Quantifying the Impact of High Fat/Sucrose Diet on Local Inflammation of the Vastus Lateralis and Soleus Muscles in Female Rats
Grade 11
Presentation
Problem
Obesity is a global epidemic affecting over 1 billion people worldwide (Wu & Ballantyne, 2017). It is associated with numerous negative health outcomes such as increased hypertension, Type II Diabetes, muscle wasting, as well as various cardiovascular diseases (Wu & Ballantyne, 2017). With the undeniable links between obesity and countless prevalent health risks, it is critical to study the effects of obesity on various body systems and organs. One of the lesser-known consequences of obesity is its effect on muscles. Obesity has been associated with muscle wasting, also known as sarcopenia, which is a process that includes intramuscular fat deposition and fibrosis coupled with the loss of contractile tissue (Collins et al., 2016). Since muscle mass is a key indicator of long-term health, it is important to understand the relationship between diet and muscle health (Srikanthan & Karlamangla, 2014). Previous work exploring diet-induced obesity-related musculoskeletal degeneration has been conducted solely on male rats. As females can differ in the presentation and mechanism behind various diseases, it is important to determine if the same biochemical and cellular factors exist. This will help us understand the impact that gender has on musculoskeletal health. A recent study done in female rats showed those fed a high fat/high sucrose diet (HFS-fed group) had more intramuscular fat infiltration than the chow-fed group in both the VL and soleus muscles, but there seemed to be no effect on fibrosis (Smith et al., 2023). Therefore, it is important to quantify local muscle inflammation to see if this can explain the lack of fibrosis in muscles from female rats fed an HFS diet.
Method
24 female Sprague-Dawley rats at 12 weeks of age were randomly assigned into two different experimental groups: 1) animals fed a chow diet (n = 12) and 2) animals fed a high fat/high sucrose (HFS) diet (n = 12). The rats' diets were maintained for a period of twelve weeks. At twenty-four weeks of age, the rats were sacrificed utilizing ethical techniques by qualified lab members, and their vastus lateralis (VL) and soleus muscles were harvested and flash-frozen in liquid nitrogen. A mid-belly muscle section was cut, mounted in an Optimal Cutting Temperature (OCT) compound, and sectioned at 10 μm using a Lecia cryostat at -20°C. The muscle sections were then dried at room temperature and stored at -20°C until used. CD68+ immunohistochemistry was completed on the muscle sections. Muscle sections were mounted on slides. Slides were first fixed in 100% cold acetone. These slides containing muscle sections were briefly blocked, first with 3% hydrogen peroxide, and then with 2.5% normal horse serum (Vector Laboratories, Burlingame, CA). The sections were then incubated overnight at 4°C with the primary antibody, ED1 mouse anti-rat CD68+ (AbD Serotec, Raleigh, NC), a marker for inflammatory macrophages at a dilution ratio of 1:100. The secondary antibody, anti-mouse IgG peroxidase (IMPRESS kit, Vector Laboratories) was applied the next morning for 30 minutes at room temperature. Slides containing muscle sections were then incubated for 20 minutes in a Cyanine 3 fluorophore (cy3) (PerkinElmer, Waltham MA) which was prepared in a Tyramide Signal Amplification (TSA) buffer. To visualize nuclei, DAPI (4',6-diamidino-2-phenylindole fluorescent stain)(Vector Laboratories) was applied, and sections were mounted in Vectashield mounting media (Vector Laboratories). Ten images per soleus cross-section and 20 images per VL cross-section were obtained using an Olympus BX51 fluorescent microscope (Olympus, Japan), with appropriate filters for cy3 and DAPI using an Olympus DP28 camera and Cell Sens software at 200x magnification.
The macrophages identified by the CD68 marker were counted per image frame using the program ImageJ (ImageJ, U.S. National Institutes of Health 2018). The first step was to take images marked with the DAPI (4',6-diamidino-2-phenylindole fluorescent stain) marker indicating nuclei, and overlap them with the CD68 marker indicating inflammatory macrophages. These overlapped images were then evaluated with the ImageJ program (ImageJ, U.S. National Institutes of Health 2018) to assess for points where the CD68 marker (red/yellow) lined up with the DAPI marker (blue). These coinciding points indicate a nucleus of an inflammatory macrophage. The inflammatory macrophage counts for each image were stored in an Excel (Microsoft Excel, 2021) sheet sorted by image number. Once all images were counted, a non-parametric statistical test (t-test) to compare group means was used to determine any difference in macrophage infiltration between the chow and HFS experimental groups for both the VL and soleus muscles. If this t-test resulted in a p-value greater than 0.05 (p > 0.05), then there was no significant difference between the compared groups' means. If the t-test resulted in a p-value less than 0.05 (p < 0.05), then there was a significant difference between the compared groups’ means.
