AyurMed: Bridging Ancient Ayurvedic Herbal Wisdom with Modern Science to Innovate Chronic Disease Management and Enhance Patient Well-Being.
Grade 10
Presentation
No video provided
Problem
1. Introduction
Chronic diseases are among the leading causes of morbidity and mortality worldwide. Their management often necessitates long-term pharmaceutical treatments, which, while effective, frequently induce adverse drug reactions (ADRs). These unintended effects compromise patient well-being, leading to additional prescriptions to counteract adverse drug reactions (ADRs), ultimately resulting in polypharmacy. This dependency on multiple medications creates a cycle that not only reduces treatment efficiency but also significantly impacts overall health and quality of life. Addressing this issue requires a paradigm shift toward safer, non-pharmacological alternatives that can mitigate adverse drug reactions (ADRs) without introducing further complications or adverse drug reactions (ADRs) of their own.
Ayurveda, an ancient Indian medicinal system, presents a promising approach through the bioactive compounds present in its natural herbs. Unlike synthetic drugs, Ayurvedic formulations work synergistically with the body's natural processes, potentially reducing adverse drug reactions (ADRs) and breaking the cycle of medication dependency by not introducing any of its own adverse drug reactions (ADRs). Despite its historical success in disease management, Ayurveda remains underutilized in modern medicine due to a lack of scientific validation. Establishing a rigorous, evidence-based framework for evaluating Ayurvedic herbs is crucial for their integration into contemporary healthcare systems.
This study aims to bridge the gap by employing molecular docking simulations and Lipinski’s Rule of Five to assess the efficacy and drug-likeness of Ayurvedic herbs through the analysis of bioactive compounds present within them. By comparing their binding affinity and oral bioavailability to conventional drugs, this research provides a systematic approach to determining their potential as viable alternatives to modern medicine for adverse drug reactions (ADRs) management.
The subsequent sections of this paper outline the burden of chronic diseases, the mechanisms underlying ADRs, and the advantages of Ayurveda, followed by a structured methodology detailing computational analysis techniques used to evaluate herbal compounds, and lastly insight into the structure of further experiments.
1.1. The Burden of Chronic Diseases
Chronic diseases are defined broadly as conditions that last one year or more and require ongoing medical attention or limit activities of daily living or both. These include cardiovascular diseases, chronic respiratory diseases, cancers, and diabetes, all of which are leading cause of illness, disability, and death around the world. As of 2021, an average across 24 Organisation for Economic Co-operation and Development countries, more than one-third of adults reported living with a chronic disease. In 2021, 45% of Canadians aged 12 and older reported having at least one major chronic disease. Approximately 12.9% of Canadians report having two or more chronic diseases, and 8.33% report having three or more. If you were to look at any group of 10 people in Canada, about 5 of them would be living with at least one major chronic condition, highlighting that chronic diseases are a widespread issue affecting a large portion of the population. These statistics aim to show the significant prevalence of chronic conditions in Canada and beyond.
1.1.1. Adverse Drug Reactions in Chronic Disease Treatment
The long-term nature of these treatment options for chronic conditions are leading to a degrading, as well as diminishing quality of life for the patients, due to the side effects, also known as Adverse Drug Reaction, that these treatments have. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, which both WHO and FDA were a part of, defined adverse drug reaction as “response to a drug which is unintended and occurs at doses normally used for treatment of a disease”. Over the years, as the prevalence of chronic conditions is growing both globally and in Canada, the adverse drug reactions of the treatment options for chronic diseases are proving to be a serious problem. Recently, in 2022, there were over 1.25 million cases of adverse drug reactions reported, alongside nearly 175 000 deaths that occurred due to these adverse drug reactions. This statistic highlights the relative significance of Adverse Drug Reactions (ADRs) in healthcare, illustrating that Adverse Drug Reactions (ADRs) are a major concern affecting millions of people annually, with a substantial impact on patient safety and public health, emphasizing that Adverse Drug Reactions (ADRs) are not just isolated events, but a pervasive issue that is deeply integrated into the healthcare system, affecting a large portion of the population.
1.1.2. The Vicious Cycle of Medication Dependency and Polypharmacy
A major consequence of adverse drug reactions is the widespread practice of prescribing additional medications to counteract the adverse drug reactions of primary treatments of chronic disease. This approach, although intended to improve patient outcomes, often leads to a phenomenon known as polypharmacy. Polypharmacy is defined as the simultaneous use of multiple medications, often five or more, to manage various health conditions or counteract treatment-induced adverse drug reactions. Using conventional medication to manage adverse drug reactions of chronic diseases treatment leads to a need for more medication to manage the adverse drug reactions of the medication already being used to manage the adverse drug reactions of chronic disease treatment, forming a cycle, since each medication has its own set of adverse drug reactions. Below is a diagram attached, which helps visualize this process:
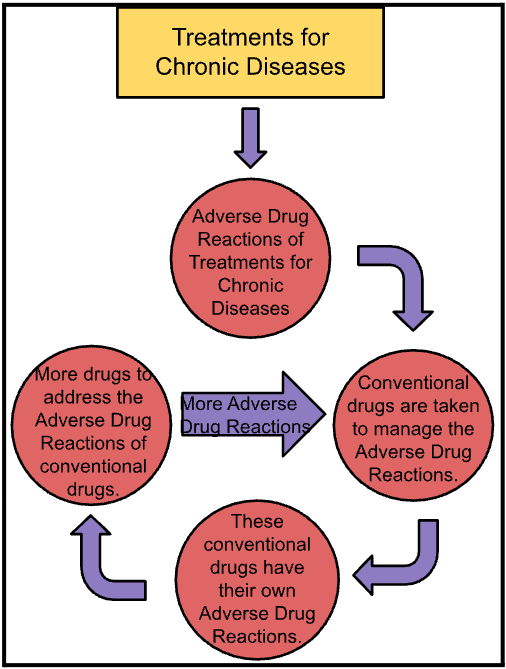
There are often multiple adverse drug reactions that have resulted from the treatment of chronic diseases, resulting in a need for multiple drugs to manage the adverse drug reactions of chronic disease treatment. This continuous dependency on multiple medications can lead to overmedication, which causes additional health problems like dependency of certain drugs, reduced cognitive function, organ damage, and further/continuous adverse drug reactions that has resulted from this long-term cycle of medication use. Additionally, the psychological impact of this cycle of medication cannot be overlooked. For patients, constantly dealing with adverse drug reactions from various drugs and the need for additional medication can be psychologically draining leading to feelings of frustration, helplessness, anxiety, and depression.
In conclusion, non-pharmacological approaches must be considered to manage the Adverse Drug Reactions of chronic disease treatments, instead of conventional drugs, which will manage the adverse drug reactions effectively of treatments for chronic diseases, with no further adverse drug reactions of its own, breaking the cycle, significantly improving the treatment outcome and overall quality of life for the patient.
1.1.3. Understanding Why Conventional Medicines Cause Adverse Drug Reactions
Adverse drug reactions occur primarily due to what is known as off-target effects. Off-target effects refer to situations in which a drug designed to interact with a specific biological target, such as a receptor or enzyme, also affects unintended areas of the body. Since the human body consists of complex, interdependent systems, it is difficult to develop pharmaceutical drugs that exclusively target a single pathway without interfering with other physiological processes. This leads to adverse drug reactions as the drug interacts with other organs or systems that weren't initially targeted. Below is a diagram attached to help visualize this concept:
1.2. The Ayurvedic Approach to Medicine
Ayurveda is a traditional system of medicine that originated in India over 5,000 years ago. The term "Ayurveda" comes from two Sanskrit words: "Ayur" meaning "life" and "Veda" meaning "knowledge" or "science," so it translates to "the science of life." Ayurveda places significant emphasis on the use of herbs for healing and maintaining health. These herbs are carefully selected for their medicinal properties, and they are used to treat a wide range of conditions, from digestive issues to skin disorders, respiratory problems, and more. Ayurvedic herbs are often considered safe and natural alternatives to conventional medicines, and they are typically consumed in various forms such as powders, teas, oils, and pastes.
Some of the most commonly used herbs in Ayurveda include Ashwagandha, known for its ability to reduce stress and enhance vitality; Tulsi (Holy Basil), which is revered for its antimicrobial and anti-inflammatory properties; Turmeric, celebrated for its powerful anti-inflammatory effects and its role in detoxification; and Triphala, a blend of three fruits used to support digestion and detoxify the body. These herbs are valued not only for their physical healing properties but also for their ability to promote overall well-being by supporting the body's natural functions.
The practice of using Ayurvedic herbs is deeply rooted in understanding the specific benefits of each herb and how it can support the body in addressing and even preventing illness. Whether used individually or in combination, these herbs are a cornerstone of Ayurveda’s approach to health and wellness.
1.2.1. How Ayurveda Differs from Modern Medicine
A major advantage of Ayurvedic herbal treatments is that they usually don't cause adverse drug reactions (ADRs), which are negative side effects often seen with modern synthetic medications. This is because Ayurvedic herbs tend to be gentler and less chemically intense than many pharmaceutical drugs, primarily because of how they interact with the body. Unlike conventional pharmaceutical drugs, Ayurvedic herbs are typically not isolated to a single active ingredient; instead, they come from whole plants or parts of plants, which contain a variety of naturally occurring bioactive compounds. A bioactive compound is a compound that has an effect on a living organism, tissue or cell. These bioactive compounds work together in a process known as synergy, where the combined effects of these natural substances enhance therapeutic outcomes while minimizing the likelihood of adverse reactions. In contrast, many pharmaceutical drugs are made by isolating one active compound, which can have a stronger and more concentrated effect on the body, often leading to more pronounced adverse drug reactions.
For instance, Turmeric contains bioactive compounds like curcumin and turmerones, both of which work together to enhance its therapeutic effects. Curcumin is the most studied compound in turmeric and is known for its strong anti-inflammatory and antioxidant properties. However, curcumin has low bioavailability, meaning the body doesn't absorb it very efficiently when consumed alone. This is where turmerones come into play. Turmerones, which are a group of volatile oils found in turmeric, have been shown to enhance the bioavailability of curcumin by increasing curcumin’s gut permeability, allowing more curcumin to pass into the bloodstream and reach its target areas more effectively, further supporting the body's ability to absorb and utilize curcumin more effectively. Together, curcumin and turmerones work synergistically: curcumin provides the powerful anti-inflammatory and antioxidant effects, while turmerones help improve the absorption of curcumin, ensuring that it reaches its full potential in the body. This balance between the two compounds ensures that the body benefits from turmeric’s healing properties in a way that is more gradual and less overwhelming than if a single compound, like isolated curcumin, were used in high doses.
In contrast, many pharmaceutical drugs focus on a single active compound, such as the synthetic anti-inflammatory drug ibuprofen. While ibuprofen effectively reduces inflammation, it can also cause significant side effects, like stomach irritation or even ulcers, especially when used over long periods. This is because ibuprofen is a concentrated, isolated compound that works intensively to block specific pathways in the body, without the moderating influence of other natural compounds that might help balance its effects. In essence, Ayurvedic herbs are considered safer with no side effects compared to conventional treatments.
1.2.2. The Need for Scientific Validation of Ayurveda
Studying Ayurvedic herbs scientifically is essential, particularly when exploring their potential to offer more efficient and safer alternatives to conventional drugs, especially in managing the adverse drug reactions (ADRs) that often accompany long-term treatments for chronic diseases. Conventional pharmaceutical drugs, while effective at treating specific symptoms or conditions, can lead to significant adverse drug reactions. Ayurvedic herbs, on the other hand, are generally considered gentler and work holistically to support the body's natural processes, which helps in addressing these harmful adverse drug reactions without further adverse drug reactions. While the effectiveness of Ayurvedic herbs has been traditionally recognized for centuries, scientific research into their true potential is still underdeveloped. By studying these herbs more deeply, we can uncover how they interact with the body and compare their efficiency to modern pharmaceutical drugs.
1.3. The Research Gap in Ayurveda
A critical issue in Ayurvedic research is the over-reliance on ancient texts, which has stalled innovation and the creation of scientific evidence. Many practitioners assume the knowledge in classical Ayurvedic scriptures is complete, which discourages the validation of traditional remedies or the exploration of new treatment approaches. This reliance has led to limited efforts to scientifically evaluate the mechanisms, safety, and effectiveness of Ayurvedic treatments. For example, multicomponent herbal formulations are often used without isolating their active compounds or studying how these compounds work together. Without rigorous clinical trials or preclinical studies to evaluate their potency, safety, and efficiency, these formulations remain unverified, which restricts their acceptance in modern healthcare. This lack of evidence-based research creates a significant gap, preventing Ayurveda from being fully integrated into contemporary medical systems.
In conclusion, the research gap in Ayurveda arises from the insufficient use of systematic, scientific methods to validate its treatments. While Ayurveda relies on centuries-old traditional knowledge, there has been little effort to isolate bioactive compounds from herbal formulations, understand their mechanisms of action at a molecular level, or evaluate their therapeutic potential through rigorous pre-clinical studies and clinical trials. This lack of empirical evidence has limited the acceptance of Ayurvedic treatments in modern medicine, as their safety, and efficacy remain largely unverified. To bridge this gap, Ayurveda must evolve beyond its reliance on tradition and embrace modern scientific methodologies. This includes isolating and characterizing active compounds in Ayurvedic formulations to identify their specific therapeutic effects, understanding their mechanisms of action through molecular and biochemical studies, and rigorously testing their safety and efficacy through structured pre-clinical research and well-designed clinical trials. By adopting these approaches, Ayurveda can generate credible, evidence-based solutions that not only validate its traditional practices but also align them with the standards of contemporary medicine. This will enable Ayurveda to remain relevant, gain wider acceptance, and integrate effectively into global healthcare systems.
1.3.1. How This Study Fills the Gap
This study helps fill the gap in Ayurvedic research by addressing the lack of scientific evidence through a modern, systematic approach that combines traditional knowledge with advanced research tools. It focuses on scientifically validating the use of Ayurvedic herbs as safer alternatives to conventional drugs for managing adverse drug reactions of treatment for chronic diseases. By utilizing molecular docking and Lipinski’s Rule of Five, this research provides a strong framework for evaluating the safety, effectiveness, and feasibility of Ayurvedic herbs as viable oral drug candidates.
One of the main challenges in Ayurvedic research is the reliance on ancient texts without scientific validation of their claims. This study addresses this issue by adopting a scientific, component-based methodology. It identifies bioactive compounds in Ayurvedic herbs and evaluates their therapeutic potential using molecular docking. This computer-based method predicts how well compounds bind to specific proteins in the body, offering insights into how effectively they might address a problem. By comparing the docking scores (a measure of binding strength) of Ayurvedic compounds with those of conventional drugs, this study establishes clear benchmarks for Ayurvedic herbs’ effectiveness, adding scientific credibility to Ayurvedic practices.
Furthermore, to ensure these herbs can be used as oral medications, this research applies Lipinski’s Rule of Five, a widely accepted checklist in drug development. This rule evaluates whether a compound has the right size, structure, and properties to be absorbed by the body when taken by mouth. By combining molecular docking with this oral drug evaluation, this study provides a comprehensive scientific basis for assessing Ayurvedic herbs, ensuring they meet modern drug development standards.
Lastly, this research directly compares Ayurvedic herbs with conventional drugs for specific and the most common adverse drug reactions of treatment for chronic diseases. It evaluates how well each works and whether Ayurvedic herbs can offer the same or better results without harmful side effects. By providing clear, evidence-based conclusions, this study provides much-needed scientific evidence, bridging the gap between traditional Ayurvedic knowledge and contemporary science. By validating the therapeutic potential of Ayurvedic herbs through modern research methods like molecular docking and oral drug evaluation, it establishes a strong foundation for integrating Ayurveda with current medical practices. This approach not only highlights Ayurveda's potential to complement modern medicine but also paves the way for further research and development, ensuring its relevance in addressing today’s healthcare challenges.
1.3.2. Significance for Doctors, Scientists, and Patients
This research is valuable for doctors, scientists, and patients because it bridges the gap between traditional Ayurvedic knowledge and modern medical practices, offering scientifically validated insights into the potential of Ayurvedic herbs as safer alternatives to conventional drugs for managing adverse drug reactions of treatments for chronic diseases.
For doctors, the findings provide evidence-based options for incorporating Ayurvedic herbs into treatment regimens, especially for patients who experience adverse drug reactions from chronic disease treatments. This can enhance patient care by improving treatment outcomes, as Ayurvedic herbs are often less likely to cause harmful effects compared to synthetic drugs, leading to improved patient satisfaction, better quality of life, and better overall physical as well as psychological health.
From a scientific perspective, this research presents a systematic, replicable framework for evaluating Ayurvedic herbs. By employing advanced tools like molecular docking and Lipinski’s Rule of Five, the study assesses the binding potential and oral bioavailability of bioactive compounds in Ayurvedic herbs. Molecular docking provides detailed insights into how these compounds interact with target proteins in the body, while Lipinski's Rule of Five ensures that the selected compounds meet the necessary criteria for oral drug development. This rigorous methodology not only validates Ayurvedic herbs but also offers a model for conducting preclinical studies and establishing a scientific basis for clinical trials and further exploration. As a result, it opens new doors for innovation in herbal medicine, contributing to drug discovery and development, and expanding the scope of natural medicine in global healthcare.
From the patient's perspective, the research holds significant promise. Patients experiencing Adverse Drug Reactions from long-term treatment for chronic diseases, may benefit from safer, more natural alternatives to conventional drugs with no side effects. The scientific validation of these herbs through molecular docking and bioavailability assessments ensures that patients can trust these alternatives, knowing that they have been rigorously tested for their therapeutic potential.
In summary, this research benefits doctors by providing evidence-based tools to incorporate Ayurvedic herbs into patient care, offers scientists a robust framework for evaluating and advancing herbal medicine, and provides patients with safer, effective treatment alternatives to treat the Adverse Drug Reactions of treatments for chronic diseases, breaking the cycle of medication. By validating the therapeutic potential of Ayurvedic herbs through modern scientific methods, this research contributes to the broader goal of integrating Ayurveda into contemporary medical practices, ensuring that patients worldwide have access to a broader range of safe, effective, and scientifically backed treatment options. Ultimately, the study establishes a foundation for further clinical trials and research, enabling Ayurveda to complement modern medicine in a scientifically validated, patient-centered way.
1.4. Research Question
Through this research, I aim to explore whether Ayurvedic herbs can effectively break the cycle of medication dependency caused by the adverse drug reactions (ADRs) of chronic disease treatments, by being a safer alternative to conventional drugs, as evaluated through molecular docking and drug-likeness assessments using Lipinski’s Rule of Five?
