Advancing Renewable Energy: The Combined Power of Saltwater and Food Waste
Grade 9
Presentation
No video provided
Problem
Throughout recent years, methane from food waste has been one of the gradually growing causes for the rise in temperature all around the globe—potentially leading to even climate change and other detrimental issues. With the production and handling of food waste, it creates a tremendous amount of carbon dioxide (CO2), and when food waste ends up in landfills, an extremely potent greenhouse gas that traps more than 100 times the amount of heat than CO2 can be generated—methane. Furthermore, another arising issue in the world that is harming our environment is the impact that desalination has on the ocean. Known as brine, desalination creates a salty byproduct that is highly concentrated, and is often disposed of back into the ocean, harming marine life, increasing salt levels and temperature, and threatening entire ecosystems. Although the harm being inflicted on the Earth is speeding up at an alarming rate, there are still many solutions to food waste and brine being disposed of in the environment and causing detriment, but rather than trying to prevent it—we should look at these as potential sources of renewable energy.
Rather than using pressure or membranes, the energy and conductive properties in brine and desalination can be utilized as batteries or fuel cells without using too much excessive energy input. Additonally, food waste can be used as well, with the citrus or food-based acids that are in all different kinds of fruits and vegetables. Utilizing food waste and salt water as renewable sources of energy is the purpose and foundation for this project, and will explore their combined power as well as the conductive properties of these two potential sources.
Method
Hypothesis & Objectives
Primary Objectives
1. To utilize food waste and salt water as potential sources of energy
2. To determine how well they work together (how the energy created is affected by adding food waste and saltwater together)
3. To discover what properties (from each of them) are needed to create the most energy
4. To demonstrate different ways to utilize them
Hypothesis
Due to the fact that different foods, as well as salt water, can act as electrolytes and have conductive properties, it is predicted that they will be a good source of energy when used together, and may be a potential source of renewable energy.
Research & Explanation
Invention of the First Battery
From the invention of the battery to today's modern technology, Luigi Galvani, in 1780, had shown that the legs of dead frogs hung on brass hooks would twitch when touched with a certain type of metal, believing that there was electrical fluid within the frogs’ tissues, and called it ‘animal electricity’. On the contrary, however, Alessandro Volta, an Italian scientist, is credited to have invented the battery that we use today, and had believed that the electricity came from the metals, and was transmitted through, not from, the frogs’ tissues. In the pivotal year of 1800, he created the first battery which consisted of copper or silver and zinc strips interspersed and separated with paper soaked in saltwater and diluted acid.
Basic Principle of the Battery
Known as an extremely beneficial and convenient device, a battery often consists of one or more electrochemical cells that stores and converts chemical energy into electricity, and is comprised of two electrodes: an anode (the positive electrode) and a cathode (the negative electrode), with an electrolyte between them. Produced by the flow or movement of electrons, electricity is a type of energy that can be harnessed from a battery for our everyday uses, while electrons are produced by a chemical reaction.
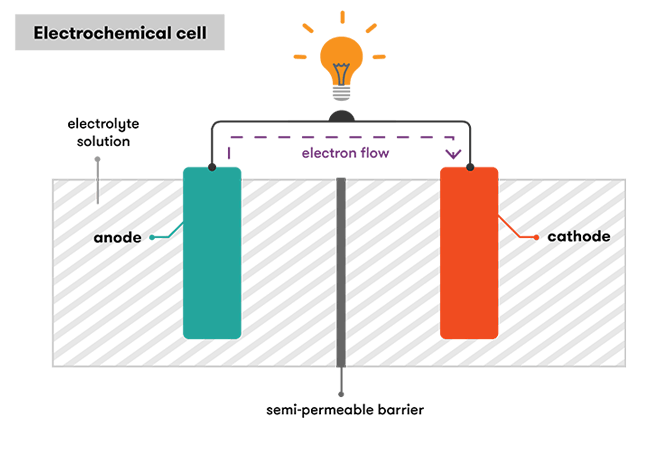
Figure 1: Diagram of a electrochemical cell or battery and its different parts. Source: https://www.science.org.au/curious/technology-future/batteries
Electrodes
When two different types of metal are immersed in the same electrolyte solution, the electrode at the anode reacts with the electrolyte and releases electrons, while another chemical reaction at the cathode occurs, enabling that electrode to accept and gain electrons. Typically called a redox reaction, the exchange of electrons involves reduction that occurs at the cathode, and oxidation at the anode. Depending on their electron affinity, the metal with low electron affinity would typically gain electrons from the negative ions of the electrolyte, and the higher one would release electrons and be added to the positive ions, with electrons being attracted to the cathode, allowing us to harness their energy through a conducting wire. With these differences and reactions, the overall electrochemical potential determines the cell's voltage, and the greater the difference between the two electrodes, the greater the electrochemical potential, leading to higher voltage. Selecting different materials for the elctrodes, some potential solutions to increase the voltage also includes stacking multiple cells together in a series for the electrons to move from the electrodes of each cell that may be added. However, combining the cells in parallel can add to the battery's current, which is the flow or movement of electrons, but, unfortunately, not its voltage.
