini P. Levonorgestrel-Releasing Intrauterine System for the Treatment of Endometriosis: Biological and Clinical Evidence. Women’s Health. 2007;3(2):207-214. doi:https://doi.org/10.2217/17455057.3.2.207
- Mirena ® (Levonorgestrel-Releasing Intrauterine System) . https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf
- Attia AM, Ibrahim MM, Abou-Setta AM. Role of the levonorgestrel intrauterine system in effective contraception. Patient preference and adherence. 2013;7:777-785. doi:https://doi.org/10.2147/PPA.S36948
- Foster RH, Wilde MI. Dienogest. Drugs. 1998;56(5):825-833. doi:https://doi.org/10.2165/00003495-199856050-00007
- Kodama M, Masayo Onoue, Otsuka H, et al. Efficacy of Dienogest in Thinning the Endometrium Before Hysteroscopic Surgery. Journal of Minimally Invasive Gynecology. 2013;20(6):790-795. doi:https://doi.org/10.1016/j.jmig.2013.04.020
- endometriosis.org. GnRH «Endometriosis.org. https://endometriosis.org/treatments/gnrh/
- Brigham and Women's Hospital. Endometriosis medical treatment - brigham and women’s hospital. www.brighamandwomens.org. https://www.brighamandwomens.org/obgyn/infertility-reproductive-surgery/endometriosis/medical-treatment-for-endometriosis
- Cho B, Roh JW, Park J, et al. Safety and Effectiveness of Dienogest (Visanne®) for Treatment of Endometriosis: A Large Prospective Cohort Study. Reproductive Sciences. 2020;27(3):905-915. doi:https://doi.org/10.1007/s43032-019-00094-5
- T. Strowitzki, Marr JM, Gerlinger C, Faustmann T, Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Human Reproduction. 2010;25(3):633-641. doi:https://doi.org/10.1093/humrep/dep469
- El Taha L, Abu Musa A, Khalifeh D, Khalil A, Abbasi S, Nassif J. Efficacy of dienogest vs combined oral contraceptive on pain associated with endometriosis: Randomized clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2021;267:205-212. doi:https://doi.org/10.1016/j.ejogrb.2021.10.029
- Piacenti I, Viscardi MF, Masciullo L, et al. Dienogest versus continuous oral levonorgestrel/EE in patients with endometriosis: what’s the best choice? Gynecological Endocrinology. 2021;37(5):471-475. doi:https://doi.org/10.1080/09513590.2021.1892632
- Saglik Gokmen B, Topbas Selcuki NF, Aydın A, Yalcin Bahat P, Akça A. Effects of Dienogest Therapy on Endometriosis-Related Dysmenorrhea, Dyspareunia, and Endometrioma Size. Cureus. Published online January 24, 2023. doi:https://doi.org/10.7759/cureus.34162
- Mehdizadeh Kashi A, Niakan G, Ebrahimpour M, et al. A randomized, double‐blind, placebo‐controlled pilot study of the comparative effects of dienogest and the combined oral contraceptive pill in women with endometriosis. International Journal of Gynecology & Obstetrics. 2021;156(1). doi:https://doi.org/10.1002/ijgo.13677
- Adachi K, Takahashi K, Nakamura K, et al. Postoperative administration of dienogest for suppressing recurrence of disease and relieving pain in subjects with ovarian endometriomas. Gynecological Endocrinology: The Official Journal of the International Society of Gynecological Endocrinology. 2016;32(8):646-649. doi:https://doi.org/10.3109/09513590.2016.1147547
- Techatraisak K, Hestiantoro A, Soon R, et al. Impact of Long-Term Dienogest Therapy on Quality of Life in Asian Women with Endometriosis: the Prospective Non-Interventional Study ENVISIOeN. Reproductive Sciences. 2022;29(4):1157-1169. doi:https://doi.org/10.1007/s43032-021-00787-w
- Carvalho N, Margatho D, Cursino K, Benetti-Pinto CL, Bahamondes L. Control of endometriosis-associated pain with etonogestrel-releasing contraceptive implant and 52-mg levonorgestrel-releasing intrauterine system: randomized clinical trial. Fertility and Sterility. 2018;110(6):1129-1136. doi:https://doi.org/10.1016/j.fertnstert.2018.07.003
- Lockhat FB. The evaluation of the effectiveness of an intrauterine-administered progestogen (levonorgestrel) in the symptomatic treatment of endometriosis and in the staging of the disease. Human Reproduction. 2004;19(1):179-184. doi:https://doi.org/10.1093/humrep/deh004
- Mariana Janini Gomes, Rui Alberto Ferriani, Julio Cesar Rosa-e-Silva, Soares A, Carolina Sales Vieira, José F. The levonorgestrel-releasing intrauterine system and endometriosis staging. 2007;87(5):1231-1234. doi:https://doi.org/10.1016/j.fertnstert.2006.11.044