1.4.1. Expected Outcomes Based on Background Information Collected Through Literature Review
One of Ayurveda’s most compelling advantages is its emphasis on safety and natural remedies, especially in addressing adverse drug reactions (ADRs) caused by chronic disease treatments. Ayurvedic therapies are derived from natural sources like herbs, roots, and minerals that have been used for centuries with minimal reported side effects. In contrast, synthetic drugs commonly used in modern medicine often lead to ADRs ranging from mild discomfort to severe organ damage. These adverse drug reactions can trap patients in a cycle of medication dependency, where additional drugs are required to manage the adverse effects of initial treatments. Ayurvedic herbs, such as Withania somnifera (Ashwagandha) and Curcuma longa (Turmeric), offer an alternative by supporting the body’s natural healing processes and modulating physiological pathways. This allows them to deliver therapeutic effects without the toxicity frequently associated with conventional pharmaceuticals.
The rich phytochemical composition of Ayurvedic herbs further highlights their potential to address adverse drug reactions. These plants are abundant in bioactive compounds such as flavonoids, alkaloids, terpenoids, and polyphenols, which possess anti-inflammatory, immunomodulatory, antioxidant, and antimicrobial properties. For instance, Tinospora cordifolia (Giloy) is traditionally used to strengthen immunity and alleviate inflammation, which can help mitigate the immunosuppressive effects of certain chronic disease treatments. Similarly, Boswellia serrata (Indian frankincense) is effective in reducing joint pain and swelling, common adverse drug reactions of long-term steroid or immunosuppressive therapies. These properties make Ayurvedic herbs uniquely suited to breaking the cycle of medication dependency.
The growing body of scientific research further supports Ayurveda’s potential to transform healthcare by offering safer alternatives. By focusing on natural compounds that work synergistically with the body’s physiological systems, Ayurveda aligns with the increasing demand for integrative approaches to health. The potential of this traditional system’s ability to mitigate adverse drug reactions positions it as a powerful tool for improving the quality of life for patients managing chronic diseases, through reducing their reliance on additional medications.
Method
2. Methods
This research investigates Ayurvedic herbs as potential alternatives to manage adverse drug reactions (ADRs) caused by conventional treatments for chronic diseases. The study began with an extensive literature review to identify common chronic diseases, their treatments, associated adverse drug reactions (ADRs), and secondary medications used to manage those adverse drug reactions (ADRs) was compiled. Ayurvedic herbs traditionally known to alleviate these adverse drug reactions (ADRs) were identified, with a focus on their bioactive compounds, and mechanisms of action. Bioactive compounds were analyzed through databases like TCMSP and Dr. Duke’s Phytochemical Database to understand their chemical composition and therapeutic potential. Next, target proteins involved in adverse drug reactions (ADRs) development were identified to establish molecular pathways for intervention. Ligand preparation followed, where 3D molecular structures of Ayurvedic compounds were retrieved and optimized through energy minimization to ensure stability. Molecular docking simulations using CB-Dock were then conducted to predict ligand-protein interactions and compare the binding efficiency of Ayurvedic herbs with conventional medications. Lastly, drug-likeness and oral bioavailability assessments of herbs were performed using Lipinski’s Rule of Five to predict absorption efficiency, given the study's limitations in conducting direct pharmacokinetic experiments. This comprehensive approach integrates traditional Ayurvedic principles with modern scientific techniques to explore safer alternatives for managing ADRs in chronic disease treatments.
2.1. Identification of Common Chronic Diseases and Their Treatment Options
As the initial step in the methodology of this research, an extensive and well-researched list of the most prevalent chronic diseases, ensuring a comprehensive selection that reflects the conditions with the highest global and clinical significance was systematically compiled. This process required extensive research into widely recognized chronic illnesses, considering factors such as their prevalence, long-term health consequences, and the necessity for continuous management. Each disease was examined in detail, focusing on its underlying causes, progression over time, and the ways it affects individuals' daily lives. By taking this approach, a broad yet precise selection of conditions that accurately represent the burden of chronic diseases was developed.
In addition to identifying these diseases, a thorough and structured documentation of the various treatment options available for each condition was conducted. This encompassed both pharmacological and non-pharmacological interventions, including widely prescribed medications, and surgical procedures. Apart from the effectiveness, these treatments’ specific role in disease management was also examined, to analyze whether they were focused on alleviating symptoms, slowing the progression of the disease, or preventing complications. By considering these aspects, I developed a well-rounded perspective on the diverse approaches to chronic disease treatment.
To expand on this further, a fourth, equally detailed list focusing on the common adverse drug reactions associated with these treatments for chronic diseases was created. Since every medical intervention carries potential risks, the unintended effects of medications, therapies, and procedures, were examined ensuring that both frequently reported and less common adverse reactions were included. This allowed for a balanced perspective on treatment approaches, acknowledging both their benefits and their drawbacks. By integrating this analysis, a clearer picture of the challenges involved in long-term disease management was provided, emphasizing the trade-offs between treatment efficacy and potential further health risks.
Through this structured approach, a strong foundation for the subsequent stages of the research was established, ensuring that the information gathered was not only comprehensive but also relevant to the broader discussion on the adverse drug reactions of chronic disease treatment, which diminish the patients’ quality of life.
2.1.1. Compilation of Adverse Drug Reactions (ADRs)
A comprehensive and structured approach was undertaken to compile a detailed list of adverse drug reactions (ADRs) commonly associated with conventional pharmaceutical treatments for chronic diseases. The goal of this step was to ensure that the most frequently reported and clinically significant adverse drug reactions (ADRs) were identified, and analyzed.
The selection process began with an extensive review of clinical pharmacology databases, drug safety reports, and peer-reviewed research articles. Reputable sources such as the U.S. Food and Drug Administration (FDA), Adverse Event Reporting System (FAERS), World Health Organization (WHO), and scientific journals focused on pharmacology and toxicology were referenced to obtain data on documented side effects. Additionally, healthcare guidelines from regulatory bodies and institutions specializing in drug safety were examined to verify the prevalence and clinical relevance of each adverse drug reaction (ADRs).
Each ADR was selected based on its frequency of occurrence, impact on patient quality of life, and relevance in clinical treatment settings. By systematically compiling and categorizing these reactions, a clear framework was established for investigating alternative treatment approaches.
2.1.2. Attached below is the table of all the information collected
|
Common Chronic Diseases |
Meaning |
Treatments |
Adverse drug reactions of these treatments |
|
Stroke |
Brain’s equivalent of a heart attack, happening when there’s an issue with blood flow to part of the brain. This can happen when blood vessels are blocked or because of bleeding in your brain. |
Thrombolytic Drugs: Dissolve blood clots that are blocking blood flow to the brain, effectively restoring circulation and limiting potential brain damage by acting quickly to break up the clot and re-establish blood supply to the affected area. Thrombectomy Procedure: Inserting a catheter (tube-like) device into a major blood vessel and steering it up to the clot in your brain. Once there, the catheter has a tool at its tip that can remove the clot. |
Bleeding in the nose, stool or urine. Bleeding or bruising at the site of IV or catheter insertion. Kidney damage, especially if you have diabetes. Low blood pressure (hypotension). Movement of the blood clot to another part of your body. Swollen tissue (angioedema). Ventricular arrhythmia: Abnormal heart rhythms that make the lower chambers of your heart twitch instead of pump. This can limit or stop your heart from supplying blood to your body. |
|
Lung Cancer |
Caused by uncontrolled cell division in the lungs, cells divide and make more copies of themselves as a part of their normal function. But sometimes, they get changes (mutations) that cause them to keep making more of themselves when they shouldn’t. Damaged cells dividing uncontrollably create masses, or tumors, of tissue that eventually keep organs from working properly. |
Surgery: Removing the tumor and a small amount of healthy tissues around it. Radiofrequency Ablation: High energy radio waves heat and destroy cancer cells. Radiation Therapy: High energy beams to kill cancer cells. Chemotherapy: Combination of multiple medications to stop cancer cells from growing. Targeted Drug Therapy: Special drugs that slow down or destroy cancer cells. Immunotherapy: Reveals cancer cells to our body, so our immune system can fight it. |
Chemotherapy: Nausea, vomiting. Diarrhea. Hair loss. Fatigue. Mouth sores. Loss of feeling, weakness or tingling (neuropathy). Immunotherapy: Fatigue. Itchy rash. Diarrhea. Nausea, vomiting. Joint pain. Complications (like pneumonitis, colitis, hepatitis and others) can have additional side effects. Radiation therapy: Shortness of breath. Cough. Pain. Fatigue. Difficulty swallowing. Dry, itchy or red skin. Nausea, vomiting. Surgery: Shortness of breath. Chest wall pain. Cough. Fatigue. |
|
Colorectal Cancer |
Develops from certain polyps (growth) in the inner lining of the colon, have to be detected before the growth can become cancerous tumors. |
Polypectomy: Surgery removing cancerous polyps. Partial colectomy: Surgery removing the section of colon that contains a tumor and some surrounding healthy tissue. Surgical resection with colostomy: Surgeons remove the section of colon containing a tumor, but they can’t connect healthy colon sections. Radiofrequency ablation: Using heat to destroy cancer cells. |
Blood clots Diarrhea or constipation Bowel obstruction Infection Paralyzed or inactive intestine (called paralytic ileus) Sexual problems (for example, men may have erectile dysfunction or women may have pain during sex) |
|
Type 2 Diabetes |
Happens when you have persistently high blood sugar levels (hyperglycemia), when the body can’t use insulin properly. Can lead to various health problems, like heart disease, kidney disease and stroke. |
Oral diabetes medication: Most common one is metformin - lowers the amount of glucose produced by the liver, improving the body's ability to use insulin, and therefore reducing blood sugar levels. GLP-1 and dual GLP-1/GIP agonists - Stimulates the pancreas to produce more insulin when blood sugar levels rise, slowing gastric emptying to regulate glucose absorption, suppressing glucagon secretion, and promoting a feeling of fullness, leading to reduced food intake and weight loss, ultimately improving blood sugar control: Common ones are Dulaglutide, Exenatide (Byetta), Semaglutide, Liraglutide (Victoza, Saxenda), Lixisenatide (Adlyxin), Semaglutide (Rybelsus). Insulin - facilitates the movement of glucose (sugar) from the bloodstream into cells, allowing the body to use it for energy, thereby lowering blood sugar levels. |
Metmorfin: Abdominal or stomach discomfort cough or hoarseness decreased appetite diarrhea fast or shallow breathing fever or chills general feeling of discomfort lower back or side pain muscle pain or cramping painful or difficult urination sleepiness Anxiety blurred vision chest discomfort cold sweats coma confusion cool, pale skin depression difficult or labored breathing dizziness fast, irregular, pounding, or racing heartbeat or pulse feeling of warmth headache increased hunger increased sweating nausea nervousness nightmares redness of the face, neck, arms, and occasionally, upper chest seizures shakiness slurred speech tightness in the chest unusual tiredness or weakness difficulty with concentrating drowsiness lack or loss of strength restless sleep unusual sleepiness GLP-1 and dual GLP-1/GIP agonists: Loss of appetite. Nausea. Vomiting. Diarrhea. Dizziness. Mild tachycardia (increased heart rate). Infections. Headaches. Indigestion (upset stomach). Insulin: Sweating Dizziness Fatigue/weakness Fast heartbeat Weight gain Hunger Blurred vision Anxiety Headache Chills |
|
Arthritis |
Causes damage in joints, where two bones meet. |
Nonsteroidal anti-inflammatory drugs (NSAIDs) - Blocks the production of prostaglandins, hormone-like chemicals that contribute to inflammation and pain in the joints, effectively reducing both swelling and discomfort associated with the condition: Most common is Ibuprofen. Corticosteroids - reduces inflammation within the joints, which is the primary cause of pain and swelling associated with the condition. Disease-modifying antirheumatic drugs (DMARDs) - reduces inflammation, slowing the progression of the disease, and preserving joint function. |
NSAIDs/acetaminophen: indigestion stomach aches diarrhoea headaches drowsiness dizziness allergic reactions Corticosteroids: Weight gain. Muscle weakness. Blurred vision. Lower resistance to infection. Acne. Osteoporosis (bone weakening disease). Onset of, or worsening of, diabetes. Onset of, or worsening of, high blood pressure. Stomach irritation. Difficulty sleeping. Cataracts or glaucoma. DMARDs: Loss of appetite. Nausea. Diarrhea. Abdominal pain. Rash, allergic reaction. Liver problems. Increased risk of infections. Low white cell count (leukopenia), red blood cell count (anemia) and platelet count (thrombocytopenia). |
|
Asthma |
Airways are blocked by excess mucus, making it narrow. |
Bronchodilators: Medicines that relax the muscles around the airways. Anti-inflammatory medicines: Reduce swelling and mucus production in the airways. |
Bronchodilators: headaches suddenly noticeable heartbeats (palpitations) muscle cramps constipation a cough headaches feeling sick (nausea) throat irritation difficulty urinating difficulty swallowing (dysphagia) vomiting a rapid heartbeat (tachycardia) an irregular heartbeat (arrhythmia) problems sleeping (insomnia) Anti-inflammatory medicines: Stomach pain Heartburn Gas Bloating Nausea Vomiting Darrhea Constipation Headaches and dizziness Feeling lightheaded Difficulty concentrating High blood pressure |
|
Chronic Obstructive Pulmonary Disease (COPD) |
Lung disease that causes restricted airflow and breathing problems. |
Bronchodilators: Reduce inflammation and open the airways. Corticosteroids: Reduce inflammation. Antibiotics: Prevent infections and exacerbations. |
Bronchodilators: headaches suddenly noticeable heartbeats (palpitations) muscle cramps constipation a cough headaches feeling sick (nausea) throat irritation difficulty urinating difficulty swallowing (dysphagia) vomiting a rapid heartbeat (tachycardia) an irregular heartbeat (arrhythmia) problems sleeping (insomnia) Corticosteroids: Weight gain. Muscle weakness. Blurred vision. Lower resistance to infection. Acne. Osteoporosis (bone weakening disease). Onset of, or worsening of, diabetes. Onset of, or worsening of, high blood pressure. Stomach irritation. Difficulty sleeping. Cataracts or glaucoma. Antibiotics: Diarrhea. Nausea and vomiting. Dizziness. Rash. |
|
Chronic Kidney Disease |
Kidneys aren’t working properly. |
Angiotensin-converting enzyme (ACE) or angiotensin receptor blocker (ARB) - lower blood pressure, reducing stress on the kidneys, and blocking hormones that can worsen kidney function. Phosphate binder - binds to phosphorus in the intestines, preventing its absorption into the bloodstream and thereby lowering serum phosphate levels, which can otherwise build up due to impaired kidney function and lead to complications like bone disease and cardiovascular issues, essentially, it helps remove excess phosphorus from the body through stool instead of letting it be absorbed into the blood. A diuretic - reduce fluid buildup in the body, lowering blood pressure, and alleviating swelling (edema) by promoting the excretion of excess salt and water through urine, which can help slow down the progression of kidney damage by mitigating the strain on the already compromised kidneys, essentially, it helps the body get rid of excess fluid that the kidneys are struggling to eliminate on their own. Erythropoietin - stimulates the bone marrow to produce more red blood cells, thereby combating anemia, a common complication of Chronic Kidney Disease where the kidneys are unable to produce sufficient erythropoietin (a hormone that stimulates the production of red blood cells) on their own due to impaired function. Vitamin D called calcitriol - promoting calcium absorption in the intestines, regulating parathyroid hormone (a hormone that helps regulate calcium and phosphorus levels in the blood and bones) levels, and preventing excessive calcium loss in the urine, thereby mitigating complications associated with mineral and bone metabolism disturbances common in Chronic Kidney Disease patients. |
Angiotensin-converting enzyme (ACE) or angiotensin receptor blocker (ARB): Dry cough. Extreme tiredness or dizziness from blood pressure going too low. Headaches. Phosphate binder: Constipation Diarrhea Intestinal gas A diuretic: Dizziness. Tiredness. Headache. Low potassium (unless you’re taking a potassium-sparing type of diuretic). Muscle cramps. Heart palpitations. Dehydration. Erythropoietin: Dizziness Drowsiness Fever Headache Muscle Joint pain Weakness Nausea Vomiting Diarrhea Vitamin D and calcitriol: Nausea Vomiting Poor appetite Weight loss Constipation Weakness Heart rhythm problems Weakness Headache Upset stomach Muscle pain Bone pain Fever or chills Stomach pain irregular heartbeat Rash Difficulty breathing or swallowing |
|
Alzeimer’s Disease |
Causes a progressive decline in memory, thinking, learning and organizing skills. |
There isn’t a cure/treatment found, but these drugs address the symptoms. Aducanuma Donepezil Rivastigmine Galantamine Memantine Antidepressants Anti-anxiety drugs Anticonvulsant drugs |
Aducanuma: Headache Confusion Dizziness Nausea Donepezil: Nausea Diarrhea Trouble sleeping Vomiting Muscle cramps Tiredness Decreased appetite Rivastigmine: Nausea Vomiting Diarrhea Stomach pain Loss of appetite Weight loss. Galantamine: Nausea Vomiting Diarrhea Loss of appetite Stomach pain Heartburn Weight loss Extreme tiredness Dizziness Headache Difficulty falling asleep or staying asleep Runny nose Memantine: Bloating or swelling of the face, arms, hands, lower legs, or feet Dizziness Headache Nervousness Rapid weight gain Slow or fast heartbeat Antidepressants: Feeling and being sick Indigestion Stomach aches Diarrhoea Constipation Loss of appetite Dizziness Not sleeping well (insomnia) Headaches Loss of libido (reduced sex drive) Difficulties achieving orgasm during sex or masturbation Difficulties obtaining or maintaining an erection (erectile dysfunction) Weight gain Excessive sweating (especially at night) Anti-anxiety drugs: Drowsiness Sedation Dizziness Loss of balance |
|
Cystic Fibrosis |
Causes sticky, thick mucus to build up in organs, including the lungs and the pancreas. |
Cystic fibrosis transmembrane conductance regulator (CFTR) protein modulators - directly target and improve the function of the defective CFTR protein, which is the underlying cause of the disease, allowing for better chloride ion flow across cell membranes and resulting in thinner, less sticky mucus in the lungs and other organs, alleviating symptoms like lung infections and digestive issues associated with Cystic Fibrosis. Antibiotics - treat bacterial infections that can cause lung damage in people with cystic fibrosis (CF). Inhaled Bronchodilators - relax the muscles surrounding the airways, effectively widening them and allowing for easier airflow, which helps clear mucus build-up and alleviate symptoms like chest tightness, making breathing easier. Inhaled hypertonic saline - draw water into the airways through osmosis, effectively thinning thick mucus produced by the disease, making it easier to cough up and clear from the lungs, thus improving lung function and reducing the risk of lung infections. Anti-inflammatory drugs - reduce inflammation and slow lung damage. Pancreatic enzymes - help with cystic fibrosis by aiding in the digestion and absorption of nutrients, compensating for the pancreas' reduced ability to secrete enzymes due to thick mucus buildup. Stool softeners - ease bowel movements, preventing constipation, and reducing the strain caused by thick, sticky stools associated with the condition. |
CFTR modulators: Headache Stomach (abdominal) pain Diarrhea Rash Increase in liver enzymes Flu (influenza) Inflamed sinuses Loss of appetite Nausea Antibiotics: Diarrhea. Nausea and vomiting. Dizziness. Rash. Inhaled bronchodilators: Headaches Suddenly noticeable heartbeats (palpitations) Muscle cramps Constipation Cough Feeling sick (nausea) Throat irritation Difficulty urinating Difficulty swallowing (dysphagia) Vomiting Rapid heartbeat (tachycardia) Irregular heartbeat (arrhythmia) Problems sleeping (insomnia) Inhaled hypertonic saline: Increased cough. Sore throat. Chest tightness. Pain or irritation in the back of your mouth and throat and discomfort when swallowing. Vomiting. Fever. Joint pain. Red, watery eyes. Rash. Dizziness. Anti-inflammatory drugs: Irritation or pain Heartburn Gas Diarrhea Constipation Nausea Vomiting
Pancreatic enzymes: Constipation Nausea Abdominal Cramps Diarrhea Stool softeners: Stomach or intestinal pain or cramps. Nausea. Diarrhea. Throat irritation Rash. Itching. Dizziness. Difficulty swallowing or breathing. Fever. |
|
Crohn’s Disease |
Causes the digestive tract to become swollen and irritated. |
Corticosteroids - reduces inflammation in the digestive system. Anti-inflammatory medications - reduces inflammation and calms the immune response. Antibiotics - treat and prevent infections caused by bacteria. |
Corticosteroids: Weight gain. Muscle weakness. Blurred vision. Lower resistance to infection. Acne. Osteoporosis (bone weakening disease). Onset of, or worsening of, diabetes. Onset of, or worsening of, high blood pressure. Stomach irritation. Difficulty sleeping. Cataracts or glaucoma. Anti-inflammatory medications: Irritation or pain Heartburn Gas Diarrhea Constipation Nausea Vomiting Antibiotics: Diarrhea. Nausea and vomiting. Dizziness. Rash. |
|
Ulcerative Colitis |
Abnormal reactions of the immune system cause inflammation and ulcers on the inner lining of your large intestine. |
Aminosalicylates - directly reduce inflammation in the lining of the colon, allowing damaged tissue to heal. Corticosteroids - suppress the immune system, which in turn reduces inflammation in the digestive tract, providing quick relief from symptoms during a flare-up. Immunosuppressants - reduces the activity of the immune system, this reduces inflammation in the gastrointestinal tract. Biologics - target specific proteins or pathways involved in inflammation within the gut, effectively reducing inflammation and allowing the intestinal lining to heal by blocking the activity of key inflammatory chemicals, like tumor necrosis factor (TNF-alpha). |
Aminosalicylates: Fever Joint pains Skin rash or itching Unusual tiredness or weakness Abdominal pain (severe) Backache Headache Lower back pain sore throat Corticosteroids: Weight gain. Muscle weakness. Blurred vision. Lower resistance to infection. Acne. Osteoporosis (bone weakening disease). Onset of, or worsening of, diabetes. Onset of, or worsening of, high blood pressure. Stomach irritation. Difficulty sleeping. Cataracts or glaucoma. Immunosuppressants Acne. Diabetes. Fatigue. Hair loss or growth. Headaches. High blood pressure. Mouth sores. Thinning bones (osteoporosis). Stomach upset and nausea and vomiting. Biologics: Headache Fever or chills Difficulty breathing Low blood pressure Stomach pain Back pain Nausea Cough Sore throat |
|
Irritable Bowel Syndrome |
Causes uncomfortable or painful abdominal symptoms. |
Antidepressants - reduce the intensity of pain signals going from gut to brain. Fibre supplements and laxatives - add bulk to stool, softening it, and promoting regular bowel movements, which can alleviate constipation symptoms often associated with Irritable Bowel Syndrome. Antidiarrheals - slow down the digestive system, this allows the body to absorb more water from the intestines, making stools firmer and less frequent. |
Antidepressants: Feeling and being sick Indigestion Stomach aches Diarrhoea Constipation Loss of appetite Dizziness Not sleeping well (insomnia) Headaches Loss of libido (reduced sex drive) Difficulties achieving orgasm during sex or masturbation Difficulties obtaining or maintaining an erection (erectile dysfunction) Weight gain Excessive sweating (especially at night) Fibre supplements and laxatives: Gas Bloating Tummy cramps Feeling sick Dehydration Anti-diarrheals: Bloating Constipation Loss of appetite Nausea Stomach pain Vomiting |
2.2. Identification of Secondary Medications used and their Adverse Drug Reactions
Once the adverse drug reactions associated with treatments for chronic diseases were identified, they were compiled into a list, selected based on their frequency and the severity of their impact on patients' well-being.
