Can Benzodiazepine Withdrawal be Alleviated via Blocking Pannexin-1 Channels?
Grade 8
Presentation
Problem
I would recommend first reading the introduction, then reading the problem.
Problem:
The issues:
-
Dependence on benzodiazepines causes benzodiazepine withdrawal syndrome.
- Tolerance after a certain amount of time requires a more potent drug to be prescribed
- Benzodiazepines are one of the most widely used prescription drugs
- Withdrawal syndrome is extremely severe and includes confusion, burning sensations, seizures, psychosis, hallucinations. It also includes suicidal thoughts, this is on the more severe end of the spectrum when it comes to drug withdrawal signs. (Mind, 2021)
- Treatment for benzodiazepine withdrawal consists of checking into a medical center for detox, which essentially helps manage withdrawal symptoms and waits for it to lessen on the patient
- Patients must stabilize with more diazepam (equivalent to the benzodiazepine taken earlier) to alleviate symptoms. The dosage is then decreased. (WHO, 2019)
- However, tolerance to the drug makes the effects it had on the body prior less severe, lessening effectiveness
Why is Withdrawal, Especially Benzodiazepine Withdrawal so Harmful?
Withdrawal is one of the main reasons why a person is trapped in the cycle of addiction for drugs, which is especially true in commonly abused drugs such as opioids. The stages for the cycle include:
- Initial use (American Addiction Centres, 2021)
- Abuse
- Tolerance (The same high cannot be achieved with the same dosage of the substance)
- Dependence (Quitting would mean various side effects, such as the symptoms of withdrawal)
- Addiction
- Relapse (Continuing abuse of the substance to either cease withdrawal symptoms, or because of cravings and other factors)
The relapse stage itself is often a result of the withdrawal symptoms, where the only way to manage withdrawal without medical care is often to continue taking the drug. This is especially prevalent in benzodiazepines due to its wide use (Hoffman 2022), as 12.5% of adults in the U.S in 2016 used benzodiazepines, 2.1% misuse benzodiazepines, and 0.2% have a benzodiazepine use disorder. In adults who have used benzodiazepines, 17.1% misuse them, and 2% misuse them. Not only do many people abuse benzodiazepines, but their use in medical practices are also extremely prevalent. Benzodiazepines are widely used primarily to treat anxiety and panic disorders, as well as seizures. The former is even more prevailing in modern society, as in the aftermath of Covid-19, 37% of U.S adults reported some form of anxiety. Anxiety symptoms can be managed without drugs, but many do turn to psychiatric treatment in the form of drugs, which is normally safe, except for when the use of the drug becomes long term and on a regular basis, defined by two or more months of benzodiazepine use. During abuse of benzodiazepines, some combine them with opioids as tolerance increases, resulting from a lack of treatment, which makes benzodiazepines even more deadly along with their rate of overdose.

Challenges of the pharmacological management of benzodiazepine withdrawal, dependence, and discontinuation. (2018, February 18). Sage Journals. Retrieved January 18, 2024, from https://journals.sagepub.com/doi/10.1177/2045125317753340
Benzodiazepine related overdose deaths in the USA.
Hoffman, K. S., & Miller, J. (2022, December 15). Benzodiazepines Addiction Statistics - Stats on Use and Abuse of Benzos. Addiction Help. Retrieved January 10, 2024, from https://www.addictionhelp.com/benzodiazepines/statistics/
Severity of Benzodiazepine Withdrawal
As discussed prior in the brief synopsis of the issues of benzodiazepine withdrawal, benzodiazepine withdrawal has some of the most severe withdrawal symptoms out of many drugs. Withdrawal acts in an opposite way to the drug's effects. For example, caffeine withdrawal leaves the person with feelings of extreme tiredness. Thus, as benzodiazepines are used to treat severe conditions like seizure, chronic insomnia, as well as anxiety, the symptoms of withdrawal include the following.
- Insomnia
- Nausea
- Seizure
- Difficulties sleeping
- Depression
- Suicidal thoughts
- Anxiety
- Muscle spasms
- Hallucinations (Mind, 2021)
As seen from the list of symptoms, these effects are not only detrimental to physical health, but also mental health for someone who withdraws from benzodiazepines. As a result of symptoms such as depression or anxiety, it could also further negatively affect social lives, and efficiency in the workplace.
Treatment for withdrawal is even more crucial than in many drugs, as a result of its high abuse and withdrawal from medical practices using benzodiazepines. This is one of the main reasons benzodiazepines are compared to the opioid crisis, as well as being called “the new opioid” due to its rate of abuse as well as its relevance in medical settings. Current practices for withdrawal consist of checking into a medical center to have a monitored withdrawal, no other drugs are taken, and instead, a patient stops taking the drug under medical supervision. (WHO, 2019) However, a patient must stabilize before withdrawing from the drug via doses of diazepam (equivalent to the amount used on a regular basis), further increasing tolerance and enabling the struggle with withdrawal symptoms. Widely used pharmacological treatments for withdrawal alleviation are not popularized as well, only using this common practice. What's more is that most agonists and other pharmacological treatments for benzodiazepine withdrawal can be addictive in the long term.
More on the Harms
This issue is also even moreso prevalent in minority populations, Alazopram abuse (commonly referred to as Xanax) is more commonly seen in non-white Hispanic populations than in any other population(Cook, 2018). The prevalence of many forms of drug abuse can be attributed to drugs being an outlet for many people to cope with existing stressors such as inequality. Stress of poverty is also a definite reason to pursue drugs. Often, they are seen as a way to duck the ills of life, so to say, as their stimulative, psychedelic, or in this case, inhibiting properties are used as a form of escapism. As seen from previous discussion on how harmful withdrawal symptoms are, both mental health which is often much worse in minority groups, and physical health, as many minorities are discriminated against and do not get proper healthcare are very much affected even more negatively for minority groups.
As withdrawal is such a prominent issue in why people cannot quit drugs, this directly affects many of these populations who are already entrenched in issues of discrimination, as well as many people within these populations living in unsafe communities
Method
Method:
The main question, "Can Benzodiazepine Withdrawal be Alleviated via Blocking Pannexin-1 Channels?" Will be kept in mind for all aspects of the project
Use 3 main guiding questions:
-
What are the underlying mechanisms for various opioid withdrawal symptoms, specifically in how it acts on the Mu receptors and its progression overtime?
-
What are the underlying mechanisms for benzodiazepine withdrawal, like with GABA receptors? Which are the main receptors it acts on, and the side effects this causes?
-
How are the two similar, is the overlap in mechanisms responsible for similar withdrawal symptoms sufficient in possibility of this new opioid therapy being used on benzodiazepines?
I will first research the two drugs withdrawal mechanisms, as well as mechanisms of use without focusing on anything in particular, rather it will primarily just be general knowledge to base my later research off of. To get a good idea of various theories and speculated mechanisms, I will source information from a large amount of scholarly articles on how benzodiazepines and opioids work. Unfamiliar terms will be placed in a "Research and Terms" document as something of a general glossary that will be included in my physical project.
After researching and compiling notes and information about withdrawal mechanisms, I will split the research in two. One half on researching whether benzodiazepines affect the locations that opioids affect, and vice versa, as well as how benzodiazepines affect pannexin-1 on microglia. My method will be able to draw conclusions from both comparing and contrasting opioids and benzodiazepines, but also looking at the method itself as a guide.
In this project, I'll have some background information, primarily about the method itself (blocking pannexin-1 via probenecid), then split the sections into research about both research about opioids and benzodiazepines on how they affect the body, build tolerance, and then create withdrawal symptoms.
There will also be two sections with what is similar and what is different, as well as how benzodiazepines affect locations that are primarily relevant to microglial pannexin-1 specifically. From this, I will draw a conclusion on whether this method could be applicable or not. In the end, I have created a model for the relations between benzodiazepines and ATP to allude towards possible relation to the pannexin-1 channel.
All of these will serve to draw conclusions from scientific literature to answer the 3 guiding questions. I will not follow them exactly, but use it as a guide for how to organize information and general research. Other things will be learned along the way.
Research
A huge acknowledgement, and thank you to Dr. Trang and Sierra Stokes-Heck, as well as the rest of the Trang laboratory. Their research and discovery is the basis for comparing these mechanisms. They have also answered various questions I have had about the topic through their expertise.
Introduction
The class of depressant drugs including diazepam, alprazolam, and lorazepam (under brand names Valium, Xanax, and Ativan respectively) are known as benzodiazepines, which slow brain signals and help sedate the body. Benzodiazepines are among the most widely prescribed drugs for anxiety and seizures (Cleveland Clinic, 2023). Acting on the GABA neurotransmitter and the mesolimbic reward system, they are addictive, contributing to further use. Tolerance, then dependence to the drug is fairly common with long term use (4-6 months). Then, when drug use is abstained, withdrawal syndrome begins to show. Benzodiazepine withdrawal is particularly dangerous as withdrawal, a compensatory mechanism which acts in an opposite way to the drug’s effects, can cause seizure, sleep disturbance, anxiety, panic attacks, and nausea. Withdrawal itself, particularly unsafe withdrawal is one of the primary reasons for the cycle of drug abuse, which perpetuates use and abuse for an individual.