Electrolyte
In the first yet remarkable battery created by Volta, the electrolyte was the pieces of paper soaked in salwater, but it can be a liquid or a solid, with the ability to allow the movement or flow of charged ions. Having a negative charge, the movement of electrons, with a negative charge, can be balanced by the electrolyte, where positive ions can flow. While electrons are produced, a matching amount of positively charged ions are also created on the anode, and are released into the electrolyte. When this occurs, the cathode, with no or very few electrons, also pulls in these ions from the electrolyte, and can produce negative ions.
Combination of Saltwater & Food
After being mixed in with brine or saltwater, fruits or food-based acids can conduct electricity better when high in salt content, helping it generate more energy. With less cost, more availability, non-flammability, and high conductivity, saltwater—along with these acids—may help with advancing renewable energy by offering alternative sources to use, even as a battery electrolyte. In the invention of the very first battery, Volta used paper dipped in both saltwater and acid, proving that these can be utilized.
Saltwater Electrolyte
Typically, the most common electrolytes are acids, bases, and salts due to the fact that they ionize in solvents such as water. Several salts that we use in cooking or our food, such as sodium chloride, which is also known as table salt, behave as electrolytes when dissolved in water. However, although it is used as a solvent, pure water will not act as an electrolyte. When salt is dissolved or added to water, the sodium and chloride ions separate temporarily and float freely, with the sodium atom becoming a positively charged ion by losing electrons and the chloride will become a negatively charged ion by gaining electrons. In spite of the fact that an ion has an electrical charge, it can carry or conduct this energy or electrical current through the water, which results in saltwater being an extremely good conductor and electrolyte for a battery.
Food & Fruit Electrolyte
Although there are several different foods from our food waste or compost that can be electrolytes, typically food or citrus fruits with high food-based acids would be the most ideal—more specifically, the acidity of them—and with stronger acids, the battery would also be stronger. When any type of metal is in direct contact with acid, it releases electrons, and the electrons are able to easily flow through the battery, with the acid acting as the conductor or electrolyte inside a battery. Typically due to salts, these foods have the right amount of electrolytes and types of acids to produce energy, and can be set up in a chain of multiple cells to create more energy as a circut inside a battery. Reacting with the acids, the anode produces ions into the food or fruit electrolyte and releases electrons that remain on the electrode, eventually flowing into the connecting wire and to the cathode. Furthermore, the electrons now on the cathode react with hydrogen ions in the acid or electrolyte to create hydrogen atoms. More importantly, using these as alternative sources of energy or electrolytes is organic and environment friendly, and can be used in places where it may be difficult to aquire electricity.
Observations & Data
Using a multimeter to accurately measure the voltage, without any special treatment, the saltwater battery had created around 0.73 volts, with 30 grams of salt dissolved in 300 milliliters of water. After increasing the salinity and concentration, it had shown that 0.80 volts was generated, using around an additional 10 grams of salt.
Using both food waste and juices from the compost as well as saltwater, it had created around 0.80 volts, and when connected in series, it had generated around 1.58 volts. Furthermore, a potato battery had created around 0.90 volts, while a lemon also created around 0.90 volts.