Additionally, a separate and detailed list was systematically compiled to document conventional medications used to mitigate the adverse drug reactions that often arise from primary treatment regimens of chronic diseases. These medications, commonly referred to as secondary medications, are prescribed or recommended alongside primary treatments to alleviate discomfort and manage unintended physiological responses that may compromise a patient’s well-being. The identification process involved an in-depth review of commonly prescribed and widely used secondary medications that target a broad spectrum of adverse drug reactions, including gastrointestinal discomfort, cardiovascular complications, respiratory issues, and neurological effects.
To ensure a comprehensive overview, both over-the-counter and prescription medications were included, covering a diverse range of drug classes. Each secondary medication was analyzed not only for its primary role in mitigating adverse drug reactions but also for its potential risks and additional adverse drug reactions of its own, which contributes to the formation of a continuous cycle of ongoing medication for the chronic disease patient.
2.2.1. Attached below is the table of all the information collected:
Modern Drugs for Gastrointestinal Adverse Drug Reactions:
|
Adverse Drug Reactions |
Modern Drugs commonly used |
Adverse Drug Reactions of these drugs |
|
Abdominal pain |
Antacids - neutralize stomach acid, providing relief from abdominal pain caused by acid reflux or indigestion: Aluminum hydroxide gel Calcium carbonate Magnesium hydroxide |
Antacids: Constipation or diarrhea. Gas (flatulence). Headache. Nausea and vomiting. Stomach cramps or pain in the abdomen. Serious side effects could include caused by long term use: Acid rebound: Antacids cause your body to produce more acid, which worsens symptoms. Neurotoxicity: An antacid changes the function of your nervous system. Microcytic anemia: Iron deficiency. Osteopenia: Weakened bones. Hypercalcemia: Too much calcium in your blood. |
|
Bloating |
Simethicone - Simethicone helps with bloating by breaking down gas bubbles in the stomach and intestines, making it easier to pass the gas and relieve discomfort. Antacids - Antacids can help with bloating if the bloating is caused by excess stomach acid, as they neutralize the acid and relieve discomfort: Aluminum hydroxide gel Calcium carbonate Magnesium hydroxide |
Simethicone: Side effects could include: Dizziness Drowsiness Tiredness Constipation Serious side effects could include: Severe constipation/nausea/vomiting Stomach/abdominal pain Uncomfortable fullness of the stomach/abdomen Fast/irregular heartbeat Severe dizziness Fainting Antacids: Side effects could include: Constipation or diarrhea. Gas (flatulence). Headache. Nausea and vomiting. Stomach cramps or pain in the abdomen. Serious side effects could include: Acid rebound: Antacids cause your body to produce more acid, which worsens symptoms. Neurotoxicity: An antacid changes the function of your nervous system. Microcytic anemia: Iron deficiency. Osteopenia: Weakened bones. Hypercalcemia: Too much calcium in your blood. |
|
Constipation |
Polycarbophil - A bulk-forming laxative that absorbs water into the intestine, creating a gel-like substance that adds bulk to stools and makes them easier to pass. Methylcellulose fiber - Another bulk-forming agent that increases stool volume, stimulating bowel movements. Wheat dextrin, Magnesium hydroxide, and Magnesium citrate - These are osmotic laxatives that draw water into the intestines, softening stools and promoting bowel movements. Lactitol and Polyethylene glycol - These osmotic laxatives increase water content in the colon, facilitating stool passage and reducing straining. |
Polycarbophil: Bloating Diarrhea Gas Nausea Stomach cramps Serious side effects: Severe dizziness, Trouble breathing Severe stomach cramps, Rectal bleeding Wheat dextrin: Bloating Flatulence Gastrointestinal distress Methylcellulose fiber: Bloating Diarrhea Gas Nausea Stomach cramps Long-term effects: Severe diarrhea. Chest pain. Magnesium citrate: loose stools, diarrhea, stomach cramps upset stomach; dizziness increased sweating. Long-term Effects: Blood in stool Unable to have a bowel movement after use a decrease in tendon reflexes Muscle weakness Mental confusion Sedation Areflexia Respiratory paralysis Hypotension Depressed myocardial conductivity Asystole Bradyarrhythmias. Magnesium hydroxide: Symptoms of high magnesium levels (such as muscle weakness, slow/irregular heartbeat, slow/shallow breathing, mental/mood changes such as confusion) Symptoms of dehydration (such as decreased urination, dizziness, extreme thirst, very dry mouth) Stomach/abdominal pain Bloody stools Rectal bleeding Lactitol: Common side effects of Lactitol include: Upper respiratory tract infection, Gas (flatulence), Diarrhea, Increased blood creatinine phosphokinase, Abdominal distension, and Increased blood pressure Serious side effects of Lactitol include: Difficulty in breathing or swallowing Fever Hives, itching, rash Nausea Reddening of the skin, especially around the ears Swelling of the eyes, face, or inside of the nose Unusual tiredness or weakness Polyethylene glycol: More common Full or bloated feeling pain in the upper stomach pressure in the stomach stomach pain swelling of the stomach area vomiting Long-term: Confusion decreased urine output dizziness dry mouth fast or irregular heartbeat headache increased thirst loss of appetite loss of consciousness muscle pain or cramps numbness or tingling in the hands, feet, fingertips, lips, or mouth seizures swelling of the face, ankles, or hands unusual tiredness or weakness |
|
Decreased appetite |
Appetite stimulants - stimulate appetite and boost energy level: Megesterol acetate Medroxyprogesteron-e acetate Steroids - improve appetite by reducing inflammation and increasing metabolism, which can stimulate hunger signals in the brain: Prednisone Dexamethasone |
Megesterol acetate: Common side effects Hot flushes and sweats High blood pressure Constipation Rounded face Raised blood sugar levels Blood clot risk Feeling sick Diarrhoea Long-Term effects Feeling tired (fatigue) Mood changes Memory and concentration Skin changes Hair thinning Swollen hands, feet and ankles Loss of sex drive Change in periods Medroxyprogesterone acetate: Headaches Feeling sick (nausea) Irregular bleeding or spotting between periods Breast tenderness Feeling nervous or depressed Difficulty sleeping (insomnia) Higher body temperature Feeling dizzy Feeling tired Itchy skin Acne Hair loss Changes to vaginal discharge Prednisone: Aggression Agitation Blurred vision Decrease in the amount of urine Dizziness Fast, slow, pounding, or irregular heartbeat or pulse Headache Irritability Mood changes Noisy, rattling breathing Numbness or tingling in the arms or legs Pounding in the ears Swelling of the fingers, hands, feet, or lower legs Trouble thinking, speaking, or walking Trouble breathing Long-term effects: Problems with the eyes, such as glaucoma or cataracts. A round face, which is sometimes called a moon face. High blood sugar, which can trigger or worsen diabetes. Increased risk of infections, especially with common bacterial, viral and fungal microorganisms. Bone fractures and thinning bones, called osteoporosis. Muscle weakness. Thin skin, bruising and slower wound healing. Dexamethasone: “let down” or withdrawal effect Flushing and sweating Difficulty sleeping (insomnia) Sexual dysfunction Personality changes or mood alterations Hyperactivity and jitters Dizziness and headaches Difficulty concentrating Increased number of white blood cells Muscle weakness Death of bone tissue (avascular necrosis) Muscle cramps Weight gain in body or face Changes affecting hair Blurred vision Cataract formation Ulcers and heartburn (dyspepsia) Gas (flatulence) Increased appetite Changes in taste Higher blood sugar levels Temporary diabetes or thyroid issues Temporary decrease in testicular size Swelling of hands, legs, or feet Acne or rashes Thinning of skin |
|
Diarrhea |
Attapulgite - works by absorbing toxins and fluids in the intestines, forming a protective coating on the intestinal lining and reducing stool frequency. Loperamide - allows for more water absorption in the intestinal, leading to firmer stools. Bismuth sub-salicylate - combines anti-inflammatory, mild antibacterial, and antacid properties to reduce diarrhea caused by infections or mild intestinal inflammation. |
Attapulgite: This medicine may cause heart rhythm problems (eg, torsades de pointes, ventricular arrhythmias). Bloating Loss of appetite Nausea Stomach pain Vomiting Loperamide: Heart rhythm changes—fast or irregular heartbeat, dizziness, feeling faint or lightheaded, chest pain, trouble breathing Redness, blistering, peeling, or loosening of the skin, including inside the mouth Severe stomach pain Trouble passing urine Bloating Blurry vision Constipation Dizziness Drowsiness Dry mouth Nausea Passing gas Vomiting Loperamide may increase your risk for stomach or bowel problems. Bismuth sub-salicylate: Anxiety Any loss of hearing Confusion Constipation (severe) Difficulty in speaking or slurred speech Dizziness or lightheadedness Drowsiness (severe) Fast or deep breathing Headache (severe or continuing) Increased sweating Increased thirst Muscle spasms (especially of face, neck, and back) Muscle weakness Nausea or vomiting (severe or continuing) Ringing or buzzing in ears (continuing) Stomach pain (severe or continuing) Trembling Uncontrollable flapping movements of the hands (especially in elderly patients) or other uncontrolled body movements Vision problems Bismuth is possibly unsafe when used in larger amounts due to the risk for kidney failure, and when taken long-term due to the risk of nerve damage. |
|
Heartburn |
Antacids - neutralize stomach acid, providing immediate relief from the burning sensation: Aluminum hydroxide gel Calcium carbonate Magnesium hydroxide H2 blockers - block histamine-2 receptors on stomach lining cells, reducing acid production for several hours: Cimetidine Famotidine Nizatidine Proton pump inhibitors - inhibit the proton pump mechanism in stomach lining cells, leading to a significant decrease in stomach acid production and longer-lasting relief: Esomeprazole |
Antacids: Side effects could include: Constipation or diarrhea. Gas (flatulence). Headache. Nausea and vomiting. Stomach cramps or pain in the abdomen. Serious side effects could include caused by long term use: Acid rebound: Antacids cause your body to produce more acid, which worsens symptoms. Neurotoxicity: An antacid changes the function of your nervous system. Microcytic anemia: Iron deficiency. Osteopenia: Weakened bones. Hypercalcemia: Too much calcium in your blood. H2 blockers Cimetidine: Side Effects Headache Dizziness Drowsiness Diarrhea Nausea/vomiting that doesn't stop Mental/mood changes (such as agitation, confusion, depression, hallucinations) Trouble urinating Muscle/joint pain Breast swelling/soreness in males Decreased sexual ability (with very high doses of this medication) Easy bruising/bleeding Signs of infection (such as sore throat that doesn't go away, fever, cough, trouble breathing) Fast/slow/irregular heartbeat Unusual tiredness Severe stomach/abdominal pain Dark urine Yellowing eyes/skin Signs of kidney problems (such as change in the amount of urine) Confusion Excitement Depression Nervousness Seeing things or hearing voices that do not exist (hallucinating) Famotidine: Anxiety Bleeding gums Blistering, peeling, or loosening of the skin Blood in the urine or stools Bloody, black, or tarry stools Cough Difficulty breathing Discouragement Fast, irregular, pounding, or racing heartbeat or pulse Feeling sad or empty Irritability Lack of appetite Loss of interest or pleasure Noisy breathing Pinpoint red spots on the skin Seeing, hearing, or feeling things that are not there Seizures Swelling around the eyes Tightness in the chest Trouble concentrating Trouble sleeping Unusual bleeding or bruising Nizatidine: Chest pain Cough or hoarseness Fever or chills Lack or loss of strength Lower back or side pain Painful or difficult urination Black, tarry stools Bloody nose Blood in the urine Chest tightness Clay-colored stools Diarrhea Difficulty swallowing Dizziness Heavier menstrual periods Hives, itching, skin rash Loss of appetite Mental confusion Nausea Pinpoint red spots on the skin Puffiness or swelling of the eyelids or around the eyes, face, lips, or tongue Sore throat Sores, ulcers, or white spots on the lips or in the mouth Swollen glands Trouble breathing Unpleasant breath odor Unusual bleeding or bruising Unusual tiredness or weakness Vomiting of blood Yellow eyes or skin Proton pump inhibitors: Esomeprazole: Headaches Feeling sick Being sick Diarrhoea Constipation Stomach pain Farting (flatulence) Low magnesium can make you feel tired, confused, dizzy and cause muscle twitches, shakiness and an irregular heartbeat. Bone fractures Gut infections Vitamin B12 deficiency |
Modern Drugs for Respiratory Adverse Drug Reactions:
|
Adverse Drug Reactions |
Modern Drugs |
Adverse Drug Reactions of these drugs |
|
Cough |
Antitussives - suppress the cough reflex by acting on the medulla in the brain: Pholcodine Codeine Dextromethorphan Benzonatate - that numbs the stretch receptors in the airways and lungs, reducing the cough reflex triggered by irritation or inflammation. |
Pholcodine: Drowsiness Nausea Vomiting Constipation Dizziness or a feeling of lightheadedness Trouble breathing Respiratory depression Codeine: Confusion Dizziness Going "on the nod" (being in and out of consciousness) Drowsiness Headaches Slowed breathing Nausea and vomiting Smaller (constricted) pupils Feeling heavy in the arms and legs Dextromethorphan: Dizziness Lightheadedness Drowsiness Nervousness Restlessness Nausea Vomiting Stomach pain Benzonatate: Nausea Constipation Drowsiness Headache Dizziness Stuffy nose Feeling chilly Burning in the eyes Tightening of the throat Difficulty breathing or swallowing Numbness of the chest Confusion Hallucinations |
|
Difficulty breathing |
Bronchodilators - medicines that open the airways (bronchi): Salbutamol Salmeterol Formoterol Vilanterol Ipratropium Tiotropium Aclidinium Glycopyrronium Theophylline - relaxes the airway muscles improving airflow and oxygen exchange. |
The main side effects of beta-2 agonists like salbutamol include: Trembling, particularly in the hands Nervous tension Headaches Suddenly noticeable heartbeats (palpitations) Muscle cramps Excessive doses can occasionally cause heart attacks and a severely low level of potassium in the blood (hypokalemia). The main side effects of anticholinergics like ipratropium include: Dry mouth Constipation Cough Headaches Feeling sick (nausea) Difficulty swallowing (dysphagia) Palpitations Throat irritation Difficulty urinating The main side effects of Theophylline include: Nausea and vomiting Diarrhea Palpitations Rapid heartbeat (tachycardia) Irregular heartbeat (arrhythmia) Headaches Problems sleeping (insomnia) Theophylline can cause serious side effects if too much of it builds up in your body. |
|
Pneumonitis |
Immunosuppressants - reduce immune system activity to prevent inflammation in lung tissue caused by autoimmune reactions: Mycophenolate Azathioprine Cortisodes - reduce inflammation in lung tissues by suppressing cytokine (signaling proteins that help the body's immune system fight infection and inflammation) production and inflammatory mediators: Prednisone Prednisolone Methylprednisolone Betamethasone Dexamethasone Triamcinolone Hydrocortisone |
Prednisone: Aggression Agitation Blurred vision Decrease in the amount of urine Dizziness Fast, slow, pounding, or irregular heartbeat or pulse Headache Irritability Mood changes Noisy, rattling breathing Numbness or tingling in the arms or legs Pounding in the ears Swelling of the fingers, hands, feet, or lower legs Trouble thinking, speaking, or walking Trouble breathing Long-term effects: Problems with the eyes, such as glaucoma or cataracts. A round face, which is sometimes called a moon face. High blood sugar, which can trigger or worsen diabetes. Increased risk of infections, especially with common bacterial, viral and fungal microorganisms. Bone fractures and thinning bones, called osteoporosis. Muscle weakness. Thin skin, bruising and slower wound healing. Mycophenolate: Constipation Nausea Vomiting Difficulty falling asleep or staying asleep Pain, especially in the back, muscles, or joints Headache Gas Prickling, tingling, or burning feeling on the skin Swelling of the hands, arms, feet, ankles, or lower legs Tremor Sudden severe stomach pain, stomach pain that doesn't go away, or diarrhea Difficulty breathing Chest pain Rash Itching Dizziness, fainting, pale skin, lack of energy, shortness of breath, or fast heartbeat Unusual bleeding or bruising; vomiting or spitting up blood or brown material that resembles coffee grounds; bloody or black, tarry Stools; or blood in urine Fever, muscle or joint stiffness or pain Mycophenolate weakens the body's immune system and may decrease your ability to fight infection and increase the risk that you will get a serious infection, including severe fungal, bacterial, or viral infections that spread through the body. Mycophenolate may increase the risk that you will develop progressive multifocal leukoencephalopathy (PML; a rare infection of the brain that cannot be treated, prevented, or cured and that usually causes death or severe disability).