The current most widely used method of alleviating benzodiazepine withdrawal is checking into an institution, and essentially withdrawing under medical supervision, which requires lorazepam stabilization and slowly decreasing doses, contributing to tolerance. Drug induced alleviation of benzodiazepine withdrawal is often addictive itself.
However, a 2017 study on alleviation of opioid withdrawal via microglial pannexin-1 blocking by Tuan Trang and associates at U of C proves promising. The study inhibits the pannexin-1 channels on microglia via probenecid, a drug previously used to treat gout, and inhibits pannexin-1 for other benefits. It found that probenecid, a pannexin-1 inhibitor, could alleviate withdrawal signs in mice, and as of 2024, clinical trials are still to be done. This is completely relevant as probenecid is non addictive, inexpensive, and has few side effects unlike many withdrawal alleviators of both opioids and benzodiazepines. (Burma et. al, 2017)
My project will primarily compare opioids and benzodiazepine use, tolerance, and withdrawal mechanisms to discuss their similarities and differences to see whether this novel method is applicable for benzodiazepines. Not only using opioid properties as a framework, but also relating back to microglial pannexin-1, as well as ATP channels and how benzodiazepines affect them.
Background: What is the Method?
The method to research has already been gone over, but how does blocking microglial pannexin-1 alleviate withdrawal of opioids? In the 2017 study, they tested withdrawal mechanisms of opioid withdrawal, and ended up able to identify two blockers of pannexin-1 that were viable. Mefloquine, and Probenecid. The former is now banned for use because of permanent side effects on the brain (VA Public Health. (2023,), but Probenecid is promising as it is not addictive, while most other alleviators of opioid withdrawal can be addictive or have other issues like side effects. Human trial approval will take a much shorter duration as well (which is still being worked on by Trang and his lab) as the drug itself is not old, but rather repurposed to block pannexin-1. There are a few important terms first. Microglia are immune cells that regulate development, repair, and other functions within the immune system (Most of their functions are very similar to those of macrophages), (Colonna, 2017). Pannexin-1 is a channel that functions as an ATP channel when formed in cells, facilitating various neuronal transmissions.
Dr. Trang's study found mice without the pannexin-1 channel present in their genome did not have an increase in ATP after an antagonist was administered after chronic morphine administration. This shows ATP is a key substrate for morphine withdrawal.
After administering probenecid to the rats, the signs of withdrawal they showed were diminished to the point where they were extremely faint. In a real world situation, the withdrawal symptoms would be rather managable for a non-medically supervised withdrawal, while keeping the side effects low and price low as well.
Doctrow, B. (2022, August 23). Learning to control microglia using CRISPR. National Institutes of Health (NIH). Retrieved March 9, 2024, from https://www.nih.gov/news-events/nih-research-matters/learning-control-microglia-using-crispr
Opioid Use
What are Opioids?
Opioids are an analgesic drug (a drug that sedates and relieves pain) commonly used in medical practices; morphine, fentanyl, and oxycodone among them (Centers for Disease Control and Prevention, 2023). They are not only used for sedation and anesthesia, but they are also used in cough medications, as well as diarrhea suppression, via essentially blocking the pain messages from locations of pain to the central nervous system. Opioids are derived from poppy plants and date back to ancient times. However, these drugs are extremely addictive, as they change the mesolimbic reward system with use, building your tolerance to the drug. Psychologically, they are considered “euphoric”, and the increase in dopamine causes cravings of the drug. Opioids are one of the most commonly abused drugs, and have some of the highest overdose related death rates. This is because they are both commonly abused illegally, but in medical practices, withdrawal can occur as a result of prolonged use as an anesthetic or pain reliever.
(morphine, prototypical opioid,)
Drug Overdose Death Rates. (2023). Drug Overdose Death Rates. NIDA.NIH.GOV | National Institute on Drug Abuse (NIDA). Retrieved March 9, 2024, from https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates
Ogura, T., & Dan, T. D. (2013). Chapter 15 - Opioid Agonists and Antagonists. ScienceDirect. Retrieved March, 2024, from https://www.sciencedirect.com/science/article/abs/pii/B9781437716795000156
As opioids change your body and brain overtime, the same dosage will not allow you to achieve the same effects as when you first take the drug. With tolerance then comes addiction, inability to stop using the drug. It is important to note dependence and addiction are not the same, as dependence, or substance use disorder as it is commonly referred to now consists of impairment associated with use of the substance. As with most drugs, they act on the CNS (central nervous system) as receptor agonists on the various receptors within the brain. In the next section on pharmacological mechanisms of opioid use, I will go into specific locations, and receptors opioids affect.
As with most drugs, there are both opioid agonists, and opioid antagonists. Agonists usually refer to the drug itself, which activates the receptor. However, antagonists such as naloxone block the effects of opioids which is especially useful in combating overdose as well as abruptly stopping effects in a scientific setting. (Berg, 2018)
Pharmacological Mechanisms
Receptors
Opioids travel through the bloodstream via injection, inhalation, or oral administration. Then, they act on the consist of the 3 major opioid receptors located in the brain, which are all a part of a family of G coupled protein receptors, which are proteins functioning via adenylyl cyclase-inhibitory GTP proteins. They include Mu, also known as μ, Delta or δ, and Kappa, κ. These receptors are concentrated in various areas such as the brain stem, and spinal dorsal horn, and are activated by opioids to produce various effects. They are each responsible for various symptoms, for example μ receptors are responsible for supraspinal analgesia, euphoria, and anorexia among other things with use, while δ is responsible for dependence, miosis, dyspnea, while generally, κ affects analgesic/ opioid induced antidepressant effects (Stoeber, 2018). When opioids act on a receptor, its effects generally cause reduced neuronal excitability (how excitable a neuron is, and its ability to generate membrane voltage change in response to small stimulus) (Timofeev et al., 2019) via various methods in different locations of the brain that will be discussed later, and causing analgesia in the body. The primary receptor I will be discussing in this project will be μ, as it is responsible for various factors like noradrenaline release.
|
Mu μ |
Delta δ |
Kappa κ |
|
Euphoria |
Any analgesic/ opioid induced antidepressant effects |
Spinal Analgesia |
|
Supraspinal analgesia |
Sedation |
|
|
Anorexia |
Miosis |
|
|
Physical dependence |
Dependence |
|
|
Pruritus |
Dyspnoea |
|
|
Gastrointestinal motility |
Respiratory depression |
Table of opioid receptors and functions
Stoeber, M. (2018, May 22). How opioid drugs activate receptors. National Institutes of Health (NIH). Retrieved February, 2024, from https://www.nih.gov/news-events/nih-research-matters/how-opioid-drugs-activate-receptors
Analgesia and use: Introduction
Opioids decrease neuronal excitability in a variety of ways via acting on certain descending pain pathways, which includes the rostral ventral medulla, the Periaqueductal Gray (PAG), and modulating various release pathways for neurotransmitters. The overall reduction in neuronal excitability then results in reduced chance of neurotransmitter firing rate. In various locations and systems of the brain, opioids are found to have a variety of effects on neuronal excitability, and neurotransmitters throughout the brain.
Useful terms:
LC - Locus Coeruleus, the region of the brain with the far-reaching noradrenergic transmitter system of the brain. It is also the main synthesizing spot of noradrenaline.
Norepinephrine/ Noradrenaline - A neurotransmitter which regulates attention, arousal, and many stress reactions [] which is synthesized in the LC. Abbreviated to NE or NA
GABA (gamma-amino butyric acid): Neurotransmitter in the brain whose primary function is to inhibit the firing of neurons.
Dopamine (DA) - Neurotransmitter which is found in many brain regions which regulate pleasure, movement, emotion.
cAMP - Cyclic adenosine 3′,5′-monophosphate (cAMP) is a messenger which plays a crucial role in responses to neurotransmitter and hormone release, which is also a derivative of ATP.
Periaqueductal Gray - PAG is a critical part of autonomic nervous system function, and is extremely key in modulation of pain behaviors, pain response, as well as visceral defense reactions like fear, anxiety, and depression.