- Barra F, Scala C, Leone Roberti Maggiore U, Ferrero S. Long-Term Administration of Dienogest for the Treatment of Pain and Intestinal Symptoms in Patients with Rectosigmoid Endometriosis. Journal of Clinical Medicine. 2020;9(1):154. doi:https://doi.org/10.3390/jcm9010154
- Lee KH, Jung YW, Song SY, et al. Comparison of the efficacy of diegnogest and levonorgestrel-releasing intrauterine system after laparoscopic surgery for endometriosis. Journal of Obstetrics and Gynaecology Research. 2018;44(9):1779-1786. doi:https://doi.org/10.1111/jog.13703
- Kim ML, Cho YJ, Kim MK, Jung YW, Yun BS, Seong SJ. The efficacy of long-term maintenance therapy with a levonorgestrel-releasing intrauterine system for prevention of ovarian endometrioma recurrence. International Journal of Gynecology & Obstetrics. 2016;134(3):256-259. doi:https://doi.org/10.1016/j.ijgo.2016.03.017
- Chen YJ, Hsu TF, Huang BS, Tsai HW, Chang YH, Wang PH. Postoperative maintenance levonorgestrel-releasing intrauterine system and endometrioma recurrence: a randomized controlled study. American Journal of Obstetrics and Gynecology. 2017;216(6):582.e1-582.e9. doi:https://doi.org/10.1016/j.ajog.2017.02.008
- Yucel N, Baskent E, Karamustafaoglu Balci B, Goynumer G. The levonorgestrel-releasing intrauterine system is associated with a reduction in dysmenorrhoea and dyspareunia, a decrease in CA 125 levels, and an increase in quality of life in women with suspected endometriosis. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2018;58(5):560-563. doi:https://doi.org/10.1111/ajo.12773
- da Costa Porto BT, Ribeiro PA, Kuteken F, Ohara F, Abdalla Ribeiro HS. Levonorgestrel intrauterine system versus dienogest effect on quality of life of women with deep endometriosis: a randomized open-label clinical trial. Women & health. 2024;64(7):551-558. doi:https://doi.org/10.1080/03630242.2024.2382418
- Gibbons T, Georgiou EX, Cheong YC, Wise MR. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Systematic Reviews. 2021;2021(12). doi:https://doi.org/10.1002/14651858.cd005072.pub4
- NILSSON CG, HAUKKAMAA M, VIEROLA H, LUUKKAINEN T, ARCANGELI P. TISSUE CONCENTRATIONS OF LEVONORGESTREL IN WOMEN USING A LEVONORGESTREL-RELEASING IUD. Clinical Endocrinology. 1982;17(6):529-536. doi:https://doi.org/10.1111/j.1365-2265.1982.tb01625.x
- Campbell S, Smeets N. Drug Delivery: Localized and Systemic Therapeutic Strategies with Polymer Systems. Polymers and Polymeric Composites: A Reference Series. Published online October 15, 2018:1-56. doi:https://doi.org/10.1007/978-3-319-92067-2_32-1
- Bao Q, Zou Y, Wang Y, Kozak D, Choi S, Burgess DJ. Drug release testing of long-acting intrauterine systems. Journal of Controlled Release. 2019;316:349-358. doi:https://doi.org/10.1016/j.jconrel.2019.11.015
- Marotta JS. Drug Delivery Systems for Localized Treatment of Disease. Springer eBooks. Published online January 1, 2004:33-60. doi:https://doi.org/10.1007/978-3-662-06108-4_2
- Serwer L, Hashizume R, Ozawa T, James CD. Systemic and Local Drug Delivery for Treating Diseases of the Central Nervous System in Rodent Models. Journal of Visualized Experiments. 2010;(42). doi:https://doi.org/10.3791/1992
- Urello MA, Luo T, Fang B, Kiick KL, Sullivan MO. Drug and Gene Delivery for Regenerative Engineering. Encyclopedia of Biomedical Engineering. Published online 2019:565-583. doi:https://doi.org/10.1016/b978-0-12-801238-3.99892-1
- Hariharan A, Tran SD. Localized Drug Delivery Systems: An Update on Treatment Options for Head and Neck Squamous Cell Carcinomas. Pharmaceutics. 2023;15(7):1844. doi:https://doi.org/10.3390/pharmaceutics15071844
- Local drug delivery. Umcutrecht.nl. Published 2024. https://www.umcutrecht.nl/en/local-drug-delivery
Acknowledgement
I am incredibly grateful to my parents for their endless support as I did this project. I woud also like to thank my sister for being so amazing and taking the time to read my work and give me feedback! Thank you to my science teacher and school’s science fair coordinator for their guidance. A special thanks to Dr. Belland for her invaluable mentorship over the past two years and to Dr. Sonja Metcalfe for judging me at the school fair and offering insightful feedback. Thank you also to reporter Jayme Doll for allowing me to be on your amazing new series and letting me show my passion to the world, it was an honor. Your support has meant so much—thank you! :)