Azathioprine: Feeling sick (mild nausea) Feel tired all the time, dizzy or sick, or you're vomiting or have diarrhoea High temperature with shivering or chills, cough or a sore throat Joints or muscles are hurting Pee changes colour or you start peeing more or less than usual – this can be a sign of kidney problems Feel confused, light-headed or weak – these can be signs of low blood pressure Bleeding or bruising more easily than usual Lumps anywhere on your body Severe stomach ache (abdominal pain) and back pain Taking azathioprine for a long time can increase your chance of getting certain types of cancer, including skin cancer. Azathioprine can also sometimes affect your liver, kidneys or bone marrow. Prednisolone: Weight gain Indigestion Problems sleeping (insomnia) Feeling restless Sweating a lot Mild mood changes A high temperature, chills, a very sore throat, ear or sinus pain, a cough, more saliva or a change in colour of saliva (yellowish and Possibly with streaks of blood), pain when you pee, mouth sores or a wound that will not heal – these can be signs of an infection Sleepy or confused, feeling very thirsty or hungry, peeing more often, flushing, breathing quickly or breath that smells like fruit – these can be signs of high blood sugar Weight gain in your upper back or belly, "moon face" (a puffy, rounded face), very bad headaches and slow wound healing – these can be signs of Cushing's syndrome Very upset stomach or you're being sick (vomiting), very bad dizziness or passing out, muscle weakness, feeling very tired, mood changes, loss of appetite and weight loss – these can be signs of adrenal gland problems Muscle pain or weakness, muscle cramps, or changes in your heart rate – these can be signs of low potassium levels Severe stomach pain, severe back pain, severe upset stomach or you're being sick – these can be signs of pancreas problems Breathlessness Swelling in your arms or legs Changes in your eyesight Any bruising or bleeding that is not normal Red or black poo You may notice mood changes and mental health problems while taking prednisolone, (feeling depressed feeling high, or moods that go up and down, feeling anxious, having problems sleeping, difficulty in thinking, or being confused and losing your memory, feeling, seeing or hearing things that do not exist (hallucinations), having strange and frightening thoughts, changing how you act, or having feelings of being alone.) Taking prednisolone for a long time can lead to side effects such as: Thinner bones (osteoporosis) Poorly controlled diabetes Eyesight problems High blood pressure (hypertension) Taking prednisolone at higher doses for a long time can slow down the normal growth of children and teenagers. Methylprednisolone: Upset stomach Stomach irritation Vomiting Headache Dizziness Insomnia Restlessness Depression Anxiety Acne Increased hair growth Easy bruising Irregular or absent menstrual periods Skin rash Swollen face, lower legs, or ankles Vision problems Cold or infection that lasts a long time Muscle weakness Black or tarry stool Betamethasone: Weight gain Indigestion Problems sleeping Feeling restless Sweating a lot a high temperature, a very sore throat, ear or sinus pain, a cough, more saliva or a change in the colour of your saliva, pain when you pee, mouth sores or a wound that will not heal – these can be signs of an infection feeling sleepy or confused, feeling very thirsty or hungry, and peeing more often – these can be signs of high blood sugar weight gain in your upper back or tummy, a puffy, rounded face (moon face) and slow wound healing – these can be signs of Cushing's syndrome Feeling or being sick, very bad dizziness or passing out, muscle weakness, feeling very tired, loss of appetite and weight loss – these can be signs of adrenal gland problems Muscle pain or weakness, muscle cramps, or changes in your heart rate – these can be signs of low potassium levels Severe stomach pain, severe back pain, feeling or being sick or diarrhoea – these can be signs of pancreas problems Swelling in your arms or legs Blurred vision Any unexplained bruising or bleeding You may notice mood changes and mental health problems, (feeling depressed feeling high, or moods that go up and down, feeling anxious, having problems sleeping, difficulty in thinking, or being confused and losing your memory, feeling, seeing or hearing things that do not exist (hallucinations), having strange and frightening thoughts, changing how you act, or having feelings of being alone.) Taking betamethasone for a long time can slow down the normal growth of children and teenagers. Taking betamethasone tablets for many months or years can lead to: Weak bones (osteoporosis) Diabetes, or worsening of your condition if you already have diabetes Eye problems Dexamethasone: “let down” or withdrawal effect Flushing and sweating Difficulty sleeping (insomnia) Sexual dysfunction Personality changes or mood alterations Hyperactivity and jitters Dizziness and headaches Difficulty concentrating Increased number of white blood cells Muscle weakness Death of bone tissue (avascular necrosis) Muscle cramps Weight gain in body or face Changes affecting hair Blurred vision Cataract formation Ulcers and heartburn (dyspepsia) Gas (flatulence) Increased appetite Changes in taste Higher blood sugar levels Temporary diabetes or thyroid issues Temporary decrease in testicular size Swelling of hands, legs, or feet Acne or rashes Thinning of skin Triamcinolone: Upset stomach Stomach irritation Vomiting Headache Dizziness Insomnia Restlessness Depression Anxiety Acne Increased hair growth Easy bruising Irregular or absent menstrual periods Swollen face, lower legs, or ankles Vision problems Cold or infection that lasts a long time Muscle weakness Black or tarry stool Hydrocortisone: Feeling dizzy, weak or tired Headaches Muscle ache Indigestion or feeling sick (nausea) Diarrhoea Swollen ankles Have a high temperature, chills, a very sore throat, ear or sinus pain, a cough, coughing up more mucus (phlegm) or a change in colour of your mucus, pain when you pee, mouth sores or a wound that will not heal – these can be signs of an infection Are sleepy or confused, feeling very thirsty or hungry, peeing more often than usual, flushing, breathing quickly or have breath that smells like fruit – these can be signs of high blood sugar (hyperglycaemia) Have a very upset stomach or vomiting, very bad dizziness or fainting, muscle weakness, mood changes, loss of appetite and weight loss or are feeling very tired – these can be signs of adrenal gland problems Have muscle pain, weakness or cramps, or your heartbeats suddenly become more noticeable (heart palpitations) – these can be signs of low potassium levels Have severe stomach pain, severe back pain, a severe upset stomach or vomiting – these can be signs of pancreas problems get breathless Have swelling in your arms or legs Have changes in your eyesight Have any bruising or bleeding that is not normal Have red or black poo You may take hydrocortisone tablets for a long time, even for the rest of your life. Over time, hydrocortisone can have several harmful effects on your body. It can lead to: Weak or fragile bones (osteoporosis) Poorly controlled diabetes Eyesight problems Slower growth in children and teenagers |
Modern drugs for Cardiovascular Adverse Drug Reactions:
|
Adverse Drug Reactions |
Modern drugs |
Adverse Drug Reactions of these drugs |
|
Angioedema (swollen tissue) |
Anti-itch drugs/Antihistamines - block H1 histamine receptors (activated by histamine, and involved in inflammation), reducing swelling, itching, and redness: Loratadine Cetirizine Diphenhydramine Fexofenadine Corticosteroid drugs - suppress inflammation and immune response, helping to reduce swelling and tissue inflammation: Prednisone |
Loratadine: Headache Dry mouth Nosebleed Sore throat Mouth sores Difficulty falling asleep or staying asleep Nervousness Weakness Stomach pain Diarrhea Red or itchy eyes Rash Hives Itching Swelling of the eyes, face, lips, tongue, throat, hands, arms, feet, ankles, or lower legs Hoarseness Difficulty breathing or swallowing Wheezing Cetirizine: Headaches Dry mouth Feeling sick (nausea) Feeling dizzy Diarrhoea Sore throat Sneezing or blocked and runny nose Diphenhydramine: Dry mouth, nose, and throat Drowsiness Dizziness Nausea Vomiting Loss of appetite Constipation Increased chest congestion Headache Muscle weakness Excitement (especially in children) Nervousness Vision problems Difficulty urinating or painful urination Fexofenadine: Feeling sick (nausea) Feeling sleepy Headaches Dry mouth Feeling dizzy Prednisone: Aggression Agitation Blurred vision Decrease in the amount of urine Dizziness Fast, slow, pounding, or irregular heartbeat or pulse Headache Irritability Mood changes Noisy, rattling breathing Numbness or tingling in the arms or legs Pounding in the ears Swelling of the fingers, hands, feet, or lower legs Trouble thinking, speaking, or walking Trouble breathing Long-term effects: Problems with the eyes, such as glaucoma or cataracts. A round face, which is sometimes called a moon face. High blood sugar, which can trigger or worsen diabetes. Increased risk of infections, especially with common bacterial, viral and fungal microorganisms. Bone fractures and thinning bones, called osteoporosis. Muscle weakness. Thin skin, bruising and slower wound healing. |
|
Arrhythmia (irregular heartbeat) |
Amiodarone - alters ion channel activity (potassium and sodium channels) in heart tissue to stabilize the electrical activity of the heart, preventing abnormal rhythms. Flecainide - slows electrical signals in the heart by blocking sodium channels, effectively treating and preventing episodes of rapid or irregular heartbeat. Sotalol - a beta-blocker and potassium channel blocker that slows the heart rate and restores a regular rhythm. |
Amiodarone: Constipation Loss of appetite Headache Decreased sex drive Difficulty falling asleep or staying asleep Flushing Changes in ability to taste and smell Changes in amount of saliva Rash Weight loss or gain Restlessness Weakness Nervousness Irritability Intolerance to heat or cold Thinning hair Excessive sweating Changes in menstrual cycle Swelling in the front of the neck (goiter) Swelling of the hands, feet, ankles, or lower legs Uncontrollable shaking of a part of the body Decreased concentration Movements that you cannot control Poor coordination or trouble walking Numbness or tingling in the hands, legs, and feet Muscle weakness Flecainide: Dizziness Changes in vision Headache Weakness Uncontrollable shaking of a part of your body Constipation Stomach pain Fast, pounding, or irregular heartbeat Chest pain Shortness of breath Extreme tiredness Nausea Loss of appetite Persistent cough with blood-tinged mucus Swelling of the hands, feet, ankles, or lower legs Confusion Unusual bleeding or bruising Pain in the upper right part of the stomach Yellowing of the skin or eyes Flu-like symptoms Sotalol: Dizziness Lightheadedness Weakness Excessive tiredness Headache Diarrhea Nausea Vomiting Shortness of breath or wheezing Swelling of the feet and lower legs Unusual weight gain Chest pain Dizziness Fainting Fast, slow, or irregular heartbeat Severe diarrhea, unusual sweating, vomiting, decreased appetite, or excessive thirst |
|
Increased risk of blood clot formation |
Anticoagulant medications - blood thinners, preventing the blood from getting clogged up: Apixaban Betrixaban Dabigatran Edoxaban Rivaroxaban Warfarin |
Apixaban: Bleeding gums Nosebleeds Heavy vaginal bleeding Red, pink, or brown urine Red or black, tarry stools Coughing up or vomiting blood or material that looks like coffee grounds Swelling or joint pain Headache Rash Chest pain or tightness Swelling of the face or tongue Trouble breathing Wheezing Feeling dizzy or faint Betrixaban: Constipation Diarrhea Nausea Frequent or painful urination Bleeding gums Frequent nosebleeds Menstrual bleeding that is heavier than normal Red, pink, or brown urine Red or black, tarry stools Coughing up blood or blood clots Vomiting blood or material that looks like coffee grounds Unexpected pain, swelling, or joint pain Headache Dizziness or weakness Dabigatran: Tiredness and lack of energy, shortness of breath, noticeable heartbeats (heart palpitations) and paler than usual skin Feeling dizzy or light-headed Feeling or being sick (nausea or vomiting) Stomach pain or indigestion
Edoxaban: Tiredness and lack of energy, shortness of breath, noticeable heartbeats (heart palpitations) and paler than usual skin Feeling dizzy or light-headed Feeling or being sick (nausea or vomiting) Stomach pain and indigestion Rivaroxaban: Muscle spasm Pain in arms or legs (in children) Vomiting (in children) Stomach pain Cough (in children) Rash (in children) Bloody, black, or tarry stools Pink, or brown urine Coughing up or vomiting blood or material that looks like coffee grounds Frequent nosebleeds Bleeding from your gums Heavy menstrual bleeding Weakness Tiredness Headache Dizziness or fainting Blurred vision Pain in arm or leg Rash Itching Difficulty breathing or swallowing Hives Pain or swelling at wound sites Decreased urination Swelling in your legs, feet, or ankles Warfarin: Gas Abdominal pain Bloating Change in the way things taste Loss of hair Feeling cold or having chills Hives Rash Itching Difficulty breathing or swallowing Swelling of the face, throat, tongue, lips, or eyes Hoarseness Chest pain or pressure Swelling of the hands, feet, ankles, or lower legs Fever Infection Nausea Vomiting Diarrhea Extreme tiredness Lack of energy Loss of appetite Pain in the upper right part of the stomach Yellowing of the skin or eyes Flu-like symptoms |
|
Chest pain |
Nitroglycerin - relaxes and dilates blood vessels, improving blood flow to the heart. Aspirin - reduces blood clotting by inhibiting platelet aggregation, ensuring better blood flow. |
Nitroglycerin: Flushing Blurred vision Dry mouth Rash, blistering, or peeling of the skin Hives Itching Difficulty breathing or swallowing Nausea Vomiting Weakness Sweating Pale skin Aspirin: Nausea Vomiting Stomach pain Heartburn Hives Rash Swelling of the eyes, face, lips, tongue, or throat Wheezing or difficulty breathing Hoarseness Fast heartbeat Fast breathing Cold, clammy skin Ringing in the ears Loss of hearing Bloody vomit Vomit that looks like coffee grounds Bright red blood in stools Black or tarry stools Aspirin can cause ulcers in your stomach or gut, especially if you take it for a long time or in big doses. |
|
Rapid heartbeat (tachycardia) |
Calcium channel blockers - slow the movement of calcium into heart cells, reducing heart rate and force of contraction: Verapamil Diltiazem Beta-Blockers - block adrenaline effects on the heart, slowing the heart rate and stabilizing rhythm: Metoprolol Nadolol Propranolol Adenosine Magnesium sulfate Digoxin - acts on the sinoatrial node, which controls the heart's rhythm. |
Verapamil: Constipation Feeling or being sick (nausea or vomiting) Flushing of the face and neck Headaches Feeling dizzy or tired Swollen hands, ankles or feet The whites of your eyes or your skin turn yellow, although this may be less obvious on brown or black skin, or you have dark pee – these can be signs of liver problems. Diltiazem: Swollen hands, ankles or feet Headaches Feeling dizzy and light-headed Feeling tired, weak and generally unwell Feeling hot (flushing) and redness of the skin Itching or burning on the skin where you use the cream or ointment Stomach pain Indigestion Constipation You get severe pain in your stomach The whites of your eyes turn yellow, or your skin turns yellow although this may be less obvious on brown or black skin – this can be a sign of liver problems Your heartbeat becomes noticeable (palpitations) Metoprolol: Headaches Feeling tired, dizzy or weak Cold hands or feet Feeling sick (nausea) Stomach pain You get shortness of breath, wheezing and tightening of the chest – these can be signs of lung problems You get shortness of breath with a cough that gets worse when you exercise (like walking up stairs), swollen ankles or legs, or an irregular heartbeat – these are signs of heart problems You have a fast heart rate, high temperature, trembling and confusion – these are signs of too much thyroid hormone in the blood The whites of your eyes turn yellow, or your skin turns yellow although this may be less obvious on brown or black skin – these can be signs of liver problems You get unexplained bruising, or you bruise more easily than usual – these can be signs of low numbers of platelets in your blood (thrombocytopenia) Nadolol: Dizziness or lightheadedness Excessive tiredness Cold hands and feet Shortness of breath Swelling of the hands, feet, ankles, or lower legs Unusual weight gain Fainting Chest pain Slow or irregular heartbeat Propranolol: Headaches Feeling tired, dizzy or weak Cold fingers or toes Feeling or being sick (nausea or vomiting), or diarrhoea Stomach pain The whites of your eyes turn yellow, your skin turns yellow although this may be less obvious on brown or black skin, or you have pale poo or dark pee – these can be signs of liver problems You get nosebleeds that last for more than 10 minutes, unexplained bruising, or you bruise more easily than usual – these can be signs of low numbers of platelets in your blood (thrombocytopenia) Adenosine: Facial flushing Difficulty breathing Chest pain Heart attack Lightheadedness Dizziness Tingling in arms Numbness Nausea Low blood pressure (hypotension) Irregular heartbeat (palpitations) Apprehension Head pressure Chest pain Blurred vision Burning sensation Heaviness in arms, neck, and back pain Metallic taste Tightness in throat Sweating Hyperventilation Elevated heart rate Rhythm disturbance (ventricular fibrillation) Transient increase in blood pressure Slow heart rate Irregular heartbeats (atrial fibrillation) Cardiac failure Infusion site pain Hypersensitivity Abnormal heart rhythm Seizure Sudden difficulty breathing (bronchospasm) Digoxin: Dizziness or lightheadedness Drowsiness Vision changes (blurred or yellow) Rash Irregular heartbeat Upset stomach Vomiting Diarrhea Loss of appetite Swelling of the feet or hands Unusual weight gain Difficulty breathing Magnesium sulfate: Stomach pain or cramps Bloating Nausea Headache Seizures Fainting Feeling confused Vomiting, especially if you can't keep down the fluids that you need for your treatment Difficulty swallowing Rectal bleeding Deceased urination Dizziness Irregular heartbeat Sudden, severe pain in one or more joints. |
|
High blood pressure |
Diuretics/water pills - increase urine production, removing excess fluid and salt from the body, which lowers blood pressure: Furosemide Bumetanide Torsemide Chlorothiazide Amiloride Hydrochlorothiazide or HCTZ Indapamide Metolazone Triamterene Beta-blockers - lower heart rate and the force of the heart's contractions, reducing the workload on the heart and lowering blood pressure: Acebutolol Atenolol Betaxolol Carvedilol Carvedilol phosphate Labetalol Metoprolol succinate Metoprolol tartrate Nadolol Nebivolol Pindolol Propranolol Angiotensin II receptor blockers - block the effects of angiotensin, a chemical that causes the arteries to become narrow. This means blood vessels stay open and blood pressure lowers: Candesartan Losartan Valsartan Alpha-2 receptor agonists - block brain signals that can increase heart rate and narrow blood vessels. This lowers blood pressure: Methyldopa Clonidine Guanfacine |