(Kosten, 2002)
Effects on cAMP signaling
Receptor activation affects cAMP signaling by reducing it, as well as reducing activity of voltage-gated calcium channels. cAMP, or cyclic adenosine 3′,5′-monophosphate plays crucial roles in neurotransmitter and hormone release, as well as being an ATP derivative (Coffey, 2023). cAMP is inhibited via μ activation, modulating release of nociceptive neurotransmitters. This is a general, widespread effect of opioid withdrawal in the brain. This inhibition, because cAMP modulates release of nociceptive neurotransmitters directly leads to decreased neuronal excitability as the production of cAMP via adenylyl cyclase is regulated by G coupled receptors. Activation of receptors with an opioid agonist dissociates subunits of the receptor to act on various intracellular pathways, which causes a few effects. A reduction in cAMP formation, modulation of potassium and calcium ion channels (Outward shift of K+ and reduced intracellular Ca2+), and altered gene expression. With chronic use, cAMP pathways are upregulated as a result of homeostasis, which is mediated by increases in adenylyl cyclase and cAMP, protein kinase A activity and cAMP response-binding protein activity. cAMP is synthesized by adenylyl cyclases from ATP. Pannexin-1 channels, the target of the opioid withdrawal therapy are an ATP channel. The extreme opioid effects on cAMP may be one of the key factors of why the treatment is effective in the first place. (Burma et. al, 2017)
The Locus Coeruleus
The Locus Coeruleus (abbreviated as the LC) is a structure in the brain that is the main synthesizer of norepinephrine, also known as noradrenaline (abbreviated as NE or NA respectively), a neurotransmitter responsible for helping to transmit signals throughout the nervous system (Hussain, 2023). The LC is also the largest group of noradrenergic neurons. NA is linked to various functions such as arousal, alertness, and stress reactions, the exact reactions opioids suppress, and the ones that are shown during withdrawal (Sci Rep, 2023). This location expresses all three receptors, but it is μ which contributes most to dependence, which the mechanisms of will be discussed later. Activation of the LC releases NA, increasing sympathetic activity, and decreasing parasympathetic activity. (Pergloizzi et al., 2020) Inhibition, which opioids do, suppresses this vital neurotransmitter. After binding, it contributes to drowsiness and many of the symptoms of opioid use such as delayed reactions. Usually, the body produces the amount of NA using opiate-like chemicals, activating adenosine cyclase which converts ATP to cAMP. Then, when a drug links to μ receptors in the LC, the enzyme converting ATP to cAMP is inhibited, less NA is released, leading to the effects of opioids. (Kosten 2002) Both inhibition of conversion of ATP to cAMP then suppresses neuronal firing rates as well as NA release. (Burma et. al, 2017)
Mesolimbic System
Much of opioid dependence and its calming as well as anxiolytic sensations can be attributed to the Mesolimbic System. This system is a CNS circuit which uses inputs from the Ventral Tegmental Area (VTA), Prefrontal Cortex (PFC), Amygdala, and Nucleus Accumbens. This is a dopamine system which “rewards” the body with dopamine for certain behaviors, and motivates the body towards certain tasks. Within this system, there are 3 main “stages”. Basal ganglia-driven binge/ intoxication (reward and incentive), extended Amygdala- and habenula-driven withdrawal (negative emotion, stress, as well as reward DEFICIT), Prefrontal cortex and allocortex driven anticipation (cravings, and impulses). Opioids affect the subsystems in a “reciprocal” fashion towards dopaminergic neurons in the VTA, inhibitory GABAergic neurons of the nucleus accumbens and corticostriatal glutamate projections, and corticotropin-releasing factor. Opioids also modulate GABA, a primary inhibitory transmitter in the brain which is like the brakes to a car, by inhibiting GABAergic transmissions. This reduction leads to further release of dopamine in the nucleus accumbens. A decrease in GABA leads to an increase in dopamine (Tan et al., 2010)
| Addiction stage and functional domain | Region of brain involved in neurocircuitry of opioid addiction |
Neurotransmitter levels associated with stage |
| Binge/intoxication | Basal ganglia (nucleus accumbens-ventral tegmental area) |
↑ Dopamine |
| Withdrawal/negative affect | Extended amygdala (including bed nucleus of the stria terminals) and habenula |
↑ Corticotropin-releasing factor |
| Preoccupation/anticipation | Prefrontal cortex, insula and allocortex |
↑ Dopamine |
Pergolizzi, J. V., Raffa, R. B., & Rosenbatt, M. H. (2020, January 27). Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: Current understanding and approaches to management. PubMed. Retrieved February 13, 2024, from https://pubmed.ncbi.nlm.nih.gov/31986228/
Other
Opioid use also causes a variety of effects on different parts of the brain that are undiscussed when looking at these three systems. The PAG, or Periaqueductal Gray is a brain structure which manages pain signals via release of monoamines. (Jang et al., 2016) Opioids, as a part of their analgesic effects activate this region during use. Which form both natural and opioid related activation then activates enkephalin releasing neurons to modulate pain. Opioids also activate the rostral ventral medulla, a structure releasing monoamines to cause analgesia as well as production of excitatory neurotransmitters.
Opioids also inhibit voltage gated calcium channels. Calcium (Ca2) is transferred through voltage gated calcium channels through the cytoplasm, thus this essential nutrient is then attained by all parts of the cell. Opioids enhance postsynaptic potassium channels, postsynaptic specifying the channels are on the receiving side of the synaptic transmission. This decreases neuronal excitability further. In total, all of these decreases in neuronal excitability decrease probability of neurotransmitter release, thus producing analgesia, and the effects of opioid use. (Burma et al., 2017)
Dependence and Withdrawal
Withdrawal, as explained here essentially has the opposite effect of the drug. Withdrawal from opioids causes the opposite effects of the opioid. During withdrawal, hyperalgesia occurs (extreme pain sensitivity), diarrhea, nausea, and insomnia. For opioids, this is because the repeated use of the drug builds tolerance, and the brain adapts to the drug. Thus, quitting the drug can be detrimental as your brain is conditioned to assume you will always have these effects on your body.
Withdrawal creates hyperexcitability in the nervous system, as opioids reduce neuronal excitability. It’s essentially “compensating” for downstream protein expression (calcium channels, and potassium channels) and the lack of opioids in its presence. Upregulation is one of the key components of opioid withdrawal, characterized by increasing response to a substance to carry its function out. Using other drugs to cancel out the effects of opioids (like an antagonist) thus leads to compensatory upregulation. Another part of opioid withdrawal is that after withdrawal, levels of extracellular glutamate and NMDAR expression increases, (Burma et al., 2017) two glutamate receptors, further increasing glutamatergic activity. Glutamate itself, as well as GABA will be further discussed in the benzodiazepine section. cAMP levels rise without an opioid to control them, as with chronic use, its pathways are upregulated to maintain its homeostasis. (Burma et al., 2017)
In the Locus Coeruleus, discontinuation of an opioid causes the body to attempt to compensate for the NA that was inhibited. However, it does this by increasing the levels to a degree that is often too high for the body, causing various symptoms. Usually, the body produces the amount of NA using opiate-like chemicals, activating adenosine cyclase which converts ATP to cAMP. Then, when a drug links to μ receptors in the LC, the enzyme converting ATP to cAMP is inhibited, less NA is released, leading to opioid these effects (Pergolizzi, 2017). Further repeated suppression increases noradrenergic tone in withdrawal, increasing alertness, as well as being one of the most key parts of withdrawal (Stoeber, 2018). Withdrawal symptoms caused by excessive NA include jitters, anxiety, and muscle cramps.
Another key structure for both withdrawal and tolerance is the Mesolimbic System. Up-regulation caused by opioids of GABA neuronal activity within this system diminishes the rewards overtime, thus building tolerance. GABA, an inhibitory neurotransmitter decreases with activation of μ receptors results in dopamine surges from dopaminergic neurons to mesolimbic targets (Stoeber, 2018). Dopamine itself is highly important to opioid craving during or after withdrawal, as this is the “reward” the body gives you with use of a drug. Chronic use of opioids dampens this release, further building tolerance.
Synaptic plasticity is also among one of the complex neuroadaptations from use. Which is ability to modify connection strength. Without an opioid, withdrawal effects like the aforementioned hyperalgesia occur.
In short, opioid withdrawal causes nervous system hyper excitability as tolerance changes your brain’s structure in various ways, primarily through upregulation throughout the relevant locations opioids act on.
Benzodiazepines
What are benzodiazepines?
Benzodiazepines are a class of similar depressant type drugs which include alprazolam, diazepam, and clonazepam. You’ve probably heard of them sold under brand names such as Xanax, Valium, and Klonopin respectively. These drugs are primarily used to treat seizure, insomnia, anxiety, and as an anticonvulsant, as well as use as a sedative. This is done by “slowing down” the nervous system rather than inhibiting it and causing analgesia like opioids do. (Annette and Marks, 2022) However, much like opioids, they’re commonly used in medical practices as anxiety medications, but also abused. The issue of benzodiazepine abuse, dependence, and withdrawal is further discussed in the problem section of this project. Benzodiazepines are extremely calming, and cause drowsiness, muscle relaxation, lightheadedness, and confusion among other things (Cleveland Clinic, 2023) In this section I will be discussing primarily how withdrawal occurs from either abuse, or long term use, as shorter acting benzodiazepines for insomnia often do not change the body enough to create a physical dependence. For opioids, I have referred to many of their effects as analgesic, alleviating pain, while for benzodiazepines, the term anxiolytic will be used, referring to alleviating anxiety symptoms.