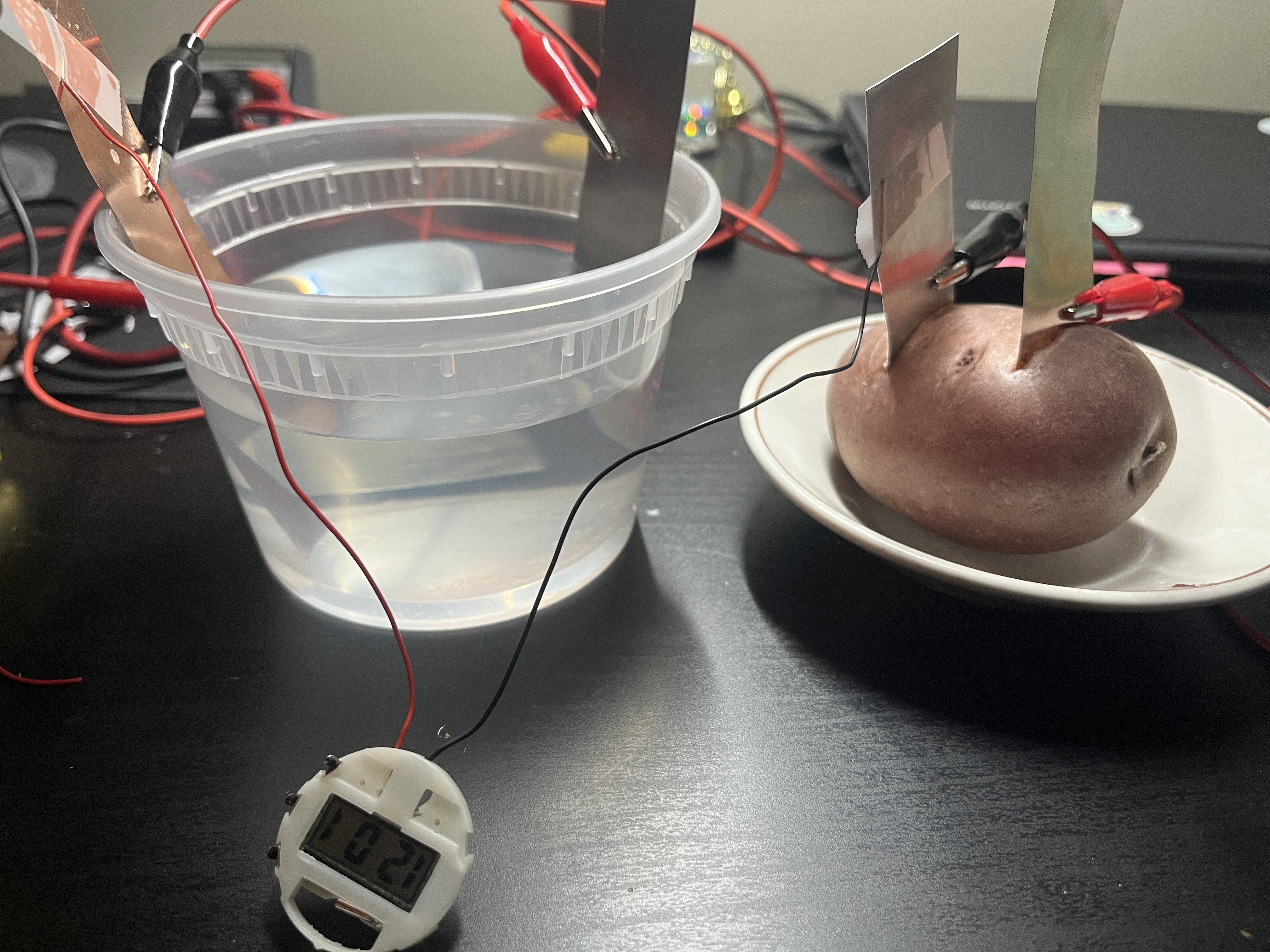
Figure 2: Image of a saltwater battery connected to a potato battery in series to power a small LED clock/watch.