Diuretics: Urinating more often. Too little sodium in the blood. Too little potassium in the blood. Dizziness. Headaches. Dehydration. Muscle cramps. A type of arthritis called gout, which causes severe joint pain, usually in the big toe. Trouble getting erections, also called erectile dysfunction or impotence. Beta-blockers: Feeling tired, dizzy or lightheaded (these can be signs of a slow heart rate) Cold fingers or toes (beta blockers may affect the blood supply to your hands and feet) Difficulties sleeping (Insomnia) Difficulty getting an erection or other difficulties with sex Feeling sick Shortness of breath and a cough that gets worse when you exercise (like walking up stairs), swollen ankles or legs, or an irregular heartbeat – these can be signs of heart problems Shortness of breath, wheezing and tightening of your chest – these can be signs of lung problems Yellowish skin or the whites of your eyes turn yellow, although this may be less obvious on brown or black skin – these can be signs of liver problems. Angiotensin II receptor blockers: Dizziness Headache Fatigue ARBs can upset the kidneys, especially if the blood vessels to your kidneys are narrowed (renal artery stenosis). Alpha-2 receptor agonists: Depression Bradycardia (low heart rate) Orthostatic hypotension (sudden drop in blood pressure when you stand from a seated or lying down position) Constipation Nausea Gastric upset Dry mouth (xerostomia) and dry nasal mucosa (caused by increased vagal activity) Impotence Fluid retention and edema with chronic use Hepatic necrosis (death of liver cells) Hemolytic anemia (anemia due to death of red blood cells) |
|
Low blood pressure (hypotension) |
Fludrocortisone - helps retain sodium in the body by acting on the kidneys, which increases blood volume and raises blood pressure. Midodrine - stimulates alpha-adrenergic receptors on blood vessels, causing them to constrict. |
Fludrocortisone: Upset stomach Stomach irritation Vomiting Headache Dizziness Insomnia Restlessness Depression Anxiety Acne Increased hair growth Easy bruising Irregular or absent menstrual periods Skin rash Swollen face, lower legs, or ankles Vision problems Cold or infection that lasts a long time Muscle weakness Black or tarry stool Midodrine: Numbness and tingling Scalp itching Goosebumps Chills Frequent urination Urgent need to urinate Difficulty urinating Rash Stomach pain Slow heartbeat Dizziness Fainting |
|
Low white cell count (leukopenia) |
Antibiotics - used to prevent or treat infections in leukopenic patients since they are at higher risk of infections due to their low immunity: Penicillins Cephalosporins Granulocyte colony-stimulating factor (G-CSF) - stimulates the bone marrow to produce more neutrophils, a type of white blood cell essential for fighting infections: Filgrastim Pegfilgrastim |
Penicillins: Nausea Vomiting Diarrhea Rash Abdominal pain Urticaria Cephalosporins: Nausea Diarrhea Vomiting Heartburn Stomach pain Rectal or genital itching Dizziness Extreme tiredness Agitation Confusion Headache Joint pain Watery or bloody stools, stomach cramps, or fever during treatment or for up to two or more months after stopping treatment Rash Itching Hives Swelling of the face, throat, tongue, lips, and eyes Difficulty breathing or swallowing Wheezing A return of fever, sore throat, chills, or other signs of infection Hallucinations (seeing things or hearing voices that do not exist) Filgrastim: Nose bleed Anemia which may require blood transfusions Bruising, bleeding Diarrhea Bone pain Fever Tiredness Hair loss High blood pressure which may cause headaches, dizziness, blurred vision Capillary Leak syndrome which may cause fluid in the body, low blood pressure, shortness of breath, swelling of ankles Chest pain, shortness of breath Cough Allergic reaction which may cause rash, low blood pressure, wheezing, shortness of breath, swelling of the face or throat Kidney damage which may cause swelling, may require dialysis Pain in limbs or muscles, muscle cramps Headache Dizziness Reduced sense of touch Rash Acute Respiratory Distress Syndrome which may cause damage to the lungs and shortness of breath. Damage to the bone marrow (irreversible) which may cause infection, bleeding, may require transfusions. Rupture of the spleen causing sudden or severe pain in the left side of abdomen spreading up to your shoulder. Pegfilgrastim: Pain in bone, arms, and legs Capillary leak syndrome which may cause fluid in the organs, low blood pressure, shortness of breath, swelling of ankles Acute respiratory distress syndrome, which may cause damage to the lungs and shortness of breath Anemia which may cause tiredness, or may require blood transfusions Rupture of the spleen causing sudden or severe pain in the left side of abdomen spreading up to your shoulder Kidney damage which may cause swelling, may require dialysis. |
Modern Drugs for Neurological Adverse Drug Reactions:
|
Adverse Drug Reactions |
Modern Drugs |
Adverse Drug Reactions of these drugs |
|
Blurred vision |
Pilocarpine hydrochloride - stimulates the contraction of the eye's ciliary muscle, which helps improve the flow of aqueous humor (the clear liquid inside the front part of the eye) and reduces intraocular pressure (the pressure of fluid within the eye that helps maintain its shape). It enhances focus and sharpens vision in conditions where muscle relaxation has caused blurring |
Pilocarpine hydrochloride: Sweating Nausea Runny nose Diarrhea Chills Flushing Frequent urination Dizziness Weakness Headache Vomiting Heartburn Stomach pain Swelling of the arms, hands, feet, ankles, or lower legs Changes in vision Fast or slow heartbeat |
|
Fatigue |
Antidepressants Tricyclics - affect serotonin and norepinephrine levels in the brain, which helps reduce fatigue and muscle tension. Modulate neurotransmitters in the brain to improve mood and energy levels: Amitriptyline Desipramine Notriptyline Selective serotonin reuptake inhibitors (SSRIs) - increase serotonin levels in the brain, which can alleviate fatigue by enhancing mood and reducing mental exhaustion: Citalopram Escitalopram Fluoxetine Paroxetine Sertraline |
Tricyclics: Dry mouth Slight blurring of vision Constipation Problems passing urine Drowsiness Dizziness Weight gain Excessive sweating (especially at night) Heart rhythm problems (arrhythmia), such as noticeable palpitations or a fast heartbeat (tachycardia) Selective serotonin reuptake inhibitors (SSRIs): Feeling agitated, shaky or anxious Feeling and being sick Indigestion and stomach aches Diarrhoea or constipation Loss of appetite Dizziness Not sleeping well (insomnia), or feeling very sleepy Headaches Loss of libido (reduced sex drive) Difficulties achieving orgasm during sex or masturbation Difficulties obtaining or maintaining an erection (erectile dysfunction) Elderly people who take antidepressants, particularly those who take SSRIs, may experience a severe fall in sodium (salt) levels, known as hyponatraemia. This may lead to a build-up of fluid inside the cells of the body, which can be potentially dangerous. Long-term use of SSRIs and TCAs has been linked to an increased risk of developing type 2 diabetes, although it's not clear if the use of these antidepressants directly causes diabetes to develop. |
Modern drugs for Dermatological Adverse Drug Reactions:
|
Adverse Drug Reactions |
Modern drugs |
Adverse Drug Reactions of these drugs |
|
Hair loss |
Oral Finasteride - treat androgenetic alopecia (male or female pattern baldness) by inhibiting the enzyme 5-alpha-reductase. This prevents the conversion of testosterone to dihydrotestosterone (DHT), a hormone responsible for hair follicle shrinkage and hair loss. Corticosteroids - reduce inflammation around the hair follicles, helping to stimulate regrowth: Clobetasol propionate Hydrocortisone |
Finasteride: Inability to have or maintain an erection Decreased sexual desire Problems with ejaculation (including decreased volume of ejaculate) Pain in the testicles Depression Changes in the breasts such as increased size, lumps, pain, or nipple discharge Rash Itching Hives Swelling of the lips and face Difficulty breathing or swallowing Taking finasteride may increase the risk that you will develop high-grade prostate cancer (a type of prostate cancer that spreads and grows more quickly than other types of prostate cancer) or breast cancer. Clobetasol propionate: Burning, itching, irritation, redness, or dryness of the skin Acne Tiny red bumps or rash around the mouth Small white or red bumps on the skin Bruising or shiny skin Red or purple blotches or lines under the skin Thin, fragile, or dry skin Changes in skin color Redness, swelling, oozing pus or other signs of skin infection in the place where you applied clobetasol Severe rash Skin sores Changes in the way fat is spread around the body Sudden weight gain Unusual tiredness Muscle weakness Depression and irritability Children who use clobetasol topical may have an increased risk of side effects including slowed growth and delayed weight gain. Hydrocortisone: Feeling dizzy, weak or tired Headaches Muscle ache Indigestion or feeling sick (nausea) Diarrhoea Swollen ankles Weak or fragile bones (osteoporosis) Poorly controlled diabetes Eyesight problems Slower growth in children and teenagers. |
Modern Drugs for Musculoskeletal Adverse Drug Reactions:
|
Adverse Drug Reactions |
Modern drugs |
Adverse Drug Reactions of these drugs |
|
Joint pain |
Opioids (severe pain) - block pain signals by binding to opioid receptors, providing strong pain relief: Oral morphine Tramadol Corticosteroid - reduce inflammation by suppressing the immune system, easing pain from inflammatory conditions: Prednisone Non-Steroidal Anti-Inflammatory Agents (NSAIDs) - inhibit COX enzymes to decrease inflammation, swelling, and pain: Ibuprofen Naproxen Diclofenac Acetaminophen Duloxetine Aspirin |
Oral morphine: Drowsiness Stomach pain and cramps Dry mouth Headache Nervousness Mood changes Small pupils (black circles in the middle of the eyes Difficulty urinating Blue or purple color to the skin Changes in heartbeat Agitation, hallucinations (seeing things or hearing voices that do not exist), fever, sweating, confusion, fast heartbeat, shivering, Severe muscle stiffness or twitching, loss of coordination, or diarrhea Nausea, vomiting, loss of appetite, weakness, or dizziness Inability to get or keep an erection Irregular menstruation Decreased sexual desire Seizures Extreme drowsiness Fainting Chest pain Hives; rash; itching; swelling of the eyes, face, mouth, lips or throat; hoarseness; difficulty breathing or swallowing Morphine may be habit forming, especially with prolonged use. Morphine may cause serious or life-threatening breathing problems, especially during the first 24 to 72 hours of your treatment and any time your dose is increased. Tramadol: Feeling sick Feeling dizzy Headaches Feeling sleepy, tired, dizzy or "spaced out" Feeling or being sick (nausea or vomiting) Constipation Dry mouth Sweating Low energy Feel dizzy, tired and have low energy – these can be a sign of low blood pressure Have hallucinations (seeing or hearing things that are not there) Feel confused Feel very sleepy Have trouble peeing or you cannot pee at all If you need to take it for a long time your body can become used to it (known as tolerance). That means you need higher doses to control your pain over time. Some people can become more sensitive to pain (hyperalgesia). It's possible to become addicted to tramadol. Prednisone: Aggression Agitation Blurred vision Decrease in the amount of urine Dizziness Fast, slow, pounding, or irregular heartbeat or pulse Headache Irritability Mood changes Noisy, rattling breathing Numbness or tingling in the arms or legs Pounding in the ears Swelling of the fingers, hands, feet, or lower legs Trouble thinking, speaking, or walking Trouble breathing Long-term effects: Problems with the eyes, such as glaucoma or cataracts. A round face, which is sometimes called a moon face. High blood sugar, which can trigger or worsen diabetes. Increased risk of infections, especially with common bacterial, viral and fungal microorganisms. Bone fractures and thinning bones, called osteoporosis. Muscle weakness. Thin skin, bruising and slower wound healing. Non-Steroidal Anti-Inflammatory Agents (NSAIDs): Gastrointestinal side effects such as indigestion, stomach upset (including nausea or feeling sick) or stomach pain are commonly caused by NSAIDs. Use of NSAIDs can also cause ulcers and bleeding in the stomach and other parts of the gastrointestinal tract (gut). Raised liver enzymes (detected by a blood test, this is more commonly associated with diclofenac than other NSAIDs) Diarrhoea Headache Dizziness Salt and fluid retention High blood pressure Ulcers of the oesophagus (food pipe) Rectal irritation (if suppositories are used) Heart failure Hyperkalaemia (high levels of potassium in the blood) Reduced kidney function Confusion Bronchospasm (difficulty breathing) Skin rash |
|
Muscle cramps |
Antispasmodics - work on the central nervous system to reduce muscle spasms and pain by blocking nerve signals or relaxing muscles: Carisoprodol Aspirin Chlorzoxazone Cyclobenzaprine Metaxalone Methocarbamol Orphenadrine Tizanidine Ibuprofen - reduces muscle cramp pain by decreasing inflammation and blocking COX enzyme. Acetaminophen - alters pain perception in the brain without reducing inflammation, providing muscle cramp relief. Naproxen - works similarly to Ibuprofen but has a longer-lasting effect in reducing inflammation and pain from muscle cramps. |
Antispasmodics: Heartburn. Constipation. Dry mouth. Difficulty passing urine. Ibuprofen: Headache Dizziness Drowsiness, fatigue and restless sleep Thirst and sweating Tingling or numbness in hands and feet Ringing in the ears Blurred vision and eye irritation Fluid retention and ankle swelling Mild allergic reaction Abdominal pain Nausea, vomiting Heartburn Diarrhoea Constipation Bladder irritation and pain, frequent urination Long term effects: Ibuprofen can cause ulcers in your stomach or gut, especially if you take it by mouth for a long time or in big doses. If you need to take it for a long time your doctor may also prescribe a medicine to help protect your stomach. Anaemia due to bleeding in the stomach Impaired hearing Kidney and liver damage Bleeding in the stomach and bowels Increased risk of heart attack Acetaminophen: Bloody or black, tarry stools Bloody or cloudy urine Fever with or without chills (not present before treatment and not caused by the condition being treated) Pain in the lower back and/or side (severe and/or sharp) Pinpoint red spots on the skin Skin rash, hives, or itching Sore throat (not present before treatment and not caused by the condition being treated) Sores, ulcers, or white spots on the lips or in the mouth Sudden decrease in the amount of urine Unusual bleeding or bruising Unusual tiredness or weakness Yellow eyes or skin Naproxen: Confusion Headache Ringing in the ears Changes in vision Feeling sleepy or tired Feeling dizzy Rashes Severe indigestion, heartburn, pains in your stomach, feeling or being sick (nausea or vomiting) or diarrhoea – these can be signs of an ulcer or swelling (inflammation) in your stomach or gut Vomiting blood or dark particles that look like coffee grounds, blood in your poo, or black poop that looks like tar – these could be signs of bleeding and perforation of your stomach or gut A frequent sore throat, nosebleeds and infections – these can be signs of problems with your blood cells, known as agranulocytosis feeling faint, tired or short of breath – these can be signs of anaemia Blood in your pee, passing less pee, feeling or being sick – these can be signs of kidney damage or infection A yellow colour to the whites of your eyes or your skin turns yellow, although this may be less obvious on brown or black skin – these can be signs of jaundice or inflammation of the liver Irregular, slow heartbeats – this can be a sign of high levels of potassium in the blood A high temperature, stomach pain and being sick – these can be signs of inflammation of the pancreas. |
|
Muscle weakness |
Corticosteroids - by reducing inflammation and immune system overactivity: Prednisone |
Prednisone: Aggression Agitation Blurred vision Decrease in the amount of urine Dizziness Fast, slow, pounding, or irregular heartbeat or pulse Headache Irritability Mood changes Noisy, rattling breathing Numbness or tingling in the arms or legs Pounding in the ears Swelling of the fingers, hands, feet, or lower legs Trouble thinking, speaking, or walking Trouble breathing Long-term effects: Problems with the eyes, such as glaucoma or cataracts. A round face, which is sometimes called a moon face. High blood sugar, which can trigger or worsen diabetes. Increased risk of infections, especially with common bacterial, viral and fungal microorganisms. Bone fractures and thinning bones, called osteoporosis. Thin skin, bruising and slower wound healing. |
|
Osteoporosis (bone weakening) |
Denosumab - a monoclonal antibody that inhibits RANKL, preventing bone resorption, thereby increasing bone density. Teriparatide - a synthetic form of parathyroid hormone (PTH) that stimulates osteoblasts (bone-forming cells that create and strengthen bones), promoting new bone formation. Abaloparatide - similar to teriparatide, it is a PTH-related peptide analog that stimulates bone-building osteoblasts (bone-forming cells that create and strengthen bones), increasing bone mass. Bisphosphonates - inhibit osteoclast (cells that break down bone to help maintain bone mass and quality)-mediated bone resorption, reducing bone loss, and improving bone strength: Alendronate Ibandronate Risedronate |