In benzodiazepines, there are three major types. Agonists, inverse agonists, and antagonists. Agonists are the typical benzodiazepine, wherein they have anxiolytic effects. Antagonists are the usual antagonists, taking up use of benzodiazepine receptors and canceling out benzodiazepine effects, an example would be flumazenil. Inverse agonists however are proconvulsant, and anxiogenic, the exact opposite of benzodiazepine effects. (Nutt, 2002)
Nutt, D. J. (2002, May 24). Pharmacological mechanisms of benzodiazepine withdrawal. PubMed. Retrieved February 13, 2024, from https://doi.org/10.1016/0022-3956(90)90041-n
Pharmacological Mechanisms
Benzodiazepines - General
Benzodiazepines are primarily known for acting on the GABAA receptor as positive allosteric modulators. GABA, or y-aminobutyric acid is the primary inhibitory neurotransmitter in the CNS, functioning like brakes (Harris, 1997) to the nervous system. It creates drowsiness, and decreased awareness. On the other hand, GABA’s precursor, glutamate, is excitatory and is somewhat like an accelerator to nervous system activities. The actions on the GABAA receptor consist of benzodiazepine binding to the receptor, and increasing the binding of GABA to this receptor, thus enhancing the effects of GABA itself (Bateson 2002). Benzodiazepines potentiate GABA-gated chloride ion opening in the presence of GABA, which is the primary mechanism of benzodiazepine use.
Vinkers, C. (2012, March). (PDF) Mechanisms Underlying Tolerance after Long-Term Benzodiazepine Use: A Future for Subtype-Selective GABA(A) Receptor Modulators? ResearchGate. Retrieved March 7, 2024, from https://www.researchgate.net/publication/224847671_Mechanisms_Underlying_Tolerance_after_Long-Term_Benzodiazepine_Use_A_Future_for_Subtype-Selective_GABAA_Receptor_Modulators
Here, as demonstrated, is the structure of the GABAA receptor. There are two GABA sites, and one benzodiazepine binding site among 5 units. Benzodiazepines bind between the A and Y units, inducing confrontational change in the GABAA chloride channel, hyperpolarizing the cell and creating the nervous system inhibition (Ochsner, 2013). A subunit causes the clinical effects, the Α1 subunit is in half of GABA receptors, which mediates sedative effects of benzodiazepines A2 and A3 mediate the anxiolytic effects of benzodiazepines. [Vinkers, 2012)
With low doses of the benzodiazepine diazepam, which is often used as the prototypical benzodiazepine, it creates anxiolysis and low receptor occupancy. High doses cause high receptor occupancy, and even sedation. Continuous use of benzodiazepines causes side effects with use, such as nervous system hyperexcitability. (Bateson, 2002)
Effects on the Locus Coeruleus
Benzodiazepines affect LC noradrenaline, as well as the HPA axis, or the hypothalamic–pituitary–adrenal axis, a stress mediator and key part of homeostatic responses as well as key for memory (Kiroujac, 2020). These are both acted on via suppression of the LC and HPA axis. These are not only a key mechanism, but also a part of benzodiazepine side effects, and how tolerance to benzodiazepines are built. One side effect of benzodiazepines is amnesia, alluding to inhibition of HPA being a central part of benzodiazepine use. Use of benzodiazepines overtime also causes less NA release, as shown in biochemical studies in both humans and animals. (Vgontzas, 2008)
The effects of benzodiazepines on noradrenaline in the LC are not a direct result of inhibiting the LC like opioids do, but rather, it’s modulating on GABA, which with an increased dosage, then indirectly inhibits the LC. (Vgontzas, 2008)
More on various other, more niche mechanisms of benzodiazepines will be discussed later when I researched overlap, but most if not all articles pointed at the increase in GABA as the primary factor for benzodiazepine mechanisms of use.
Dependence and Withdrawal
Benzodiazepine withdrawal consists of various mechanisms that are not extremely well understood compared to other drugs. Tolerance is adaptive, much like in other drugs. The most key part of building tolerance to benzodiazepines is downregulation of GABA, or reduction in cellular response because benzodiazepines have the most prominent roles on GABA (Authier, 2009). This is the opposite of upregulation. As with other withdrawals, the opposite effects occur. For benzodiazepines, this includes anxiety, sleep disturbance, panic attacks, tension, and even life threatening seizures. (WHO, 2021)
In GABA receptors, A2 and A3 receptor subunits do not contribute to withdrawal or tolerance (Authier, 2009). There are various theories to attempt to understand both dependence and withdrawal, but a few things are understood. Monoamines and neurosteroids have a role in early stages of tolerance. Glutamatergic sensitization is also said to have a role in this, as it is the opposite of GABA, and rather is an excitatory neurotransmitter (Hood and Davis, 2023). This is moreso only slightly related to tolerance, though, and other information on it hasn’t been discovered. Tests on whether withdrawal and dependence is a result of receptor number have results with varying success. (Nutt, 2002)
In a study using 40mg/kg/day using water soluble flurazepam, after 7 days rats were introduced to an inverse agonist (which used the receptor, but produced opposite effects), the majority had seizures, while a control group that did not receive benzodiazepines did not. Short benzodiazepine use makes users more sensitive to inverse agonists. Chronic usage of benzodiazepines in the long term and seizure rate reduces, essentially will reduce agonist effects as a result of tolerance, and increase inverse agonist effects This shift in receptor function is what causes tolerance, and then withdrawal. However, this is mostly to do with the benzodiazepine receptors, but not receptors like GABA. Receptors like the peripheral benzodiazepine receptor, for example. (Nutt, 2002)
Decrease in GABAa reactivity to ligands or alteration in benzodiazepine coupling to GABA sites is among one of the mechanisms of tolerance as use continues. Chronic benzodiazepine use includes uncoupling of GABA and BZD sites, suggesting changing the GABAa’s receptor function (Foitzick et al., 2020)
In withdrawal, various changes to certain receptors were observed. NMDA, N-methyl-D-aspartic acid, a glutamate receptor which also has a role in memory (Yamamoto, 199), has increased expression during benzodiazepine withdrawal, as well as AMPA (Bateson, 2002). Both are a result of adaptation to downstream regulation from GABAA overstimulation. Blocking NMDA and AMPA is also shown to help withdrawal symptoms in the PAG (Periaqueductal Gray). Withdrawal enhances high voltage activated calcium channel activity preceding AMPA potentiation, this process may be central to benzodiazepine withdrawal, while blocking calcium channels can have an alleviating effect on certain withdrawal symptoms.
Mechanism Comparisons
Introduction
After looking at specific mechanisms of opioids and benzodiazepines, I have concluded that I should base my next steps in research on looking at how benzodiazepines affect the locations opioids affect. This is because much of my research on benzodiazepines focused on one location, the mesolimbic system and GABAa receptors. Opioid withdrawal and tolerance are also much more well known, as upregulation of channels is a widely agreed consensus, while benzodiazepine mechanisms of tolerance still have speculation and theories.
Mesolimbic system
Benzodiazepines and opioids both increase dopamine levels in the Mesolimbic System in two different ways (Van der Laan et al., 1992). This is because their effects on GABA are the opposite of each other. Opioids inhibit GABA, while benzodiazepines potentiate GABA to produce the calming effect it’s associated with. Benzodiazepines in one study were found to decrease dopamine turnover, the ratio of dopamine and metabolites (Ruth, T., Vesna Sossi (2002). This is done by activating the GABA-ergic system, a system usually activated in stressful situations to help “calm” the body.
Opioids also altered dopamine turnover, with morphine increasing dopamine turnover rate in the frontal cortex, but decreasing it in the neostriatum (Deyo et al., 1979) . Overall, the effects in increasing turnover rate are more prominent than their slight decrease. (Ghosh, & Patel, 1998). This is one of the ways opioids are dissimilar, which makes sense as their effects on GABA are the exact opposite.
Via positive modulation of GABA-A, dopamine is increased in the VTA (Tan et al., 2010) . While opioids increase dopamine by conditioning the brain to release dopamine as a reward for “positive” behavior. Both trigger synaptic plasticity, ability to modify connections between neurons, which is an underlying mechanism. Benzodiazepines do this via increasing dopamine levels through disinhibition, which is an underlying mechanism for reinforcement of drugs. Although mesolimbic dopamine is required for benzodiazepine addiction, there are many more mechanisms involved. (Tan et al., 2010)
Calcium Channel Activity
Diazepam creates selective transcriptional down-regulation of GABAA using a mechanism dependent on L-type voltage gated calcium channels (Foitzick et al., (2020), the same voltage gated calcium channels opioids inhibit as a part of use. This suggests that benzodiazepine induced stimulation of calcium influx through L type voltage-gated calcium channels results in activation of the signaling pathway, leading to uncoupling and alteration of subunit receptor expression to the receptors it binds to. With benzodiazepine use, calcium channels are upregulated, with an increase in calcium in the long term. (Earl, 2011). This is because benzodiazepines are direct inhibitors of calcium channel activity, decreasing neuronal excitability with use.