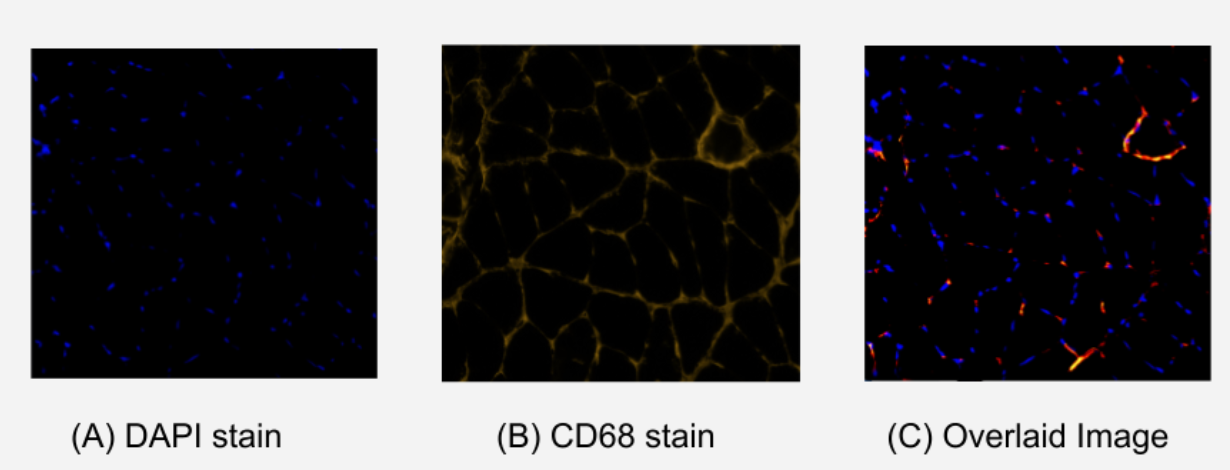
Figure 1. Comparison of Stained Immunohistochemistry Images of Soleus Muscle. (A) image using DAPI stain which indicates viable nuclei with royal blue fluorescence. (B) image using CD68 stain which indicates inflammatory M1 macrophages with yellow/red fluorescence. (C) overlaid image of (A) and (B) showing matching points of DAPI and CD68 fluorescence. These matching points indicate the localization of inflammatory M1 macrophages.
Research
One of the lesser-known consequences of obesity is its effect on muscles. Obesity has been associated with muscle wasting, also known as sarcopenia, which is a process that includes intramuscular fat deposition and fibrosis coupled with the loss of contractile tissue (Collins et al., 2016). Since muscle mass is a key indicator of long-term health, it is important to understand the relationship between diet and muscle health (Srikanthan & Karlamangla, 2014). Previous studies on adipose tissue have found that chronic inflammation is a response to obesity, caused by the release of inflammatory molecules called cytokines (Wu & Ballantyne, 2017). Studies looking at diet-induced obesity in male rats have shown that a similar process of inflammation occurs in muscle in response to a high fat/high sucrose (HFS) diet (Collins et al., 2016). Normally, muscle cells will release cytokines in response to exercise and activity (Wu & Ballantyne, 2017). These molecules generally have a positive effect on glucose metabolism and muscle repair. For example, IL6, a common cytokine, improves glucose uptake and insulin sensitivity while increasing lipolysis (breakdown of fat) and reducing inflammation (Wu & Ballantyne, 2017). However, if chronically increased, as in the case of a HFS diet, these cytokines can result in excessive and unhealthy inflammation (Wu & Ballantyne, 2017). Although these molecules play a fundamental role in muscle repair and regeneration, they become a part of the inflammatory cascade and can impair normal inflammatory responses and regenerative capacities of skeletal muscles.