Denosumab: Muscle spasms/cramps Mental/mood changes (such as irritability or confusion) Numbness/tingling (especially around lips/mouth or in fingers/toes) Fast/irregular heartbeat Severe dizziness/fainting Seizures Fever/chills Red/swollen/tender/warm skin (with or without pus) Severe abdominal pain Ear pain/discharge Trouble hearing Frequent/painful/burning urination Pink/bloody urine Jaw pain New or unusual thigh/hip/groin pain Bone/joint/muscle pain May cause low calcium levels, especially if you have kidney problems. Denosumab can affect your immune system. You may be more likely to get a serious infection, such as a skin, ear, stomach/gut, or bladder infection. After your treatment with denosumab is stopped, you may be at increased risk for bone fracture, including bones in your spine. Can cause skin problems such as dryness, peeling, redness, itching, small bumps/patches, or blisters. Teriparatide: Pain Weakness Heartburn or sour stomach Leg cramps Dizziness Depression Redness, pain, swelling, bruising, a few drops of blood or itching at the injection site Back spasms Chest pain Fainting Difficulty breathing Nausea Vomiting Constipation Lack of energy Muscle weakness Purple net-like pattern, painful lumps, or sores on the skin Abaloparatide: Dizziness Sense of spinning Headache Tiredness Upper stomach pain or bloating Nausea Diarrhea Pain in joints Redness, pain, or swelling in the area where the medication was injected Signs of high blood calcium: nausea, vomiting, constipation, lack of energy, and muscle weakness Pain in the lower back or lower stomach Painful urination Blood in the urine Alendronate: Nausea Stomach pain Constipation Diarrhea Gas Bloating or fullness in the stomach Change in ability to taste food Headache Dizziness Swelling of the joints, hands, or legs Muscle spasms, twitches, or cramps New or worsening heartburn Difficulty swallowing Pain on swallowing Chest pain Bloody vomit or vomit that looks like coffee grounds Black, tarry, or bloody stools Fever Blisters or peeling skin Rash (may be made worse by sunlight) Itching Hives Swelling of eyes, face, lips, tongue, or throat Difficulty breathing Hoarseness Painful or swollen gums Loosening of the teeth Numbness or heavy feeling in the jaw Poor healing of the jaw Eye pain Dull, aching pain in the hips, groin, or thighs Taking a bisphosphonate medication such as alendronate for osteoporosis may increase the risk that you will break your thigh bone(s). You may feel pain in your hips, groin, or thighs for several weeks or months before the bone(s) break, and you may find that one or both of your thigh bones have broken even though you have not fallen or experienced other trauma. You should know that alendronate may cause severe bone, muscle, or joint pain. You should know that alendronate may cause osteonecrosis of the jaw (ONJ, a serious condition of the jaw bone), especially if you have dental surgery or treatment while you are taking the medication. Ibandronate: You should know that ibandronate may cause osteonecrosis of the jaw (ONJ, a serious condition of the jaw bone), especially if you have dental surgery or treatment while you are taking the medication. You should know that ibandronate may cause severe bone, muscle, or joint pain. Nausea Stomach pain Diarrhea Constipation Weakness Dizziness Headache Fever, sore throat, chills, cough, and other signs of infection Frequent or urgent need to urinate Painful urination New or worsening heartburn Difficulty swallowing Pain on swallowing Upper chest pain Rash Painful or swollen gums Loosening of the teeth Numbness or heavy feeling in the jaw Poor healing of the jaw Dull, aching pain in the hips, groin, or thighs Taking a bisphosphonate medication such as ibandronate for osteoporosis may increase the risk that you will break your thigh bone(s). You may feel pain in your hips, groin, or thighs for several weeks or months before the bone(s) break, and you may find that one or both of your thigh bones have broken even though you have not fallen or experienced other trauma. Risedronate: Constipation Diarrhoea Indigestion, bloating, stomach pain or wind Feeling sick (nausea) Headaches Mild muscle, bone or joint pain Heartburn (or heartburn that gets worse), or problems or pain when swallowing – these may be signs of ulcers in your food pipe. A loose tooth, mouth sores, or swelling or pain in your mouth or jaw Pain, weakness or discomfort in your thigh, hip or groin Severe pain in the joints, muscles or bones Ear pain, discharge from your ear or an ear infection – these can be signs of damage to the bones in your inner ear Black or red poo – these can be signs of an ulcer or bleeding from your gut Blurred vision, light sensitivity, or painful or red eyes – these can be signs of swelling of the eye Muscle cramps or spasms, a tingling sensation in your fingers or around your mouth – these can be symptoms of low calcium levels in your blood Taking risedronate for more than 2 years can increase their chances of getting a rare type of bone damage in their inner ear and certain types of breaks to their thigh bones. |
Modern drugs for Infectious Adverse Drug Reactions:
|
Adverse Drug Reactions |
Modern drugs |
Adverse Drug Reactions of these drugs |
|
Fever |
Acetaminophen - lowers body temperature by targeting the brain's heat-regulating center (hypothalamus), and increasing the body's pain threshold. Ibuprofen - reduces the body's ability to make prostaglandins, these are chemicals in the body that trigger the feeling of pain and produce inflammation. With fewer prostaglandins in the body, the pain, fever and inflammation decrease. Aspirin - inhibit an enzyme needed to make prostaglandins, natural chemicals in the body that produce pain, inflammation and fever. |
Acetaminophen: Bloody or black, tarry stools Bloody or cloudy urine Fever with or without chills (not present before treatment and not caused by the condition being treated) Pain in the lower back and/or side (severe and/or sharp) Pinpoint red spots on the skin Skin rash, hives, or itching Sore throat (not present before treatment and not caused by the condition being treated) Sores, ulcers, or white spots on the lips or in the mouth Sudden decrease in the amount of urine Unusual bleeding or bruising Unusual tiredness or weakness Yellow eyes or skin Ibuprofen: Headache Dizziness Drowsiness, fatigue and restless sleep Thirst and sweating Tingling or numbness in hands and feet Ringing in the ears Blurred vision and eye irritation Fluid retention and ankle swelling Mild allergic reaction Abdominal pain Nausea, vomiting Heartburn Diarrhoea Constipation Bladder irritation and pain, frequent urination Long term effects: Ibuprofen can cause ulcers in your stomach or gut, especially if you take it by mouth for a long time or in big doses. If you need to take it for a long time your doctor may also prescribe a medicine to help protect your stomach. Anaemia due to bleeding in the stomach Impaired hearing Kidney and liver damage Bleeding in the stomach and bowels Increased risk of heart attack Aspirin: Nausea Vomiting Stomach pain Heartburn Hives Rash Swelling of the eyes, face, lips, tongue, or throat Wheezing or difficulty breathing Hoarseness Fast heartbeat Fast breathing Cold, clammy skin Ringing in the ears Loss of hearing Bloody vomit Vomit that looks like coffee grounds Bright red blood in stools Black or tarry stools Aspirin can cause ulcers in your stomach or gut, especially if you take it for a long time or in big doses. |
Modern Drugs for Psychological Adverse Drug Reactions:
|
Adverse Drug Reactions |
Modern Drugs |
Adverse Drug Reactions of these drugs |
|
Anxiety |
Benzodiazepines - enhance the activity of the neurotransmitter gamma-aminobutyric acid (GABA), a chemical in the brain that helps you to feel calm: Clonazepam Alprazolam Lorazepam Bromazepam Oxazepam Chlordiazepoxide Clorazepate Diazepam |
Benzodiazepines: Dizziness Confusion Drowsiness Constipation Memory loss Slurred speech Muscle weakness Loss of coordination and balance Delusions Hallucinations Skin reactions Sudden anxiety Euphoria (a feeling of well-being) Restlessness and agitation Irritability and aggressiveness Physical dependence Problems learning or concentrating Developing tolerance means that, over time, you need more of the drug to get the same effect. |
|
Depression |
Antidepressants - increase neurotransmitters like serotonin and noradrenaline, these are chemicals in the brain which can improve mood and emotion: Fluoxetine Paroxetine Fluvoxamine Citalopram Escitalopram Sertraline Venlafaxine Duloxetine Levomilnacipran Desvenlafaxine |
Antidepressants: Decreased alertness Headaches Nausea (feeling sick) Sexual problems (e.g., delayed orgasm, inability to orgasm, reduced sexual desire) Tooth decay and oral health issues Risk of diabetes Gastrointestinal bleeding Serotonin syndrome Neuroleptic malignant syndrome (NMS) Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) Suicidal feelings Hypomania or mania Priapism (painful, prolonged erection) Memory problems and difficulty concentrating (linked to SIADH) Convulsions (fits) Agitation, confusion, and hallucinations Rapid heartbeat and high blood pressure Coma (loss of consciousness) Antimuscarinic Effects: Blurred vision Confusion and agitation Constipation (can become life-threatening) Difficulty urinating Drowsiness Dry mouth (long-term risk of tooth decay) Erectile dysfunction Hallucinations Hot or dry skin, and decreased sweating Increased pressure in the eye Low blood pressure (exacerbated by hot baths) Rapid heartbeat and disturbed heart rhythm Elderly people who take antidepressants, particularly those who take SSRIs, may experience a severe fall in sodium (salt) levels, known as hyponatraemia. This may lead to a build-up of fluid inside the cells of the body, which can be potentially dangerous. Long-term use of SSRIs and TCAs has been linked to an increased risk of developing type 2 diabetes, although it's not clear if the use of these antidepressants directly causes diabetes to develop. |
|
Difficulty sleeping (Insomnia) |
Benzodiazepines - enhance the activity of the neurotransmitter Gamma-aminobutyric acid (GABA), a chemical in the brain that helps you to feel calm. Lorazepam Nitrazepam Oxazepam Temazepam Triazolam Flurazepam |
Benzodiazepines: Dizziness Confusion Drowsiness Constipation Memory loss Slurred speech Muscle weakness Loss of coordination and balance Delusions Hallucinations Skin reactions Sudden anxiety Euphoria (a feeling of well-being) Restlessness and agitation Irritability and aggressiveness Physical dependence Problems learning or concentrating Developing tolerance means that, over time, you need more of the drug to get the same effect. |
2.3. Target Protein Identification
A comprehensive investigation into target proteins, which are specific proteins in the body that play a role in the development of adverse drug reactions (ADRs), was conducted through an extensive literature review using sources from the National Library of Medicine (NLM). Key biological molecules involved in the physiological processes that lead to adverse drug reactions caused by pharmaceutical treatments were identified. Understanding these target proteins is crucial for determining how certain drugs interact with the body at a molecular level, as well as identifying potential intervention points where alternative therapies, such as Ayurvedic herbs, may be effective in mitigating these ADRs.
The literature review focused on identifying proteins that serve as enzymes (catalysts that speed up chemical reactions in the body), receptors (proteins that receive and transmit biological signals), transporters (proteins that move molecules across cell membranes), and signaling molecules (proteins involved in communication between cells). These proteins were examined in relation to their involvement in drug-induced physiological responses, such as inflammation, neurochemical imbalances, gastrointestinal disturbances, cardiovascular complications, and immune system dysregulation. By systematically analyzing peer-reviewed research and pharmacological studies from the National Library of Medicine, a comprehensive understanding of the molecular pathways underlying adverse drug reactions of treatments for chronic diseases was developed.
2.3.1. Obtaining 3D Structures of Target Proteins for Comparative Molecular Docking Studies
In parallel to the identification of target proteins associated with adverse drug reactions of treatments for chronic diseases, the 3D structures of these target proteins were obtained from the AlphaFold Protein Structure Database in PDB (Protein Data Bank) format, a highly reliable resource for molecular docking studies. Molecular docking refers to a computational technique used to predict the interaction between a drug and its target protein. It simulates how small molecules (such as drug candidates) bind to a receptor or enzyme, providing insights into the molecular mechanisms underlying drug action and potential side effects. Obtaining an accurate 3D structure of the target protein is essential for comparing conventional and Ayurvedic medicines in molecular docking studies, as it provides a detailed foundation for understanding how both types of treatments interact with specific proteins in the body. By knowing the precise structure of the protein, researchers can simulate how conventional drugs and bioactive compounds from Ayurvedic herbs bind to the target, revealing their potential efficacy and mechanisms of action. This allows for a side-by-side comparison of how both conventional pharmaceuticals and Ayurvedic compounds may modulate the same protein targets, offering insights into their relative effectiveness, safety profiles, and potential for reducing adverse drug reactions. Without this 3D structural information, it would be difficult to assess and compare how these different therapeutic approaches affect the body at the molecular level.
2.4. Ayurveda-Based Herbal Alternatives for Adverse Drug Reactions Management
Once the list of adverse drug reactions of treatments for chronic diseases was finalized and the conventional drugs to address them were identified, the next step involved identifying Ayurvedic herbs that have traditionally been used to alleviate these adverse effects.
To systematically match Ayurvedic herbs to specific adverse drug reactions (ADRs), a multi-layered approach was employed, focusing on biochemical composition, pharmacological activity, and scientific validation, ensuring credibility and accuracy through the use of peer-reviewed pharmacological studies and clinical trials on medicinal plants were examined. This process ensured that the proposed herbal interventions were grounded in both traditional knowledge and modern research, allowing for a more integrative approach to adverse drug reactions of treatments for chronic diseases management. The selection of herbs was based on the following criteria:
- Traditional usage: To establish a historical foundation for the selection of Ayurvedic herbs in mitigating adverse drug reactions (ADRs) of treatments for chronic diseases, an extensive review of classical Ayurvedic texts, ethnobotanical records, and traditional healing practices was conducted. This process involved systematically examining ancient compendiums such as the Charaka Samhita, Sushruta Samhita, and Bhavaprakasha Nighantu, which document the medicinal applications of herbs, including their role in counteracting the side effects of long-term treatments.
- Phytochemical composition: A key factor in identifying the right Ayurvedic herbs for adverse drug reactions was analyzing their bioactive compounds, which are naturally occurring chemical substances responsible for the plant’s medicinal effects. Each herb contains a distinct combination of phytochemicals, which are plant-derived compounds with therapeutic properties, such as flavonoids, alkaloids, tannins, glycosides, saponins, and polyphenols. These compounds interact with biological pathways in ways that help counteract or alleviate the adverse drug reactions of treatments for chronic diseases. For example, Ajwain (Carum copticum) contains thymol, a bioactive compound with carminative properties, meaning it helps relieve gas and bloating by stimulating digestion and reducing intestinal discomfort. By examining the specific bioactive molecules present in each herb, their medicinal effects on the body could be mapped, providing a strong foundation for their use in alleviating the adverse drug reactions of treatments for chronic diseases.
- Mechanism of action: Beyond identifying the chemical composition of these herbs, it was also essential to understand how they interact with the body at a molecular level. Adverse drug reactions of treatments for chronic diseases often occur when a treatment affects the normal functioning of enzymes (proteins that speed up chemical reactions in the body), receptors (proteins on the surface of cells that receive signals and trigger biological responses), or inflammatory mediators (substances in the body that regulate inflammation and immune responses). Each herb was matched to an adverse drug reaction based on its ability to restore balance to these systems and counteract unwanted drug effects. For example, many nonsteroidal anti-inflammatory drugs (NSAIDs) cause gastrointestinal irritation by inhibiting cyclooxygenase-1 (COX-1), an enzyme that protects the stomach lining. However, NSAIDs also reduce inflammation by blocking COX-2, another enzyme responsible for pain and swelling. Certain Ayurvedic herbs, such as Shallaki (Boswellia serrata) and Haridra (Curcuma longa, or turmeric), act as natural COX-2 inhibitors, meaning they reduce inflammation without harming the stomach, making them safer alternatives for managing pain and swelling. By understanding these biological interactions, ayurvedic herbs could be scientifically matched to adverse drug reactions of treatments for chronic diseases in a way that aligns both with traditional healing principles and modern pharmacology.
- Scientific validation: To validate the effectiveness of Ayurvedic herbs in treating adverse drug reactions of treatments for chronic diseases, extensive scientific research was reviewed. This included clinical trials, laboratory experiments, and pharmacological studies that examined how these herbs function within the body. Sources such as PubMed, the National Institutes of Health (NIH), and the AYUSH Ministry (India) provided a wealth of peer-reviewed studies supporting the medicinal properties of various Ayurvedic herbs.
2.4.1. Identification of Bioactive Compounds
This study employs a component-based research approach, which focuses on analyzing individual bioactive compounds found in ayurvedic herbs. Bioactive compounds are naturally occurring chemical substances in plants that are responsible for their medicinal effects. Rather than evaluating Ayurvedic herbs as whole entities, this method systematically breaks them down into their chemical components, their bioactive compounds, to determine which specific molecules contribute to their therapeutic properties. By adopting this approach, the study aims to establish a direct connection between these bioactive compounds and their role in mitigating adverse drug reactions (ADRs).
To achieve this, detailed information on the bioactive compounds present in the previously selected Ayurvedic herbs was systematically collected. The herbs were chosen based on their historical use in Ayurveda for addressing symptoms comparable to modern adverse drug reactions (ADRs), as well as their documented efficacy in scientific literature. The primary sources for data collection were the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database and Dr. Duke’s Phytochemical and Ethnobotanical Database, both of which are widely recognized and reputable repositories for medicinal plant research.
The Traditional Chinese Medicine Systems Pharmacology (TCMSP) database is an extensive online resource that provides comprehensive information on the chemical makeup of medicinal plants, as well as how effectively a compound is processed and utilized by the human body. In addition, the database identifies the biological targets of these compounds, helping to determine their interactions with enzymes (proteins that accelerate chemical reactions in the body), receptors (proteins on the surface of cells that receive chemical signals and trigger biological responses), and signaling pathways (the series of chemical processes by which cells communicate with one another to regulate bodily functions). By leveraging the TCMSP database, detailed data was extracted on each compound’s molecular structure, drug-likeness, and known biological effects.
To ensure accuracy and comprehensiveness, the information obtained from Traditional Chinese Medicine Systems Pharmacology (TCMSP) database was cross-verified using Dr. Duke’s Phytochemical and Ethnobotanical Database, a well-established resource developed by the United States Department of Agriculture (USDA). This database contains an extensive catalog of phytochemicals (plant-derived chemical compounds), their traditional medicinal applications, and their scientifically documented physiological effects. By comparing the data from both databases, the study ensured consistency in the reported medicinal properties of each compound, thereby strengthening the reliability of its findings.
Through this rigorous methodology, a structured and scientifically validated dataset of bioactive compounds present in Ayurvedic herbs was developed. Each compounds’ therapeutic effects (the specific benefits it provides in addressing adverse drug reactions (ADRs), mechanisms of action (the biological processes through which it produces its effects), and potential interactions with human biological pathways (its influence on critical bodily functions such as digestion, inflammation, and neurotransmitter activity), were researched, facilitating a deeper understanding of how natural bioactive compounds found in ayurvedic herbs play a key role in adverse drug reactions (ADRs) management.