There is also more research suggesting that calcium channel activity is a key part in building withdrawal in benzodiazepines. Neuronal activity modulates GABAergic transmission strength by different mechanisms involving calcium channels (Foitzick et al., 2020). There are a few mechanisms to take note of.
- Prolonged exposure benzodiazepines regulates voltage gated calcium channels
- Sustained benzodiazepine use potentiates calcium currents through these channels
- GABAA receptor mediated currents in certain Ca1 (calcium) neurons are reduced as a result of chronic benzodiazepine usage
- Benzodiazepine disruption of GABA synapses mediated by calcium mobilization
These suggest the tolerance mechanism involves an increase in calcium.
Uncoupling is mediated by a mechanism involves binding diazepam to the specific site of benzodiazepine, then activation of GABAa
Foitzick, M. F., & Medina, N. B. (2020, March 16). Benzodiazepine exposure induces transcriptional down-regulation of GABAA receptor α1 subunit gene via L-type voltage-gated calcium channel activation in rat cerebrocortical neurons. ScienceDirect. Retrieved February 26, 2024, from https://www.sciencedirect.com/science/article/abs/pii/S0304394020300719
See here, uncoupling of GABA/benzodiazepine site interactions is produced by sustained exposure to diazepam.
Locus Coeruleus
Previously, I have discussed benzodiazepine mechanisms to do with the Locus Coeruleus in general mechanisms. However, as noradrenaline upregulation is so key to opioid withdrawal, I will look further into the LC, and benzodiazepines.
In a study (Filinger, 1989), it was found both diazepam and clonazepam reduced noradrenaline release by about 50%, as the catecholaminergic system (referring to neurotransmitters/ biological amines which refers to noradrenaline, and dopamine) (Farina & Aschner, 2020) is linked to benzodiazepine effects.
Filinger, E. J., Elgoyhen, A. B., & Alder-Graschinsky, E. (1989). Benzodiazepines decrease the release of [3H]noradrenaline and of [3H]acetylcholine in the cat superior cervical ganglion. PubMed. Retrieved February 27, 2024, from https://pubmed.ncbi.nlm.nih.gov/2577222/
NA release figure. Number of experiments is indicated between brackets.
From this, diazepam reduces noradrenaline release. They also reduced ACh, the excitatory neurotransmitter also known as Acetylcholine by about 50%. It’s also shown that diminution of NA is a result of reduction of liberation in the ACh. Morphine, however, acts via a cause in SCG which causes diminution of NA.
Finally, benzodiazepines are not shown to be direct modulators of the A1A‐ and A1B‐adrenoceptors, receptors activated by NA. (Williams & He, 2018). In fact there was no evidence that pertained to their multiple experiments, rather it was simply decreasing NA levels instead of ligand binding.
Periaqueductal Gray
The Periaqueductal Gray (PAG), as mentioned earlier is said to not only have a role in pain, but also anxiety. This could possibly allude to a benzodiazepine mechanism being focused here to create anxiolytic effects. It works via triggering fight or flight response in mammals. Responses of the PAG are lessened by drugs facilitating GABA-ergic transmission, which includes benzodiazepines. The results indicated that the PAG has one role of many in the anxiolytic effects of benzodiazepines, as a full agonist benzodiazepine (diazepam) injected directly into the PAG caused many anxiolytic effects. (Russo et al., 1993)
NMDA
Benzodiazepines were found to also increase NMDA and AMPA receptor expression (Authier et al., 2009). This is one of many mechanisms of benzodiazepine withdrawal, a loosely speculated mechanism. This increase overall is said to be a downstream regulation. Opioids were also in fact found to modulate NMDA in the same exact mechanism. With tolerance, there is a downstream regulation that increases NMDA expression as it is used (Mao, 2000).It also affects synaptic plasticity in both opioids and benzodiazepines.
cAMP
cAMP is inhibited by benzodiazepines (Cherry & Thompson, 2000). Although this mechanism is not commonly discussed, for usually mechanisms of use involve primarily looking into their effects on GABAA, the effects on cAMP are still existent and relevant. There is a clear correlation between cAMP and benzodiazepines, as they inhibit NA. NA increases cAMP via an increase in Adenylyl Cyclase, a building block of cAMP and ATP (Phyllis & Wu, 1980). With use, and inhibition of NA, Adenylyl Cyclase amount lowers.The actions of benzodiazepines and may be a key factor in possibility of using this method to alleviate withdrawal.
Benzodiazepines and Pannexin-1/ ATP related mechanisms
After looking at the specific mechanisms to each drug, as well as the comparisons between the drugs. To further attempt to solidify findings, this section will focus on how benzodiazepines can specifically affect pannexin-1, as well as various mechanisms opioids act on ATP to see whether benzodiazepines affect these mechanisms. These aspects will likely be slightly more vague, such as effects on receptor ligands, or peripheral receptors. This section is considerably shorter compared to the other two, but will still offer valuable insight to add to the theory itself.
Study 1: Human erythrocytes release ATP by a novel pathway involving VDAC oligomerization independent of pannexin-1 (Ostuni, 2018)
One study that seems promising is by Ostuni M, published in 2018 discusses the peripheral benzodiazepine receptor (referred to as TSPO). This receptor is not discussed as much, as development of withdrawal and tolerance is primarily focused on the CNS rather than the PNS. Activation of β-adrenergic receptors via agonists stimulates ATP, leading to cAMP activity increases (), which is an interesting mechanism that has not been discussed previously. Use of pannexin-1 inhibitors, like probenecid, diminishes ATP release from red blood cells specifically from adrenergic stimulation. Something that may occur with increased levels of NA during withdrawal.
Ostuni, M. (2018, August 1). (PDF) Human erythrocytes release ATP by a novel pathway involving VDAC oligomerization independent of pannexin-1. ResearchGate. Retrieved February 25, 2024, from https://www.researchgate.net/publication/326690577_Human_erythrocytes_release_ATP_by_a_novel_pathway_involving_VDAC_oligomerization_independent_of_pannexin-1
Seen here is the proposed figure. TSPO (benzodiazepine receptor) ligands bind to TSPO2/VDAC/ANT, inducing calcium entry, enhancing cAMP signaling, and creating ATP. Activating PKA and PKC, releasing ATP through pannexin-1.
Study 2: Influence of adenosine receptor agonists on benzodiazepine withdrawal signs in mice (Listos et al., 2005)
This study primarily focuses on adenosine receptors and withdrawal. Both Adenosine A and 2A receptors are involved in withdrawal of benzodiazepines (Listos et al., 2005). Adenosine receptors are G coupled protein receptors modulating adenosine actions. These actions include those on cAMP, as well as ATP, by convesion of ATP to cAMP. (Sheth, 2014). Adenosine is also a modulator of GABA, NMDA, and dopamine. Activation of adenosine A1 and A3 inhibits adenylyl cyclase, while activation of A2a and A2b receptors activate adenylyl cyclase release.
Proof for its relation is that in vitro, it was demonstrated that BZDs inhibited adenosine uptake by rat cerebral cortical synaptosomes. While chronic diazepam usage downregulated the A1 adenosine receptor. Observed suppression of withdrawal syndrome via adenosine receptor agonists may be related to antagonist relation between NMDA and adenosinergic system.
Study 3: Investigation of the inhibitory effects of the benzodiazepine derivative, 5-BDBD on P2X4 purinergic receptor by two complementary methods (Zsembery and Balaz, 2013)
5-BDBD is a benzodiazepine derivative that was found to selectively inhibit purinergic receptors, primarily P2XR receptors. These are a receptor family of 19 receptors. 4 adenosine activated receptors, 4 adenosine activated P1 receptors, 7 ATP gated P2X receptors (P2XR), and 8 P2Y receptors by adenosine and tri/diphosphates. The focus is on the P2X4 receptor, part of the 7 ATP gated P2XR receptors. 5BDBD thus inhibits ATP induced calcium signals through the inhibition of P2XR, as ATP induced calcium influx is inhibited. 5BDBD shifts ATP concentration curve rightwards (changing the equilibrium into a certain direction). (Zsembery and Balaz, 2013)
P2X7 specifically is key to opening pannexin-1 channels. P2X7R-Panx1 in the study is identified to be a microglial signaling ensemble required for morphine withdrawal. It was determined P2X7R expression was selectively increased in morphine dependent rats’ spinal microglia. (E. Burma et al., 2017). ATP’s role in pain consists of activating P2X in sensory neurons of mammals, while blocking of P2X with opioids directly inhibits this (Chizhmakov et al., 2005).