Immune cells such as macrophages are activated in response to cell inflammation. Macrophages are a heterogeneous population of immune cells that function in tissue-specific immune responses (Yao et al., 2022). In muscle, macrophages have a major role in muscle regeneration, healing, and fibrosis. Their job is to clear necrotic muscle cells and promote remodeling and regeneration of muscle cells (Stepien et al., 2020). In diet-induced obesity, affected muscle and fat cells release an important molecule known as Monocyte Chemoattractant Protein 1 (MCP-1) which recruits macrophages into the damaged tissue. There are two types of activated macrophages identified in fat and muscle tissue; M1 and M2 (Yao et al., 2022). Depending on local environmental cues, macrophages polarize to one of two types. In chow-fed mice, the main population of macrophages are the anti-inflammatory M2 cells which produce anti-inflammatory cytokines (IL10 and TGF-beta) (Yao et al., 2022). These resolve tissue inflammation and promote tissue homeostasis. In obese mice fed an HFS diet, the second type of macrophage dominates and is known as the proinflammatory M1 cells (Yao et al., 2022). These M1 cells release various cytokines (IFN gamma and TNF alpha) and MCP - 1, which attract additional macrophages and induce further inflammation (Yao et al., 2022). These various inflammatory cytokines activate fibroblasts and alter the collagen dynamic. This process of healing and repair can become dysregulated and lead to fibrosis and abnormal function. Importantly, these proinflammatory M1 macrophages can be identified by certain cell markers such as Cluster of Differentiation 68 (CD68) (Yao et al., 2022). Studies have shown an association between increased local muscular CD68 and systemic MCP-1 and increased fat infiltration and fibrosis in response to an HFS diet (Yao et al., 2022). This shows that an increased M1 cell population alters muscle healing and leads to fibrosis in tissue.
Previous studies have shown differential effects of an HFS diet on glycolytic and oxidative muscle types. The two muscles considered in these studies are the glycolytic vastus lateralis (VL) and the oxidative soleus muscle (Collins et al., 2017). The VL is a glycolytic muscle that creates ATP through anaerobic glycolysis and is capable of quick bursts of movement (Collins et al., 2017). The soleus muscle is an oxidative muscle that generates ATP through aerobic metabolism and can sustain high oxidative capacities, therefore having stronger endurance (Collins et al., 2017). In previous studies conducted on male Sprague-Dawley rats, intramuscular fat infiltration (IMF), inflammatory changes, and fibrosis were preferentially seen in the VL than the soleus, indicating a possible protective mechanism of the oxidative capacity of the soleus muscle (Collins et al., 2017). A recent study done in female rats showed the HFS-fed group had more intramuscular fat infiltration than the chow-fed group in both the VL and soleus muscles, but there seemed to be no effect on fibrosis (Smith et al., 2023). Therefore, it is important to quantify local muscle inflammation to see if this can explain the lack of fibrosis in muscles from female rats fed an HFS diet.
Data
The average macrophage counts for the VL and soleus muscles for the animals exposed to the chow and HFS diets were counted and stored in excel sheets. A t-test was used to determine if there was a significant difference between group means. The data is visualized in figures 1 and 2 below. In the vastus lateralis (VL) muscle, there was no statistically significant difference between the chow-fed diet and the HFS diet (Chow mean = 32, ±SD = 8.69, HFS mean = 34, ±SD = 7.37, p > 0.05) (Figure 1). In the soleus muscle, there was also no statistically significant difference between the chow-fed diet and the HFS diet (Chow mean = 48, ±SD = 8.46, HFS mean = 47, ±SD = 2.66, p > 0.05) (Figure 2). Therefore, the data suggest that diet does not play a significant role in altering macrophage count and, by extension, does not affect local muscle inflammation in the VL or the soleus muscle.
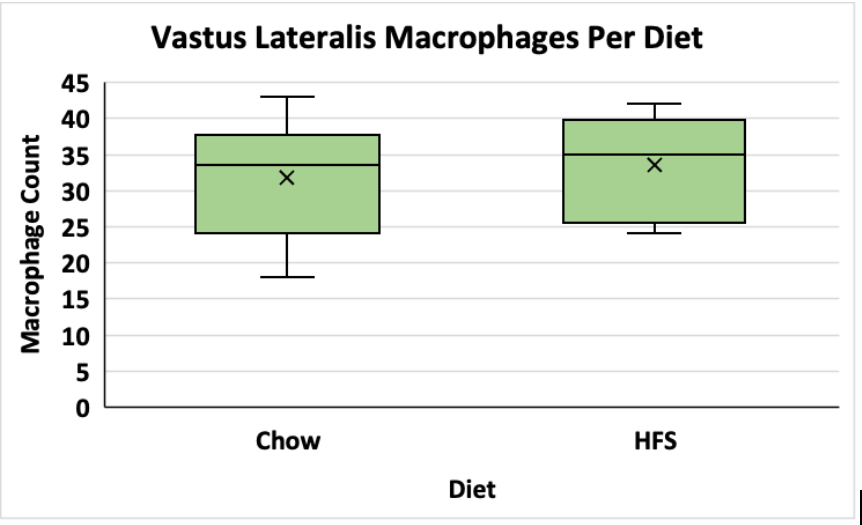
Figure 1. Comparison of Dietary Effect on Macrophage Count in the Vastus Lateralis of Female Sprague-Dawley Rats. This graph compares the difference between the chow-fed diet and the HFS diet on local VL muscle inflammation. Data is shown in a box and whisker plot where the x represents the mean, the line across the box represents the median, and the whiskers extending from the top of the box represent the upper quartile to the maximum value and the whiskers extending from the bottom represent the minimum value of the lower quartile.