Analysis
Expansion & Analysis of Project
To continue and expand my project, I would like to find and test or study different methods to increase and extend their longevity, as well as different methods to extract and construct the saltwater and food waste needed into easily used and more convenient batteries without using too much energy input to manufacture them. Furthermore, I would like to find more uses for these two sources, and eventually find ways to use them to power bigger and more complicated appliances.
Application of Project
With the several advantages that saltwater and acids from different food waste have, these alternative sources of energy would be able to create the most environmentally friendly battery, due to the fact that they do not create any toxic chemicals or harmful substances, and are compostable, leading to them being safer to transport. Even more remarkable, they can be left unused for long periods of time without causing harm. In today's modern world, the most commonly used batteries made with lithium, which is extremely detrimental for the environment. Although lithium batteries may help with storing energy and acting as a stable power supply as we begin to transition into cleaner energy solutions, they contain flammable and toxic electrolytes that can cause the battery to catch fire—whereas saltwater is fire resistant—or even explode if they are damaged or exposed to high temperatures, releasing toxic fumes that are damaging to both humans and nature around us. With the extraction of lithuim being immensely harmful to the environment, lithium mining can destroy thousands of habitats and wildlife, and chemicals used in other methods can leak into the soil and ground, which may cause irreversible detriment. Requiring around 2.2 million liters of water, lithium extraction has been known to cause extreme water shortages, and even contamination, releasing toxic chemicals in the air, ground, and water.
According to a study from Virginia Tech researchers, food waste has a significant fiber component that can be used to create carbon materials for a battery anode and other materials needed for a battery, which is more cost-effective and environment-friendly. By utilizing this waste, it also helps decrease its significant greenhouse gas footprint that is currently contributing to the climate change crisis. In our massive agriculture industry, this can also help with the utilization of agricultural waste and the money that food producers to lose due to food waste. All across the globe to today's arising environmental issues, food waste is often sent to landfills, which can generate methane as they decompose. Furthermore, there is a tremendous abundance of food waste, whereas graphite or other metals used to make battery anodes are limited resources. Although food waste is not being used as batteries in today's world yet, more research and testing may help it eventually become a common material in batteries, and harness the energy we need for our everyday uses.
Without using pressure, membranes or too much energy input, utilizing saltwater—an awaiting renewable power source—in batteries could capture an immense amount of energy that we may eventually be able to use daily, especially since saltwater is less costly, fire-resistant and conductive. Considering that the process of desalination produces tremendous amounts of brine, saltwater and useful chemicals may be extracted from it, rather than diposing it back into the ocean and harming marine life, and could potentially be used to power the desalination plant someday. With a small carbon footprint and more simplicity, there is no need for backup batteries or constant maintaining, due to the fact that the electrodes can be prevented from corrosion and has no moving parts. Although using saltwater may not solve many of our problems and climate change at the global scale, it continues to hold much potential for eventually being used for our common electrical appliances and could later lead to many more advances.
Alternative Methods
Using different alternative methods to generate energy from these sources and materials, today's modern scientists and researchers from countries all over the world are continuously trying to find different and better ways to utilize potential sources, such as saltwater and food waste.
With millions of tons of food waste left in landfills, they could cause immense detriment to the environment, and can generate large amounts of methane, especially when these wastes are not managed properly. However, there are some other alternative methods, aside from utilizing it in a battery, by using a process known as anaerobic digestion. After organic materials, such as plant and animal products, are broken down, anaerobic digestion can be used to turn these materials into biogas—energy that can be created from organic waste—and can be controlled and contained using an anaerobic digester. When biogas is captured and harnessed, it can generate heat and electricity.
Through osmotic energy, some technologies use a semi-permeable membrane that lets water through and captures salts, with freshwater on one side and seawater on the other side. After the freshwater is attracted by the salt and moves to the saltwater with the process of osmosis occuring, a turbine, driven by the water's movement, can generate power, although a massive amount of osmotic membrane would be required to gain enough energy from salt water.