Data
No data was collected for this research.
Conclusion
Discussion
This section will be discussing my findings, and whether I think that the findings are enough to solidify my hypothesis. It will compile all that I know about benzodiazepines and opioids, and give proof either against, or for the hypothesis. The first part will be a comparison, while the second part will be an overview of my thoughts overall on if this can apply.
Sources of Error
This project, in my opinion held very few sources of error. Aside from one article I looked into on TSPO ligands and how it would be unapplicable for this project (more on that later), the only other ones I can really think to identify are that some of the articles are a bit older (pre-2000s). While the newer articles do not discuss these findings. This primarily applies to benzodiazepines, as I really had to look deep into the internet to find information I needed to look at both mechanism overlap and ATP related functions.
Similarities and differences
Effects on the Mesolimbic System
Both benzodiazepines and opioids achieve a dopamine increase in the body via acting in the mesolimbic system. However, the mechanism of this is very different. Opioids receive dopamine via a method of affecting how the body rewards behaviors. Opioids create reward and incentive for further use, which for every time opioids are used, dopamine is released. This is because its inhibitory effects on GABA cause a surge in dopamine without GABA “taking up space”, so to say. On the other hand, benzodiazepines modulate GABAA, a receptor, not the neurotransmitter. The positive allosteric modulation causes increased dopamine in the VTA. The overall result is the same, but the mechanisms to achieve this are completely different.
Bennett, C. (2019, July 18). GABA Activation and Dopamine Suppression. News-Medical. Retrieved March 2, 2024, from https://www.news-medical.net/health/GABA-Activation-and-Dopamine-Suppression.aspx
A dopaminergic neuron, diagram of the dopamine neurotransmitter between synapses.
Benzodiazepines as specified earlier potentiate and strengthen GABAergic transmission, and cause more of this inhibitory neurotransmitter to be released. GABAergic transmission strengthening is done by binding to GABAA, and The opposite effect is done by opioids, as opioids inhibit GABA from the Mesolimbic System. This is one key difference between opioids and benzodiazepines, being opposites to each other. Another thing to note is the key way benzodiazepines act on the body is through the Mesolimbic System. Modulation of GABA itself is the main way to produce their effects.
Locus Coeruleus
In both benzodiazepines and opioids, effects on the Locus Coeruleus were observed. For benzodiazepines, NA release decreased about 50% due to the fact the catecholaminergic system of GABA, dopamine, and NA are all modulated via benzodiazepine use. Opioids bind to their respective receptors in the LC to inhibit NA release, and therefore reduce attention. Dependence and withdrawal in opioids also incorporates NA as a primary mechanism for both. NA release in withdrawal attempts to compensate for NA that was not released prior. Benzodiazepines also inhibit NA, although with a different mechanism, but eventually creates the same result. Through increasing GABA-ergic transmission, this inhibits various parts of the brain, thus also inhibiting the LC and release of NA.
Calcium Channels
Although ATP and calcium channels are fully independent in mechanisms, the study of benzodiazepine effects on calcium channels is still important (Petersen and Verkhratvasky, 2016). Both opioids and benzodiazepines act on calcium channels, specifically voltage gated calcium channels. Opioids decrease calcium channel activity, and so do benzodiazepines. Transcriptional down-regulation of GABAA is dependent on these channels. For both benzodiazepines and opioids, calcium channels play an important role in tolerance via inhibition/ reduction of its activity.
cAMP
cAMP inhibition may be one of the most important shared factors of opioids and benzodiazepines for their applicability of using pannexin-1 blockers to alleviate withdrawal, as it is an ATP derivative. Both inhibit cAMP, although benzodiazepine cAMP inhibition is mostly seen through their inhibition of NA release, thus inhibiting Adenylyl Cyclase and formation of cAMP. Adenylyl Cyclase modulation is also seen in both benzodiazepines and opioids to inhibit cAMP.
Other Similarities/ Differences
The Periaqueductal Gray (PAG) has a role in both benzodiazepine and opioid use, as it’s not only a modulator of pain, but also anxiety. During opioid and benzodiazepine use, this brain region is activated.
Benzodiazepines and opioids also both increase NMDA expression with a downstream regulatory effect. The same mechanism is true for both, and is a part of tolerance development for both drugs.
Overall Thoughts on Applicability, and can this Achieve the Desired Result?
After analyzing my information that I’ve compiled for this project, I personally think that the method of blocking pannexin-1 channels to alleviate withdrawal could have great promise when applying to benzodiazepines. Benzodiazepines and opioids share many of the same mechanisms of tolerance, and the locations they act on. Although for some locations they act in very different ways, the result is often the same. For example, opioids and benzodiazepines act in completely opposite ways when it comes to GABA. While benzodiazepines increase GABA, opioids inhibit it to allow for more dopamine. Benzodiazepines on the other hand increase dopamine through positive allosteric modulation of the GABAA receptor. Some of the most relevant mechanisms of withdrawal such as inhibition of noradrenaline, as well as cAMP are shared in nearly identical ways to both benzodiazepines and opioids, creating great promise if you were to look at their similarities alone.
However, when looking at benzodiazepines, there are also very seldom talked about correlations to ATP and benzodiazepines that could be noteworthy, as pannexin-1 is an ATP release channel. The study on 5-BDBD acting on purinergic receptors is very interesting, as P2X7, part of the P2X receptors that 5-BDBD was found to inhibit, is key to opening pannexin-1 channels. P2X7R-pannexin-1 was identified as a system required for morphine withdrawal. This itself is very much in favor of the theory I have, as benzodiazepine compounds are all a part of this class for their similarities.
It was also found that Adenosine receptors had roles in benzodiazepine withdrawal, which is an interesting mechanism. Adenosine receptors modulate actions of adenosine, which include actions on cAMP as well as ATP. Benzodiazepines were found to inhibit adenosine uptake in rats in this study, which is a huge breakthrough in favor of the theory I have proposed, as blocking pannexin-1 is directly related to ATP and ATP release. cAMP is required for ATP release in response to stressful stimuli, as well as requiring adenylyl cyclase (Sridharan et al., 2010).
One study also found that adenosine uptake was inhibited by benzodiazepines. As ATP is a derivative of adenosine (Adenosine Triphosphate), this study directly supports my theory.
The final study which is slightly different, as it addresses the ligands of the peripheral benzodiazepine receptor shows use of pannexin-1 inhibitors diminishes ATP release. This study also discusses how activation of β-adrenergic receptors stimulates ATP, which benzodiazepines do indirectly.
From both being extremely similar to opioids, which have been tested with this method, as well as very obscure but interesting properties that affect ATP, I believe that benzodiazepines could benefit from this method of withdrawal alleviation with probenecid. Here I will create a model to simulate how that might work.
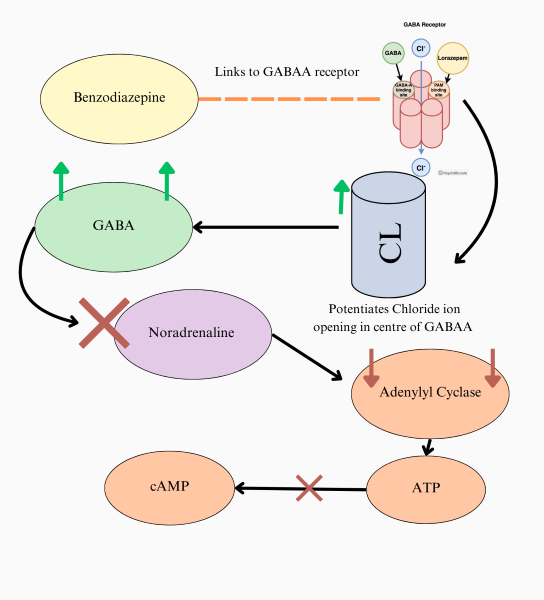
I have here, a diagram I have created for my theory on how benzodiazepines affect ATP. Arrows mean "causes", while a large X means inhibit. The smaller X means conversion cannot happen.
This diagram states as follows: Benzodiazepines link to GABAA, potentiating chloride ion opening. Thus, more GABA is released into the nervous system. The inhibitory effects of GABA inhibit noradrenaline, which then causes lowered adenylyl cyclase due to their correlation. A lack of adenylyl cyclase, the catalyst for ATP to cAMP conversion thus also creates inhibition of ATP to cAMP conversion.
ATP also requires cAMP and adenylyl cyclase to be released outside of a cell in response to stressful situations. During withdrawal, opposite effects occur, and if benzodiazepines are much like opioids, the body is conditioned to regulate these processes as they see the drug use as the new "normal". Then, when drug use is abstained, there is often too much NA, as well as not enough GABA for benzodiazepines specifically. Targetting the channels, pannexin-1 that allow for ATP transfer out of the cell could prove beneficial, for this relation, which may look a bit loose does exist.