2.4.2. Below is an attached table, consisting of information about target proteins and ayurvedic alternatives for adverse drug reactions (combination of part 2.3. - 2.4.).
|
Adverse Drug Reactions of Chronic Disease Treatments |
Target Protein Involved |
Ayurveda Herb Traditionally Used |
|
Abdominal Pain |
Cyclooxygenase-2 (COX-2) is an enzyme that produces prostaglandins, which mediate inflammation and pain. Inhibiting COX-2 can reduce inflammation and alleviate pain. |
Triphala’s anti-inflammatory and antioxidant properties help reduce gastrointestinal inflammation. Additionally, Triphala exhibits mild laxative effects, promoting regular bowel movements and relieving constipation-related discomfort. These combined actions contribute to its effectiveness in managing abdominal pain. |
|
Bloating |
Guanylate cyclase-C (GC-C) is an enzyme in the intestinal lining that regulates fluid secretion and gut motility. Activation of GC-C increases fluid secretion into the intestines, which can alleviate constipation and reduce bloating. |
Ajwain (Carum copticum), commonly known as carom seeds, has been traditionally used to alleviate bloating and other gastrointestinal discomforts, because of its active compounds, such as thymol, which possess carminative properties that help reduce gas formation and promote digestion. Additionally, ajwain has demonstrated antimicrobial and anti-inflammatory effects, which can further aid in relieving bloating and supporting overall digestive health. |
|
Constipation |
Guanylate cyclase-C (GC-C) is an enzyme in the intestinal lining that regulates fluid secretion and motility. Reduced GC-C activity leads to decreased chloride and water secretion into the intestines, disrupting fluid balance and resulting in harder, drier stools. This contributes to constipation by impairing intestinal motility and stool softening. |
Triphala stimulates peristalsis, the series of wave-like muscular contractions that move food through the intestines. Also its natural laxative properties help promote regular bowel movements and alleviate discomfort, contributing to relieving constipation. |
|
Decreased Appetite |
The ghrelin receptor (GHS-R1a) is primarily involved in stimulating hunger when activated by the hormone ghrelin, which signals to the brain to increase appetite. Decreased GHS-R1a activity can reduce appetite, as the signal to eat is weakened. |
Chitrak (Plumbago zeylanica) is an Ayurvedic herb traditionally used to stimulate appetite.It is believed to work through its carminative and digestive properties, which help increase gastric acid secretion and improve digestion. Notably, its active compound, plumbagin, is believed to enhance digestive fire by increasing gastric acid secretion, thereby improving digestion and promoting hunger. |
|
Diarrhea |
The 5-HT₃ receptor is a serotonin receptor subtype that plays a significant role in gastrointestinal function. Overactivation of this receptor can lead to increased gut motility and secretion, contributing to diarrhea. Conversely, inhibiting this receptor, reduces motility and secretion, which can help alleviate diarrhea. |
Bael (Aegle marmelos) treats diarrhea due to its antimicrobial properties, attributed to compounds like aegeline, which help combat harmful bacteria and viruses. Its astringent effects come from ellagic acid and gallic acid, specific tannins that tighten the intestinal walls and reduce fluid loss. Additionally, rutin and quercetin, specific flavonoids in bael, possess anti-inflammatory properties, soothing gut irritation and restoring digestive balance. |
|
Heartburn |
The H⁺/K⁺ ATPase, also known as the gastric proton pump, is an enzyme located in the stomach's parietal cells responsible for secreting gastric acid. It functions by exchanging hydrogen ions (H⁺) from the cytoplasm with potassium ions (K⁺) from the stomach lumen, leading to increased stomach acidity. Overactivity of this enzyme can result in excessive acid production, contributing to conditions like heartburn. Inhibiting the H⁺/K⁺ ATPase, effectively reducing acid secretion and alleviating heartburn symptoms. |
Amla (Phyllanthus emblica) helps treat heartburn by reducing stomach acid production and protecting the stomach lining. It contains tannins and flavonoids, which have anti-inflammatory effects that soothe irritation in the esophagus. Amla is also rich in Vitamin C, which acts as an antioxidant to reduce oxidative stress that can worsen acid reflux. Studies suggest that Amla can improve symptoms of acid reflux like heartburn and regurgitation. |
|
Cough |
The TRPV1 (Transient Receptor Potential Vanilloid 1) channel plays a key role in chronic cough by increasing sensitivity to irritants in the airway. It is activated by heat, acid, and inflammatory mediators, leading to an exaggerated cough reflex. In chronic cough conditions, TRPV1 is often overexpressed or hypersensitive, making the airway more reactive to stimuli like cold air, smoke, or capsaicin. This contributes to persistent coughing even in the absence of infection. |
Tulsi (Ocimum sanctum), commonly known as holy basil, is traditionally used to treat coughs due to its antimicrobial, anti-inflammatory, and antitussive (cough-suppressing) properties. These properties help reduce inflammation in the respiratory tract, combat respiratory infections, and suppress the cough reflex. |
|
Difficulty breathing |
The Beta-2 Adrenergic Receptor (β₂AR) plays a crucial role in relaxing airway muscles. It is found on smooth muscle cells in the lungs, and when activated, it relaxes the airway muscles, making breathing easier. Reduced β₂AR activity can lead to airway constriction, causing difficulty breathing, wheezing, and shortness of breath. |
Pippali (Piper longum) helps treat difficulty breathing by acting as a bronchodilator (a substance that relaxes airway muscles to widen the airways and improve airflow). It also has decongestant (a substance that reduces nasal and airway congestion) and expectorant (a substance that helps clear mucus from the respiratory tract) properties, making breathing easier. |
|
Pneumonitis |
The TNF-α receptor contributes to pneumonitis by promoting lung inflammation and immune activation. When TNF-α binds to its receptors (TNFR1 and TNFR2) on lung cells, it triggers the release of more inflammatory molecules (cytokines) and attracts immune cells to the lungs. This leads to swelling, fluid buildup, and lung tissue damage, making it harder to breathe. In conditions like radiation pneumonitis, drug-induced pneumonitis, and autoimmune lung diseases, TNF-α and its receptors are often overactive, worsening inflammation and lung injury. |
Haridra (Curcuma longa), commonly known as turmeric, is traditionally used in Ayurvedic medicine to manage respiratory conditions like pneumonitis due to its potent anti-inflammatory and antioxidant properties. The active compound curcumin in Haridra helps reduce lung inflammation and oxidative stress, which are key factors in pneumonitis. |
|
Angioedema (swollen tissue) |
The bradykinin B2 receptor increases vascular permeability (the ability of blood vessel walls to allow fluids and molecules to pass through) when activated by bradykinin, making blood vessels more leaky. This causes fluid to leak into surrounding tissues, leading to swelling (angioedema), especially in the skin and mucous membranes (the moist tissue lining body cavities such as the mouth and nose). Excessive activation of this receptor results in more severe swelling. |
Turmeric, particularly its active compound curcumin, is used to treat angioedema due to its anti-inflammatory and antioxidant properties. Curcumin can help reduce inflammation by inhibiting the bradykinin receptor, which is responsible for increased blood vessel permeability and swelling. |
|
Arrhythmia (irregular heartbeat) |
The Nav1.5 sodium channel, encoded by the SCN5A gene (Sodium Voltage-Gated Channel Alpha Subunit 5), is essential for initiating and conducting electrical impulses in the heart. Mutations or dysfunctions in Nav1.5 can disrupt these electrical signals, leading to various arrhythmias. |
Arjuna (Terminalia arjuna) has been traditionally used in Ayurvedic medicine to manage cardiovascular conditions, including arrhythmias (irregular heartbeats). Its bark contains bioactive compounds such as triterpenoids and flavonoids, which exhibit antioxidant and anti-inflammatory properties. These compounds help protect the heart muscle from oxidative stress and support overall cardiac function. Additionally, studies suggest that Arjuna may have a hypotensive effect, helping to lower blood pressure, which can be beneficial in managing certain types of arrhythmias. |
|
Increased risk of blood clots |
Factor Xa is a key enzyme in the blood coagulation cascade (a series of steps leading to blood clot formation). It converts prothrombin into thrombin, which then helps form fibrin, the main structural component of blood clots. When Factor Xa activity is too high, it accelerates clot formation, increasing the risk of thrombosis (dangerous blood clots), which can lead to conditions like deep vein thrombosis (DVT), pulmonary embolism, or stroke. |
Bibhitaki (Terminalia bellirica) may help reduce blood clot risk by exhibiting anticoagulant (blood-thinning) and anti-platelet properties (reduces the clumping of platelets, which are blood cells that help form clots). Its bioactive compounds, such as ellagic acid and tannins, help regulate blood coagulation (the process by which blood forms clots to stop bleeding) and reduce lipid buildup (accumulation of fats in blood vessels that can contribute to clot formation). |
|
Chest pain |
Troponin I plays a critical role in chest pain as it is a marker of heart muscle damage. When the heart muscle is injured, such as during a heart attack, troponin I is released into the bloodstream. This elevation in troponin levels is directly associated with cardiac injury and helps clinicians assess the severity of the damage and confirm whether chest pain is due to a heart attack or other cardiac issues. |
Arjuna (Terminalia arjuna) may help alleviate chest pain by improving heart function, reducing symptoms of angina (chest pain or discomfort that occurs when part of your heart muscle does not get enough oxygen-rich blood), and supporting healthy blood flow. Its bioactive compounds, such as flavonoids and tannins, help strengthen the heart, reduce cholesterol, and promote overall cardiovascular health. |
|
Rapid heartbeat (tachycardia) |
The β1-adrenergic receptor plays a key role in regulating heart rate. Overactivation of β1 receptors, due to stress, stimulants, or medical conditions, can result in sustained tachycardia, which may strain the heart. |
Arjuna (Terminalia arjuna) may help manage rapid heartbeat (tachycardia) by acting as a natural cardio-protective agent (a substance that supports heart health and protects against heart disease). Its bioactive compounds, such as flavonoids, tannins, and saponins, help strengthen the heart muscle, regulate blood pressure, and promote beta-blocking effects (reducing the activation of β1-adrenergic receptors, which slows heart rate and decreases heart workload). Additionally, its antioxidant and anti-inflammatory properties support overall heart function and reduce cardiac stress. |
|
High blood pressure (Hypertension) |
Angiotensin-converting enzyme (ACE) plays a key role in regulating blood pressure by converting angiotensin I into angiotensin II, a potent vasoconstrictor (a substance that narrows blood vessels). Increased ACE activity leads to higher levels of angiotensin II, causing blood vessels to constrict and raising blood pressure (hypertension). |
Arjuna (Terminalia arjuna) may help manage high blood pressure (hypertension) by acting as a natural ACE inhibitor (reducing angiotensin-converting enzyme activity to lower blood pressure). Its bioactive compounds, such as flavonoids and tannins, promote vasodilation (widening of blood vessels), improving blood flow and reducing strain on the heart. Additionally, Arjuna's antioxidant and diuretic properties (helping remove excess sodium and water) further support blood pressure regulation. |
|
Low blood pressure (Hypotension) |
The Alpha-1 Adrenergic Receptor plays a key role in maintaining blood pressure by causing vasoconstriction (narrowing of blood vessels) when activated. If alpha-1 receptor activity is reduced or blocked, blood vessels remain dilated (widened), leading to low blood pressure (hypotension) due to decreased vascular resistance and reduced blood flow to vital organs. |
Ashwagandha (Withania somnifera) may help manage low blood pressure (hypotension) by supporting the autonomic nervous system (the part of the nervous system that controls involuntary functions like heart rate and blood pressure) and enhancing alpha-1 adrenergic receptor activity (which helps constrict blood vessels to stabilize blood pressure). Its withanolides (bioactive compounds with anti-stress and neuroprotective effects) and alkaloids contribute to this effect by promoting vasoconstriction (narrowing of blood vessels) and improving circulation. Additionally, its adaptogenic properties (helping the body resist stress) regulate cortisol and prevent sudden drops in blood pressure. |
|
Low white cell count (leukopenia) |
Granulocyte colony-stimulating factor (G-CSF) is a growth factor that stimulates the production, survival, and activation of white blood cells (granulocytes) in the bone marrow. When G-CSF levels are low, the body produces fewer white blood cells, leading to low white cell count (leukopenia), which weakens the immune system and increases infection risk. |
Guduchi (Tinospora cordifolia) may help treat low white cell count (leukopenia) by stimulating the immune system and promoting white blood cell production. Its bioactive compounds, such as alkaloids, flavonoids, and polysaccharides, enhance Granulocyte colony-stimulating factor (G-CSF) activity (which stimulates the production of white blood cells in the bone marrow). Additionally, Guduchi’s immunomodulatory properties (ability to regulate immune function) help improve overall immunity and protect against infections. |
|
Blurred Vision |
Vascular endothelial growth factor (VEGF) plays a key role in abnormal blood vessel growth (neovascularization – the formation of new, fragile blood vessels) and increased vascular permeability (leakiness of blood vessels, allowing fluid to escape into surrounding tissues) in the retina. Excess VEGF activity can lead to fluid leakage and swelling (edema – accumulation of excess fluid) in the retina, resulting in blurred vision. |
Amla (Phyllanthus emblica) may help treat blurred vision due to its antioxidant properties (protecting cells from damage) and anti-inflammatory effects. Amla is rich in vitamin C, which supports vascular health and may help regulate VEGF activity, potentially reducing fluid leakage in the retina. Additionally, its antioxidant-rich nature helps protect the retina from oxidative stress, improving overall eye health. |
|
Fatigue |
The A2A adenosine receptor plays a key role in regulating energy metabolism and promoting sleep-wake cycles. When adenosine binds to the A2A receptor, it typically induces relaxation and fatigue by promoting sleepiness and reducing alertness. During the day, adenosine levels build up, and increased activation of A2A receptors can contribute to feelings of tiredness and fatigue. This process is naturally reversed during sleep, when adenosine levels decrease. Overactivation of A2A receptors can contribute to persistent feelings of fatigue. |
Ashwagandha (Withania somnifera) is often used to treat fatigue due to its adaptogenic properties (ability to help the body manage stress). It can regulate the HPA axis (hypothalamic-pituitary-adrenal axis), which controls the body's response to stress, thereby reducing cortisol (stress hormone) levels and preventing the energy-depleting effects of chronic stress. Additionally, withanolides, the active compounds in ashwagandha, have been shown to enhance energy levels, improve physical endurance, and promote restful sleep, helping alleviate fatigue and restore vitality. |
|
Hair loss |
The 5-alpha reductase enzyme is responsible for converting testosterone (a hormone that plays a key role in male and female sexual development) into dihydrotestosterone (DHT) (a more potent form of testosterone). DHT binds to androgen receptors (proteins on the surface of cells that interact with hormones like testosterone) in hair follicles, particularly in the scalp, leading to the miniaturization (shrinking) of hair follicles. This process shortens the hair growth phase and causes hair thinning, contributing to male pattern baldness (androgenic alopecia) and female pattern hair loss. Reducing 5-alpha reductase activity can lower DHT levels, potentially preventing or slowing hair loss. |
Bhringraj (Eclipta alba) is used to treat hair loss because it contains bioactive compounds such as lactones, flavonoids, and alkaloids, which may inhibit 5-alpha reductase (an enzyme that converts testosterone into dihydrotestosterone (DHT), a hormone that contributes to hair follicle miniaturization and hair loss). By lowering DHT levels in the scalp, Bhringraj can help prevent or slow down hair loss, particularly in cases of androgenic alopecia (a type of hair loss caused by hormonal imbalances). Additionally, Bhringraj improves scalp circulation (blood flow to the scalp), providing nourishment to hair follicles, and its anti-inflammatory (reduces inflammation) and antioxidant (protects cells from oxidative damage) properties further support healthy hair growth. |
|
Joint pain |
Tumor Necrosis Factor-alpha (TNF-α) is a pro-inflammatory cytokine (a signaling protein that promotes inflammation) that plays a major role in joint pain by contributing to inflammation, swelling, and cartilage damage (damage to the smooth tissue that protects and cushions the body's joints). TNF-α binds to its receptors on joint tissues, triggering the release of other inflammatory mediators, which lead to pain, stiffness, and joint degradation over time. Blocking TNF-α activity (such as with TNF inhibitors) can help reduce joint inflammation and pain. |
Shallaki (Boswellia serrata) is used to treat joint pain because it contains boswellic acids (bioactive compounds) that act as anti-inflammatory agents. These compounds help inhibit TNF-α (Tumor Necrosis Factor-alpha), which is responsible for triggering inflammation, swelling, and cartilage breakdown. |
|
Muscle cramps |
Ryanodine receptor 1 (RyR1) is a protein in muscle cells that helps control calcium levels, which are needed for muscles to contract and relax. If RyR1 doesn't work properly due to genetic mutations or damage, calcium can leak out uncontrollably, causing muscle cramps, stiffness, and pain. This calcium leak makes muscles overactive and tired, leading to cramping. Proper RyR1 function is essential for maintaining normal muscle contraction and relaxation cycles, and its impairment is directly linked to muscle cramps and related symptoms. |
Nirgundi (Vitex negundo), commonly known as the five-leaved chaste tree, is utilized in Ayurvedic medicine to alleviate muscle cramps due to its potent anti-inflammatory and analgesic (pain-relieving) properties. The plant contains bioactive compounds that help reduce inflammation and pain by inhibiting the production of certain mediators responsible for inducing inflammation. This action helps in relaxing muscle spasms and providing relief from cramps. Additionally, Nirgundi's muscle relaxant effects contribute to easing muscle stiffness and discomfort. It is often applied topically as an oil or consumed in various forms to manage musculoskeletal pain and inflammation. |
|
Muscle weakness |
Dystrophin is a key protein that helps maintain the strength and stability of muscle cells by linking the internal muscle structure to the surrounding support system. This connection protects muscle fibers from damage during movement. When dystrophin is absent or deficient, muscle cells become weak and easily damaged, leading to progressive muscle weakness and degeneration. Without dystrophin, muscle fibers break down more quickly, triggering inflammation and scarring (fibrosis), which further reduces muscle function. Over time, this results in severe mobility issues, making dystrophin essential for maintaining healthy muscle function. |
Ashwagandha (Withania somnifera) is used in Ayurvedic medicine to help manage muscle weakness, due to its rejuvenating and regenerative properties. It supports muscle strength and mass, potentially alleviating weakness and fatigue commonly seen in these conditions. Studies have demonstrated that Ashwagandha supplementation can enhance muscle mass and strength, especially when combined with resistance training |
|
Osteoporosis (bone weakening) |
RANKL (Receptor Activator of Nuclear Factor Kappa-Β Ligand) plays a key role in the regulation of bone resorption, a process in which bone tissue is broken down. RANKL binds to its receptor RANK on osteoclasts (cells responsible for bone resorption), promoting their formation, activation, and survival. This leads to an increase in bone breakdown. In conditions like osteoporosis, an imbalance occurs where bone resorption outpaces bone formation, leading to weakened bones that are more prone to fractures. Elevated levels of RANKL contribute to this imbalance, making it a critical factor in the development of osteoporosis. Therefore, targeting RANKL to inhibit its action can be an effective strategy for preventing or treating osteoporosis by reducing excessive bone resorption. |
Hadjod (Cissus quadrangularis) is used in traditional medicine to support bone health and manage osteoporosis (a condition where bones become weak and brittle, making them more prone to fractures) due to its regenerative properties. Research shows that Hadjod inhibits RANKL-induced osteoclastogenesis (the process where RANKL—a protein—stimulates the formation of osteoclasts—cells that break down bone), reducing bone resorption (bone breakdown) and enhancing bone density (the amount of mineral in bones, which contributes to bone strength). Additionally, Hadjod accelerates fracture healing (the process of bone repair after a break) by promoting a balance between bone restoration and formation, leading to increased bone mineral density (the concentration of minerals in the bone) and strength. These effects make Hadjod valuable in managing osteoporosis, aiding in bone strengthening and fracture recovery. |
|
Fever |