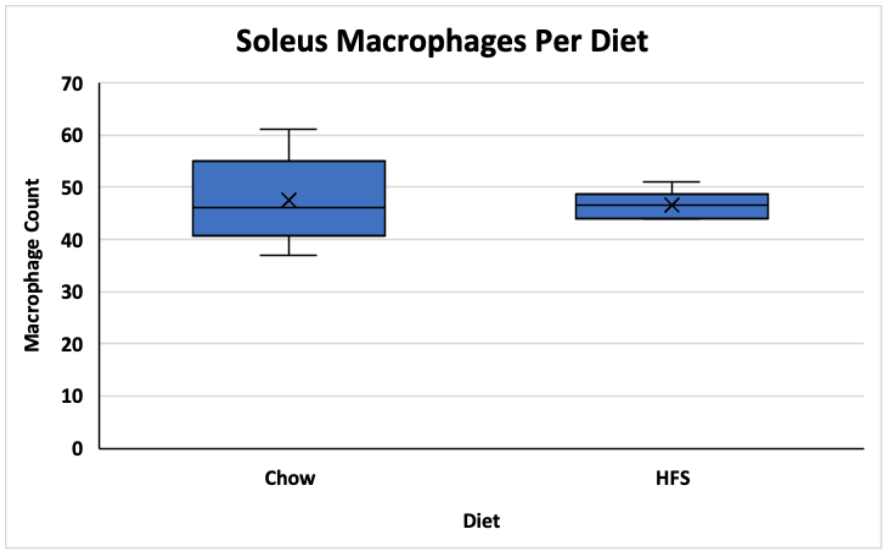
Figure 2. Comparison of Dietary Effect on Macrophage Count in the Soleus of Female Sprague-Dawley Rats. This graph compares the difference between the chow-fed diet and the HFS diet on local soleus muscle inflammation. Data is shown in a box and whisker plot where the x represents the mean, the line across the box represents the median, and the whiskers extending from the top of the box represent the upper quartile to the maximum value and the whiskers extending from the bottom represent the minimum value of the lower quartile.
Although there was no significant difference between the diet groups (HFS and chow-fed) in either muscle, there was a statistically significant difference between the two muscles (p < 0.05). The VL muscle had a much lower average macrophage count (VL total mean = 32.6, ±SD = 1.30) and therefore, a lower inflammatory response (Figure 3). The soleus muscle had a much higher average macrophage count (soleus total mean = 47.1, ±SD = 0.589) and therefore increased local inflammation (Figure 3).
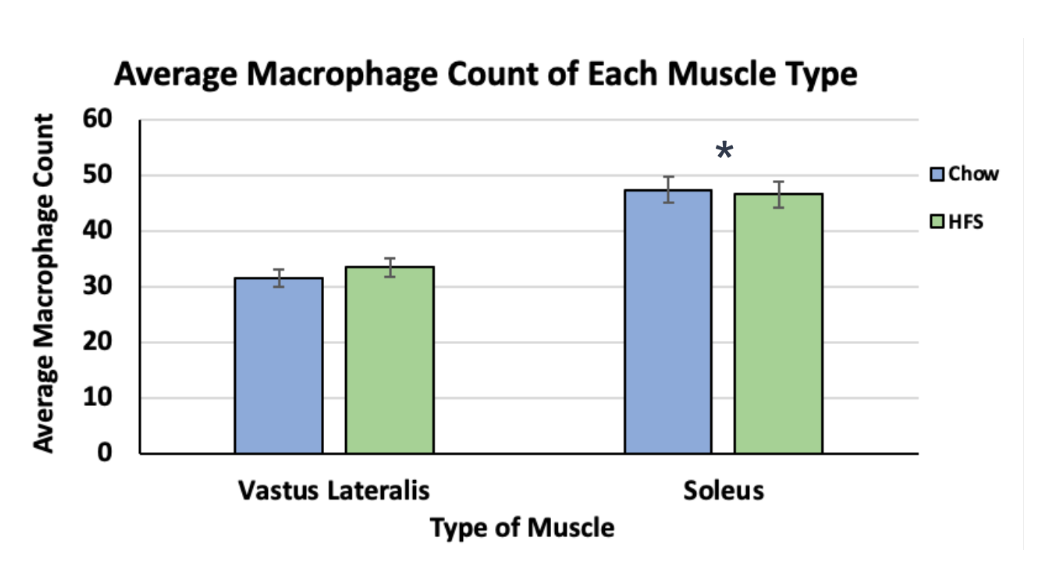
Figure 3. Comparison of Macrophage Counts between the Vastus Lateralis and Soleus Muscles of Female-Sprague Dawley Rats. This graph compares local inflammation in the two muscles. It also showcases diets' response as represented by the blue colour (chow-fed diet) and the green colour (HFS diet). The asterisk indicates a significance level of p < 0.05.