Application
Probenecid is a gout medication that was invented in 1949 by Miller et al. (1949). There are a few primary benefits to probenecid. Not only is it already an approved drug, speeding up clinical approval, but it is also easily accessible at pharmacies, and cheap. For people who are recovering from addiction, and facing withdrawal for either opioids or benzodiazepines, this makes it very accessible, and helps ameliorate the issue of relapsing due to withdrawal. Probenecid also has relatively few side effects, and is not addictive, unlike many other withdrawal treatments. Pharmacologically, probenecid blocks pannexin-1, which was found to be effective in alleviating opioid withdrawal.
Finding that benzodiazepines not only share very similar mechanisms to opioids, with differences that still lead to the same result (a certain neurotransmitter’s release) allows for possible future usage when clinical trials for using probenecid to alleviate opioid withdrawal go forward. Using the same method to block pannexin-1 creates a new way to combat the issue of withdrawal in the cycle of drug abuse, by using an easily accessible drug.
What’s Next?
So far, I do not have too many plans on what’s next aside from presenting my finds to the CYSF. The main issue is that the method on opioids is still awaiting clinical trials to date. Possibly, in future years I could expand on this by attempting to make this experimental, and receiving approval to work on lab rats.
Conclusion
Using pannexin-1 blockers like probenecid has benefits on opioid withdrawal, but also based on my research on both mechanism overlap and the role of ATP in benzodiazepines, on benzodiazepines too. Benzodiazepines, as discussed in the “discussion” portion, share some mechanisms that are identical to those of opioids, and ones which are different, such as their effects on GABA still lead to the same result. For my first "part" of my research, I think it is sufficient to say the two drugs are similar enough to illicit attention to look into this method of withdrawal. Benzodiazepine role in ATP primarily consists of their functions on the Locus Coeruleus and NA release, thus modulating cAMP as well as ATP. In my model/ diagram, I have demonstrated how it affects ATP, which further contributes to my theory. This model could potentially also apply to opioids due to their very similar effects on the LC, leading to decreased ATP production as well.
Thus, in conclusion, with my research I firmly believe benzodiazepine withdrawal can be alleviated via blocking pannexin-1 channels. After trials for this method on opioids go through, this may be the next step for both this treatment specifically, and the future of combating drug abuse.
Citations
Citations are all done via APA format.
Authier, N., Balayssac, D., & Zangarelli, A. (2009, September 18). Benzodiazepine dependence: focus on withdrawal syndrome. PubMed. Retrieved February 23, 2024, from https://doi.org/10.1016/j.pharma.2009.07.001
Bateson, A.N. (2002). Basic Pharmacologic Mechanisms Involved in Benzodiazepine Tolerance and Withdrawal. Sciencedirect. Retrieved February 22, 2024, from https://doi.org/10.2174/1381612023396681
Benzodiazepines: What They Are, Uses, Side Effects & Risks. (2023, January 3). Cleveland Clinic. Retrieved January 8, 2024, from https://my.clevelandclinic.org/health/treatments/24570-benzodiazepines-benzos
Beyer, K. H., Miller, K. A., Russo, H. F., Patch, E. A., & Verwey, W. F. (1947). The inhibitory effect of Carinamide on renal elimination of penicillin. American Journal of Physiologic, 149, 355–368.
Burma, N. E., Bonin, R. P., Leduc-Pessah, H., Baimel, C., Cairncross, Z. F., Mousseau, M., Shankara, J. V., Stemkowski, P. L., Baimoukhametova, D., Bains, J. S., Antle, M. C., Zamponi, G. W., Cahill, C. M., Borgland, S. L., De Koninck, Y., & Trang, T. (2017). Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nature medicine, 23(3), 355–360. Retrieved December 18, 2023 https://doi.org/10.1038/nm.4281
Burma, N.E., Kwok, C. H., & Trang, T. (2017, November 10). Therapies and mechanisms of opioid withdrawal. FutureMedicine. Retrieved January 19, 2024, from https://www.futuremedicine.com/doi/10.2217/pmt-2017-0028
Cardinali, D. (1985). Benzodiazepines decrease norepinephrine release from rat pineal nerves by acting on peripheral type binding sites. PubMed. Retrieved February 27, 2024, from https://pubmed.ncbi.nlm.nih.gov/2940804/
Centers for Disease Control and Prevention. (2023, August 8). Opioid Basics | Opioids | CDC. Centers for Disease Control and Prevention. Retrieved March 3, 2024, from https://www.cdc.gov/opioids/basics/index.html
Cherry, J. A., & Thompson, B. (2000, December). Diazepam and rolipram differentially inhibit cyclic AMP-specific phosphodiesterases PDE4A1 and PDE4B3 in the mouse. ScienceDirect. Retrieved February 21, 2024, from https://www.sciencedirect.com/science/article/abs/pii/S0167478101001646
Chizhmakov, I., Yudin, Y., Mamenko, N., & Prudikenov, I. (2005, April). Opioids inhibit purinergic nociceptors in the sensory neurons and fibres of rat via a G protein-dependent mechanism. ScienceDirect Retrieved February, 2024, from https://www.sciencedirect.com/science/article/abs/pii/S0028390805000134
Colonna, M. (2017, February 9). Microglia Function in the Central Nervous System During Health and Neurodegeneration. NCBI. Retrieved November, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8167938/
Connors, E. (2023, May 10). Americans Express Worry Over Personal Safety in Annual Anxiety and Mental Health Poll. American Psychiatric Association. Retrieved January 8, 2024, from https://www.psychiatry.org/news-room/news-releases/annual-anxiety-and-mental-health-poll-2023
Cook, B. (2018, June 1). Examining racial/ethnic differences in patterns of benzodiazepine prescription and misuse. PubMed. Retrieved January 11, 2024, from https://pubmed.ncbi.nlm.nih.gov/29626743/
Cruz, M. T., & Bajo, M. (n.d.). Shared Mechanisms of Alcohol and Other Drugs. NCBI. Retrieved February 26, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3860454/
Doctrow, B. (2022, August 23). Learning to control microglia using CRISPR. National Institutes of Health (NIH). Retrieved March 9, 2024, from https://www.nih.gov/news-events/nih-research-matters/learning-control-microglia-using-crispr
Earl, D. E., & Tietz, E. I. (2011). Inhibition of Recombinant L-Type Voltage-Gated Calcium Channels by Positive Allosteric Modulators of GABAA Receptors. NCBI. Retrieved February 26, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3063747/
Farina, M., & Aschner, M. (2020). The catecholaminergic neurotransmitter system in methylmercury-induced neurotoxicity. NCBI. Retrieved February, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7188191/
Filinger, E. J., Elgoyhen, A. B., & Alder-Graschinsky, E. (1989). Benzodiazepines decrease the release of [3H]noradrenaline and of [3H]acetylcholine in the cat superior cervical ganglion. PubMed. Retrieved February 27, 2024, from https://pubmed.ncbi.nlm.nih.gov/2577222/
Foitzick, M. F., & Medina, N. B. (2020, March 16). Benzodiazepine exposure induces transcriptional down-regulation of GABAA receptor α1 subunit gene via L-type voltage-gated calcium channel activation in rat cerebrocortical neurons. ScienceDirect. Retrieved February 26, 2024, from https://www.sciencedirect.com/science/article/abs/pii/S0304394020300719
Gibson, K. M., & Crunelli, V. (n.d.). Aberrant GABAA Receptor-Mediated Inhibition in Cortico-Thalamic Networks of Succinic Semialdehyde Dehydrogenase Deficient Mice. PLOS. Retrieved February 19, 2024, from https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0019021
Graeff, F., & Russo, A. S. (1993). Role of benzodiazepine receptors located in the dorsal periaqueductal grey of rats in anxiety. PubMed. Retrieved February 29, 2024, from https://pubmed.ncbi.nlm.nih.gov/7870885/
Griffin, C. E., & Kane, A. M. (2013). Benzodiazepine Pharmacology and Central Nervous System–Mediated Effects. NCBI. Retrieved February 24, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3684331/
Harris, R. (1997). GABA and the GABAA receptor. PubMed. Retrieved February 18, 2024, from https://pubmed.ncbi.nlm.nih.gov/15704348/
Hood, S. D., Norman, A., & Alice, D. A. (2023, May 19). Benzodiazepine dependence and its treatment with low dose flumazenil. OnlineLibrary. Retrieved February 22, 2024, from https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.12023
J Neuro Sci Mini-Symposium: Extrasynaptic GABAA Receptors: Form, Pharmacology, and Function. (2009). NCBI. Retrieved February 22, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2784229/
Johnston, G. A. (2013). Advantages of an antagonist: bicuculline and other GABA antagonists. NCBI. Retrieved February 20, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3651659/
Kelly A Berg (6 August 2018)“Making Sense of Pharmacology: Inverse Agonism and Functional Selectivity.” NCBI, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6165953/. Accessed 24 November 2023.