According to a study by a group of Standford researchers, each meter of freshwater that combines with seawater generates approximately 0.65 kilowatt-hours of energy, and uses a process where it releases ions from the electrodes into the solution, with energy being recovered or saved during a freshwater and seawater flush, meaning that the battery can discharge and recharge—with no extra energy input. Rather than using membranes or pressure, this method is the first to use battery electrochemistry.
Conclusion
Amidst the modern world of today and the approaching future of renewable energy, utilizing this combination of potential sources of saltwater or brine and food waste could ultimately benefit the environment and provide true insight into our rapidly changing world of climate change and global warming. From the harmful byproduct of desalination to the immensely detrimental methane generated from waste when left abandoned in landfills, we may soon unearth new ways to use these in our everyday essentials, and advance toward a cleaner and thriving environment, powering not just mere appliances, but also the world. As our future swiftly arrives, we may find ourselves needing to take action, and although we have only taken a small step with these batteries, it is the beginning to a new generation of eco-friendly practices.
Citations
Moseman, A. (2024, January 4). Why do we compare methane to carbon dioxide over a 100-year timeframe? Are we underrating the importance of methane emissions?. MIT Climate Portal. https://climate.mit.edu/ask-mit/why-do-we-compare-methane-carbon-dioxide-over-100-year-timeframe-are-we-underrating
Simon, M. (2019, January 14). Desalination Is Booming. But What About All That Toxic Brine?. Wired. https://www.wired.com/story/desalination-is-booming-but-what-about-all-that-toxic-brine/
Bhatt, A. Forsyth, M. Withers, R. Wang, G. (2016, February 16). How a battery works. Australian Academy of Science. https://www.science.org.au/curious/technology-future/batteries
Fantozzi, J. (2018, May 16). Why Do Some Fruits and Vegetables Conduct Electricit?. Live Science. https://www.livescience.com/62570-potato-battery-conduct-electricity.html
(2023, July 10). Why do lithium-ion batteries catch fire?. Fire Protection Association. https://www.thefpa.co.uk/advice-and-guidance/advice-and-guidance-articles/why-do-lithium-ion-batteries-catch-fire-
Anderson, K. (2024, July 23). The Harmful Effects of our Lithium Batteries. Greenly Earth. https://greenly.earth/en-us/blog/industries/the-harmful-effects-of-our-lithium-batteries
(2021, September 13). Food Waste Could Be Converted Into Rechargeable Batteries. Technology Networks. https://www.technologynetworks.com/applied-sciences/news/food-waste-could-be-converted-into-rechargeable-batteries-353567
(2017, October 3). Biogas: Converting Waste to Energy. Environmental and Energy Study Institute. https://www.eesi.org/papers/view/fact-sheet-biogasconverting-waste-to-energy
(2021, January 29). Turning Food Waste into Energy to Power Homes. Biogas World. https://biogasworld.com/news/turning-food-waste-into-energy-to-power-homes/
Ye, M. Pasta, M. Xie, X. Dubrawski, K. Xu, J. Liu, C. Cui, Y. Criddle, C. (2019, July 8). Charge-Free Mixing Entropy Battery Enabled by Low-Cost Electrode Materials. ACS Publications. https://pubs.acs.org/doi/10.1021/acsomega.9b00863
(2022, November 25). Salt Water, Driving Osmotic Energy. Planete Energies. https://www.planete-energies.com/en/media/article/salt-water-driving-osmotic-energy
Chu, J. (2014, August 20). The power of salt. MIT News. https://news.mit.edu/2014/energy-from-salt-water-0820
Acknowledgement
With the utmost appreciation, I would like to express my deepest gratitude to the many people who have supported me throughout this project. Even when I had my own doubts, they encouraged me to keep going and to try my hardest. Forever caring and always supportive, my dad has created such a postive and motivating environment for me, and I would like to thank him for helping me push through this project and ensuring that I could get the materials I need. I would also like to thank my science teacher for helping me with any questions that I asked him, as well as coordinating and organizing our science fair club. Uplifting and inspirational, my friends, classmates, teachers, family and anyone else who had constantly supported and motivated me are truly the best people I could have in my life, and I would also like to thank them as well as anyone who had helped me with this project in any way. Thank you so much!