Conclusion
The first conclusion is that there is no significant difference between the chow-fed group and the HFS group in both the VL and soleus muscles. This supports my original hypothesis which was based on the lack of fibrosis seen in female Sprague-Dawley rats in a previous study. Since fibrosis is directly caused by inflammation, a lack of fibrosis supports the lack of local inflammation. One possible explanation for similar inflammation between the diet groups could be similar systemic MCP-1 levels. Review of the literature reveals one previous study that focused on systemic MCP-1 levels in female Sprague-Dawley rats and found no significant difference between the two diets (Smith et al., 2023). Since MCP-1 is the cytokine that recruits macrophages into the tissue, it makes sense that the macrophage counts will be similar between the two diets. The second observation is that there is a significantly higher inflammation found in the soleus muscle when compared to the VL muscle. There are two possible reasons for this higher inflammatory response. One reason could be a difference in muscle usage. Soleus muscles are more chronically stimulated muscles and the VL muscles are used in short outbursts. It is possible that the rats engaged in physical activity that required them to use their soleus muscles to a greater extent, which could explain the higher inflammatory response. Another reason could be an increased passive delivery of macrophages into the soleus through the blood supply. The soleus muscles have higher capillary density which means that they will have more blood pumped into them. Since blood contains and delivers macrophages into soft tissue, this supports the higher inflammatory response seen in the soleus muscle.
Citations
Collins, K. H., Hart, D. A., Smith, I. C., Issler, A. M., Reimer, R. A., Seerattan, R. A., Rios, J. L., & Herzog, W. (2017). Acute and chronic changes in rat soleus muscle after high-fat high-sucrose diet. Physiological Reports, 5(10), e13270. https://doi.org/10.14814/phy2.13270
Collins, K. H., Paul, H. A., Hart, D. A., Reimer, R. A., Smith, I. C., Rios, J. L., Seerattan, R. A., & Herzog, W. (2016). A High-Fat High-Sucrose Diet Rapidly Alters Muscle Integrity, Inflammation and Gut Microbiota in Male Rats. Scientific Reports, 6, 37278. https://doi.org/10.1038/srep37278
Smith, H., Gazaleh N.A., Seerattan R.A., & Herzog, W. (2023). Intramuscular fat infiltration, but not fibrosis, increases in rat vastus lateralis and soleus muscle in a diet-induced obesity model. Scientific Reports, 127(5). Unpublished manuscript.
Srikanthan, P., & Karlamangla, A. S. (2014). Muscle mass index as a predictor of longevity in older adults. The American Journal of Medicine, 127(6), 547–553. https://doi.org/10.1016/j.amjmed.2014.02.007
Stepien, D. M., Hwang, C., Marini, S., Pagani, C. A., Sorkin, M., Visser, N. D., Huber, A. K., Edwards, N. J., Loder, S. J., Vasquez, K., Aguilar, C. A., Kumar, R., Mascharak, S., Longaker, M. T., Li, J., & Levi, B. (2020). Tuning Macrophage Phenotype to Mitigate Skeletal Muscle Fibrosis. Journal of Immunology , 204(8), 2203–2215. https://doi.org/10.4049/jimmunol.1900814
Wu, H., & Ballantyne, C. M. (2017). Skeletal muscle inflammation and insulin resistance in obesity. The Journal of Clinical Investigation, 127(1), 43–54. https://doi.org/10.1172/JCI88880
Yao, J., Wu, D., & Qiu, Y. (2022). Adipose tissue macrophage in obesity-associated metabolic diseases. Frontiers in Immunology, 13, 977485. https://doi.org/10.3389/fimmu.2022.977485
Acknowledgement
I would like to thank Hannah Smith and Dr. Walter Herzog for their support, direction, and advice. I would also like to thank Dr. Garcia Diaz for her guidance and teaching.