Kirouac, G. J. (2020, December 10). The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Frontiers. Retrieved March 9, 2024, from https://www.frontiersin.org/articles/10.3389/fnbeh.2020.601939/full
Kosten, T. R., & George, T. P. (2002, 7 1). The Neurobiology of Opioid Dependence: Implications for Treatment. NCBI. Retrieved February 7, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851054/
Laila S. Hussain et al. Physiology, Noradrenergic Synapse - StatPearls. (2023). NCBI. Retrieved February 6, 2024, from https://www.ncbi.nlm.nih.gov/books/NBK540977/
Listos, J., Malec, D., & Fidecka, S. (2005, October 13). Influence of adenosine receptor agonists on benzodiazepine withdrawal signs in mice. Science Direct. Retrieved February 28, 2024, from https://www.sciencedirect.com/science/article/abs/pii/S0014299905007582
Mao, J. (2000, August 17). NMDA and opioid receptors: their interactions in antinociception, tolerance and neuroplasticity. ScienceDirect. Retrieved March, 2024, from https://doi.org/10.1016/S0165-0173(99)00020-X
McSwine, D. (2023, November 7). .,. Emergency Medicine news. Retrieved January 8, 2024, from https://journals.lww.com/em-news/fulltext/2001/12000/benzodiazepine_withdrawal__potentially_fatal,.13.aspx
Michael H. Grider Physiology, Action Potential - StatPearls. (2023). NCBI. Retrieved February 20, 2024, from https://www.ncbi.nlm.nih.gov/books/NBK538143/
Mussetto, V., Teuchmann, H. L., Heinke, B., Trofimova, L., Sandkühler, J., Drdla-Schutting, R., & Hogri, R. (2023). Opioids Induce Bidirectional Synaptic Plasticity in a Brainstem Pain Center in the Rat. The journal of pain, 24(9), 1664–1680. Retrieved February, 2024 from https://doi.org/10.1016/j.jpain.2023.05.001
Non-synaptic Interneuronal Communication: Physiological and Pharmacological Implication. (2023, May 19). Science Direct. Retrieved February 20, 2024, from https://www.sciencedirect.com/science/article/abs/pii/B9780080297897500119
Nutt, D. J. (2002, May 24). Pharmacological mechanisms of benzodiazepine withdrawal. PubMed. Retrieved February 13, 2024, from https://doi.org/10.1016/0022-3956(90)90041-n
Ogbru, Annette, and Jay W. Marks. “Benzodiazepines: Uses, Side Effects, Types, Interactions, Addition, Withdrawal.” RxList, 2022, https://www.rxlist.com/benzodiazepines/drug-class.htm. Accessed February 2024.
Ostuni, M. (2018, August 1). (PDF) Human erythrocytes release ATP by a novel pathway involving VDAC oligomerization independent of pannexin-1. ResearchGate. Retrieved February 25, 2024, from https://www.researchgate.net/publication/326690577_Human_erythrocytes_release_ATP_by_a_novel_pathway_involving_VDAC_oligomerization_independent_of_pannexin-1
Pergolizzi, J. V., Raffa, R. B., & Rosenbatt, M. H. (2020, January 27). Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: Current understanding and approaches to management. PubMed. Retrieved February 13, 2024, from https://pubmed.ncbi.nlm.nih.gov/31986228/
Phillis, J. W., & Wu, P. H. (1980). Interactions Between the Benzodiazepines, Methylxanthines and Adenosine. Canadian Journal of Neurological Sciences / Journal Canadien Des Sciences Neurologiques, 7(3), 247–249. doi:10.1017/S0317167100023271
Ruth, T. (2002). Increase in dopamine turnover occurs early in Parkinson's disease: evidence from a new modeling approach to PET 18 F-fluorodopa data. PubMed. Retrieved February 26, 2024, from https://pubmed.ncbi.nlm.nih.gov/11823721/
Schelp, S. A., Brodnik, Z. D., & Rakowski, D. R. (2018). Diazepam Concurrently Increases the Frequency and Decreases the Amplitude of Transient Dopamine Release Events in the Nucleus Accumbens. NCBI. Retrieved February 24, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5741045/
Sci Rep. Noradrenergic 'Tone' Determines Dichotomous Control of Cortical Spike-Timing-Dependent Plasticity. (2012, May 23). NCBI. Retrieved January 25, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3358707/
Sheth, S. (2014). Adenosine Receptors: Expression, Function and Regulation - PMC. NCBI. Retrieved March, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3958836/
Sung Ho Jang, So Min Park Relation between injury of the periaqueductal gray and central pain in patients with mild traumatic brain injury: Observational study. (2016, July 1). NCBI. Retrieved January 22, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4937934/
Tan, K. R., Brown, M., & Labouèbe, G. (2010, Aug 11). Neural bases for addictive properties of benzodiazepines. NCBI. Retrieved February 27, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2871668/
Taylor, D. (n.d.). Electrophysiological actions of benzodiazepines. PubMed. Retrieved February 25, 2024, from https://pubmed.ncbi.nlm.nih.gov/6252064/
The Addiction Cycle: What Are The Stages of Addiction? (2022, October 21). American Addiction Centers. Retrieved January 10, 2024, from https://americanaddictioncenters.org/the-addiction-cycle
Timofeev, I., & Chauvette, S. (2019, June 21). Chapter 29 - Sleep-Wake and Cortical Synaptic Plasticity. Sciencedirect. Retrieved January 15, 2024, from https://www.sciencedirect.com/science/article/pii/B9780128137437000293
VA Public Health. (2023, October 12). Mefloquine (Lariam®) - Public Health. VA Public Health. Retrieved November, 2024, from https://www.publichealth.va.gov/exposures/mefloquine-lariam.asp
Van der Laan, J. W., & Eigman, L. (1992, December). Benzodiazepines preferentially affect mesolimbic dopamine turnover in rats. ScienceDirect. Retrieved February 26, 2024, from https://doi.org/10.1016/0924-977X(92)90005-S
Vgontzas, A. N., Kales, A., & Brixier, E. O. (2008, June 10). Benzodiazepine Side Effects: Role of Pharmacokinetics and Pharmacodynamics. Karger. Retrieved February 24, 2024, from https://karger.com/pha/article-abstract/51/4/205/270392/Benzodiazepine-Side-Effects-Role-of
Vinkers, C. (2012). (PDF) Mechanisms Underlying Tolerance after Long-Term Benzodiazepine Use: A Future for Subtype-Selective GABA(A) Receptor Modulators? ResearchGate. Retrieved February 23, 2024, from https://www.researchgate.net/publication/224847671_Mechanisms_Underlying_Tolerance_after_Long-Term_Benzodiazepine_Use_A_Future_for_Subtype-Selective_GABAA_Receptor_Modulators
WHO Withdrawal effects of benzodiazepines. (2021, April). Mind. Retrieved January 8, 2024, from https://www.mind.org.uk/information-support/drugs-and-treatments/sleeping-pills-and-minor-tranquillisers/withdrawal-effects-of-benzodiazepines/
Williams, L. M., & He, X. (2018, December 26). Diazepam is not a direct allosteric modulator of α1‐adrenoceptors, but modulates receptor signaling by inhibiting phosphodiesterase‐4. NCBI. Retrieved February 28, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6306559/
Withdrawal Management - Clinical Guidelines for Withdrawal Management and Treatment of Drug Dependence in Closed Settings. (2019). NCBI. Retrieved January 9, 2024, from https://www.ncbi.nlm.nih.gov/books/NBK310652/
Yamamoto, T. (1999). The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. PubMed. Retrieved February 23, 2024, from https://pubmed.ncbi.nlm.nih.gov/9892356/
Zsembery, A., & Balaz, B. (2013). Investigation of the inhibitory effects of the benzodiazepine derivative, 5-BDBD on P2X4 purinergic receptors by two complementary methods. PubMed. Retrieved February 28, 2024, from https://pubmed.ncbi.nlm.nih.gov/23867750/
Acknowledgement
I would like to thank first and foremost, Professor Tuan Trang at the University of Calgary for not only doing the research on opioids which was the framework for this project’s conceptual idea, but also releasing the paper for free online to read, which led to beginning this project, as well as meeting with me to discuss my questions, and project concept. I’d also like to thank Sierra Stokes-Heck for also answering my questions on the logistics of opioids, as well as many of the questions I had, as well as discussing with me methods of finding reliable research and looking for review articles to continue with my research. I would also like to thank the rest of all contributors to: “Blocking pannexin-1 channels alleviates opioid withdrawal in rodents”, for the same reasons of the amazing research done that inspired me to begin my project. I want to thank Ms. Friesen, my science fair coordinator not only for organizing the science fair at Tom Baines school, but also for answering some broader questions about opioid receptors.