Cyclooxygenase-2 (COX-2) is an enzyme that plays a critical role in inflammation and fever regulation. When the body experiences an infection or injury, COX-2 is activated and promotes the production of prostaglandins (lipid compounds). Specifically, prostaglandin E2 (PGE2), which is produced through COX-2 activity, acts on the hypothalamus (the part of the brain that controls temperature regulation), causing it to raise the body's set point temperature. This results in fever, which is a common response to infections and inflammation. By inhibiting COX-2, the production of PGE2 is reduced, which can help lower fever. Therefore, COX-2 inhibitors are often used to manage fever and related inflammatory conditions. |
Giloy (Tinospora cordifolia) is a plant used in traditional medicine to help reduce fever. It has antipyretic (fever-reducing) properties, meaning it can lower body temperature when it's too high. Giloy works by boosting the body's immune system (the body's defense against illness), helping it fight infections that cause fever. It also has anti-inflammatory (reducing swelling) and antioxidant (protecting cells from damage) effects, which can help reduce the symptoms of fever. In summary, Giloy is used to treat fever because it helps the body fight infections, reduces swelling, and protects cells from damage. |
|
Anxiety |
The serotonin 5-HT1A receptor is a protein in the brain that binds to the neurotransmitter serotonin (a chemical messenger involved in mood regulation). This receptor plays a significant role in anxiety (feelings of worry or fear). Research indicates that lower levels of 5-HT1A receptor activity are associated with increased anxiety. Conversely, activating these receptors can produce anxiolytic (anxiety-reducing) effects. This is why some antidepressant (mood-lifting) and anxiolytic medications target the 5-HT1A receptor to help alleviate anxiety symptoms. |
Ashwagandha (Withania somnifera) is an adaptogen (a natural substance that helps the body manage stress) traditionally used in Ayurveda to treat anxiety (feelings of worry or fear). It works by influencing gamma-aminobutyric acid (GABA) (a neurotransmitter that promotes calmness) and serotonin (a neurotransmitter that regulates mood), helping to reduce excessive nerve activity linked to anxiety. Additionally, it lowers cortisol (a stress hormone) levels, which helps control the body's stress response. Clinical studies suggest that ashwagandha supplementation can significantly reduce anxiety and perceived stress, making it a natural and effective option for anxiety relief. |
|
Depression |
The serotonin 5-HT1A receptor plays a key role in depression (a mood disorder characterized by persistent sadness and loss of interest) by regulating serotonin (a neurotransmitter that affects mood, emotions, and stress). When 5-HT1A receptor activity is low, serotonin signaling decreases, which can lead to depressive symptoms. Activation of this receptor enhances serotonin transmission, promoting feelings of well-being and reducing stress. Many antidepressants (medications that treat depression) work by stimulating the 5-HT1A receptor to improve mood and emotional stability. |
Ashwagandha (Withania somnifera) is an adaptogen (a natural substance that helps the body manage stress) traditionally used in Ayurveda to treat anxiety (feelings of worry or fear). It works by influencing gamma-aminobutyric acid (GABA) (a neurotransmitter that promotes calmness) and serotonin (a neurotransmitter that regulates mood), helping to reduce excessive nerve activity linked to anxiety. Additionally, it lowers cortisol (a stress hormone) levels, which helps control the body's stress response. Clinical studies suggest that ashwagandha supplementation significantly reduces symptoms of depression and stress, making it a promising natural remedy for mental well-being |
|
Difficulty sleeping (Insomnia) |
The GABA(A) receptor is integral to sleep regulation. Activation of these receptors by gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the central nervous system, promotes sleep by reducing neuronal excitability. Insomnia, characterized by difficulty falling or staying asleep, has been linked to impaired GABAergic function. Studies have shown that individuals with insomnia may exhibit decreased expression of GABA(A) receptor, leading to reduced inhibitory signaling and heightened arousal. Consequently, many sleep aids, such as benzodiazepines and certain hypnotics, target GABA(A) receptors to enhance inhibitory effects and facilitate sleep onset and maintenance. |
Tagara (Valeriana wallichii), commonly known as Indian valerian, is traditionally used in Ayurvedic medicine to treat insomnia (difficulty sleeping). Its effectiveness is attributed to its sedative (calming) properties, primarily due to compounds like valepotriates and valerenic acid, which interact with the GABA(A) receptor in the brain. Activation of these receptors enhances the inhibitory effects of gamma-aminobutyric acid (GABA), a neurotransmitter that reduces neuronal excitability, thereby promoting relaxation and facilitating sleep. Clinical studies have demonstrated that Tagara significantly improves sleep initiation, duration, and quality, making it a valuable natural remedy for managing insomnia. |
2.5. Ligand Preparation - Retrieval of 3D Structures of Bioactive Compounds and Pharmaceutical Drugs
After identifying the bioactive compounds through component-based research, and conventional medicine for adverse drug reactions, the next step focused on preparing ligands, which are molecules that can attach to specific target proteins in the body and influence biological processes. In simpler terms, ligands act like keys that fit into particular locks (proteins), triggering certain effects within cells.
Since this is a comparative study, meaning it is comparing two methods, these ligands are the active chemical constituents or called bioactive compounds found in Ayurvedic herbs, and the common drugs used to treat adverse drug reactions. These natural compounds would be computationally analyzed to see how they interact with proteins that are linked to adverse drug reactions (ADRs), the unwanted side effects caused by treatments for chronic diseases.
To facilitate computational analysis, the ligands, which in this case are the three-dimensional (3D) molecular structures of bioactive compounds and pharmaceutical drugs, were retrieved from PubChem, a widely recognized and freely accessible database maintained by the National Center for Biotechnology Information (NCBI). PubChem is an extensive repository of small molecules, offering comprehensive data on molecular weight, structural properties, and chemical composition of compounds. By obtaining structural data from this authoritative source, defined as a trusted, credible, and well-recognized source of information that is widely accepted by experts in a particular field, the study ensured that each compound was accurately represented, which is crucial for understanding how it interacts with proteins at an atomic level.
By computationally studying and comparing how well conventional drugs bind to target proteins compared to how well bioactive compounds bind to and affect target proteins, insights into how these bioactive compounds influence biological pathways, the series of chemical reactions that take place in the body to maintain health, can be gained. If an herbal compound can modulate (adjust or regulate) a pathway in a beneficial way, better than conventional drugs, it could help counteract the adverse drug reactions.
2.6. Ligand Preparation - Energy Minimization of 3D Structures of The Bioactive Compounds Retrieved
Energy minimization is especially important for bioactive compounds from Ayurvedic herbs compared to conventional drugs, because drugs are typically synthesized and often pre-optimized. In the case of conventional drugs, the compounds are often designed and synthesized in a laboratory with a specific focus on achieving a stable, low-energy structure before they're tested for biological activity. These drugs are usually optimized using well-established methodologies and computational tools for energy minimization. This means their 3D structures are already considered to be in their most stable, biologically relevant form when tested. However, bioactive compounds from Ayurvedic herbs are usually complex, natural products that haven't been synthesized or specifically designed for therapeutic purposes. These compounds often come in raw, unoptimized forms, and their 3D structures can be far more varied and flexible. Natural compounds from plants like those used in Ayurveda might not always adopt the most energetically favorable conformations in their initial state because they are derived from biological systems that don’t necessarily prioritize drug-like properties (such as stability or ease of binding to a specific target). Energy minimization, in this case, is essential because it helps refine these naturally occurring compounds into their most stable and biologically relevant 3D structures. Therefore, energy minimization is especially important for these compounds because they are often not as pre-optimized as synthetic, conventional drugs.
2.6.1. Importance of Energy Minimization
Energy minimization is a critical process in molecular modeling that follows the thermodynamic principle of reaching a state of minimum energy. In real-life systems, molecules naturally tend to evolve from a high-energy conformation to eventually the lowest-energy state, seeking stability. In thermodynamics, this concept is closely related to a system moving toward equilibrium, where all forces within the system are balanced. At equilibrium, there is no net change in energy, meaning energy is neither increasing nor decreasing, resulting in a stable state. In simple terms, the system settles into a condition where its energy is as low as possible, and no further energy transitions occur unless external factors, such as temperature or pressure change.
In the context of specifically molecular systems, reaching the lowest energy state requires the reduction of the molecule’s potential energy. A molecule’s potential energy refers to the stored energy within a molecule, which arises from the forces acting within and between its atoms. These forces include bond stretching (how far atoms are from each other within a bond), van der Waals forces (weak attractions between atoms or molecules), and electrostatic interactions (attractions or repulsions between charged regions of molecules). When these forces are unbalanced, the molecule has higher potential energy and is less stable, then according to the thermodynamic principle of reaching a state of minimum energy, these forces are naturally balanced, moving towards the equilibrium, lowering their potential energy state which represents a more stable and favorable conformation.
Unlike in real-world environments, such as within cells or during drug interactions, where molecules naturally adopt low-energy conformations that maximize stability and minimize unfavorable interactions, the 3D structure of molecules obtained from databases are unstable or unrealistic in a high-energy state. If it remains in a high-energy state, it may not accurately represent how the molecule would behave under physiological conditions, leading to unreliable predictions in molecular docking. Low-energy conformations are better because they represent the most stable, realistic forms that molecules naturally adopt in real-life biological systems.
Therefore, in computational molecular simulations, the process of energy minimization is performed, using algorithms that systematically adjust unstable forces like bond stretching, van der Waals forces, and electrostatic interactions, to ensure a low potential energy, thus a low overall energy state of the 3D structure of the molecule. Energy minimization helps the 3D structures of molecules to reach a local minimum, resulting in a structurally stable, chemically realistic conformation, increasing the likelihood that the modeled structure is biologically relevant, meaning it will behave similarly in real-world biological processes and interactions.
2.6.2. Potential Energy Surface
Energy minimization refines the molecular structure step by step, guiding it toward a local minimum on the potential energy surface (PES), which is a mathematical model that visualizes how the energy of a molecule changes as its atomic arrangement is altered. The potential energy surface can be imagined as a multidimensional landscape, where each point represents a possible atomic conformation, and the height at that point corresponds to the molecule’s energy level. In this landscape, peaks represent high-energy, unstable structures, and valleys represent low-energy, stable conformation. As the molecule’s structure is adjusted, it moves across this surface, seeking the lowest possible energy. The goal of energy minimization is to reach a valley on the potential energy surface, specifically a local minimum, which is a conformation that is more stable than surrounding conformation. In other words, the local minimum is the lowest-energy structure relative to its immediate neighbors. However, while a local minimum is stable compared to nearby structures, it is not necessarily the global minimum. The global minimum represents the absolute lowest-energy conformation a molecule can adopt, and it is the most stable form of the molecule in the entire potential energy surface landscape. Since energy minimization algorithms adjust atomic positions incrementally, they often settle into the first local minimum they encounter, rather than continuing the search for the global minimum. Thus, while energy minimization does not guarantee reaching the global minimum, it ensures that the molecule reaches a stable conformation that is sufficiently low in energy for practical applications, such as molecular simulations or drug design.
2.6.3. Atomic and Molecular Structure
At the most fundamental level, all matter is composed of atoms, which are the smallest units that retain the chemical properties of an element. Each atom consists of a nucleus, a dense central core containing positively charged protons and neutrally charged neutrons, surrounded by a cloud of negatively charged electrons that orbit in discrete energy levels known as electron shells. These electrons determine an atom’s valency, or bonding capability.
Atoms bond to form molecules through chemical bonds, which are attractive forces that hold atoms together. The main types of chemical bonds are:
- Covalent Bonds: These involve the sharing of electron pairs between atoms to achieve a stable electron configuration. Covalent bonds can be single bonds (one shared electron pair), double bonds (two shared pairs), or triple bonds (three shared pairs), with triple bonds being the shortest and strongest.
- Ionic Bonds: These occur when electrons are completely transferred from one atom to another, resulting in oppositely charged ions that attract each other.
- Metallic Bonds: Found in metals, these involve a "sea" of delocalized electrons that flow freely between atoms.
2.6.4. Key properties of the 3D structures of molecules that energy minimization affects:
Bond Lengths: Bond length refers to the distance between the nuclei of two bonded atoms. This distance is governed by a delicate balance between attractive forces (resulting from shared electrons pulling the atoms together) and repulsive forces (arising from the positively charged nuclei pushing against each other). Each type of bond has an ideal bond length: Single bonds (e.g., C-C) are the longest because they involve one shared electron pair, Double bonds (e.g., C=C) are shorter than single bonds due to increased electron sharing. Triple bonds (e.g., C≡C) are shortest because three shared electron pairs create stronger attraction. When bond lengths deviate from these equilibrium values, meaning they’re stretched or compressed beyond its equilibrium length, the molecule experiences bond strain, which increases its potential energy. If a bond is stretched or compressed beyond its equilibrium length, the system's energy rises, making the molecule less stable. Energy minimization corrects this strain by iteratively adjusting atomic positions, preventing excessive strain that could lead to instability or inaccurate molecular behavior.
Steric Strain (Van der Waals Interactions): The principle of steric strain is based on the idea that molecules experience stress when atoms are forced into positions that are too close to each other. This closeness leads to repulsive forces between the electron clouds of the atoms, causing them to repel each other. This repulsion arises from Van der Waals interactions, which occur due to the transient dipoles (a temporary moment that occurs when electrons in an atom or molecule are not evenly distributed, making molecule’s one end partially positive and one partially negative end) created by the movement of electrons around atoms. These temporary dipoles result in weak attractions and repulsions between molecules. When atoms are too close, the repulsive forces increase, raising the energy of the molecule and making it less stable. Conversely, if atoms are too far apart, the attractive forces between them weaken, also reducing stability. Thus, for a molecule to be stable, the atoms need to be at an optimal distance from each other, balancing these repulsive and attractive forces. Energy minimization ensures atoms remain within an optimal distance, preventing steric clashes and enhancing molecular stability.
Electrostatic Interactions: Electrostatic interactions are fundamental forces that arise between charged particles. These interactions are governed by the principle that opposite charges attract and like charges repel. When atoms or groups with like charges (e.g., two positive or two negative charges) are positioned too closely, they exert a repulsive force, which increases the energy of the system and makes the molecule less stable. This repulsion occurs because the negatively charged electrons on one atom or group push away those on another, causing stress within the molecule. Conversely, when atoms or groups with opposite charges (e.g., a positively charged ion and a negatively charged ion) come into proximity, they experience an attractive force. This attraction stabilizes the molecule, helping to hold it together. Energy minimization optimizes charge distributions by adjusting atomic positions to reduce repulsions and maximize stabilizing attractions. Energy minimization optimizes charge distributions by adjusting atomic positions, to reduce repulsions and maximize stabilizing attractions.
2.6.5. Process of energy minimization:
1. Load the Molecular Structure: Open Avogadro and Import the PDB File of the Molecule - The first step is to load the molecular structure into a software program capable of visualizing and manipulating it. In this case, Avogadro, a popular molecular visualization tool, is used. The PDB (Protein Data Bank) file format is commonly used for storing 3D structures of proteins and other biological macromolecules. The PDB file contains the atomic coordinates of a molecule, along with other structural information (e.g., heteroatoms, secondary structure elements). When this file is opened in Avogadro, the program reads these coordinates and generates a 3D representation of the molecule. This step is crucial because the structure being worked on is the starting point for further manipulation, analysis, and optimization. Without a properly loaded structure, any subsequent simulations or analyses would be inaccurate.
2. Apply a Force Field: Choose an Appropriate Force Field (e.g., MMFF94, UFF) - A force field is a mathematical model, its key function is to help identify and fix high-energy conformations. They provide predefined parameters, such as bond lengths, angles, and van der Waals radii, that describe atomic interactions. By calculating the potential energy of the system and applying optimization techniques, the force field can guide the molecule toward a more stable, lower-energy conformation. For organic molecules, the MMFF94 (Merck Molecular Force Field) is commonly used, while the UFF (Universal Force Field) is more general and applicable to a broader range of elements. When minimizing the energy of bioactive compounds found within Ayurvedic herbs, which are organic molecules, the MMFF94 force field is typically applied because it provides the necessary parameters that govern the behavior of atoms in the system. For energy minimization, the force field adjusts atomic positions to eliminate unrealistic, high-energy structures, ultimately leading to a more stable molecule. However, force fields typically find a local minimum on the potential energy surface, meaning that they may not always identify the global minimum energy conformation. Using an inappropriate force field could result in inaccurate results, as the parameters would not be suited to the molecule's specific properties.
3. Monitor Energy Convergence: Observe the Energy Graph to Ensure Stabilization - During energy minimization, it is important to monitor the energy graph to confirm that the process has successfully converged to a stable, low-energy state. The energy graph plots the total energy of the system against the number of iterations or time, and as minimization progresses, the energy should steadily decrease and stabilize at a minimum value. Convergence (the point where the energy minimization process has stabilized, and no further significant reductions in energy are achievable) is indicated when the energy plateaus, highlighting that a stable value has been achieved, meaning further iterations are unlikely to improve the energy further. If the energy continues to fluctuate, it suggests the minimization has not fully converged. Confirming energy stabilization is crucial because a structure that isn’t fully optimized, leads to errors in further analysis like docking or binding studies. Stabilization indicates that the system has reached a physically realistic and low-energy state.
4. Export the Final Structure: Save the Minimized Structure for Molecular Docking Simulations - Once the structure has been optimized, the final step is to export it for use in other simulations, such as molecular docking, where the molecule's interaction with a target (e.g., a protein or receptor) will be tested. After ensuring the geometry is satisfactory, the structure is saved in a format compatible with docking software such as PDB format (Protein Data Bank), containing the atomic coordinates and necessary data for the docking process. This saved structure is crucial for further computational analysis, including docking, which is essential for drug design and understanding molecular interactions. If the structure isn't properly saved or if the minimization was incomplete, the docking results may be unreliable, unrealistic, and inaccurate.
2.6.6. Limitations of Energy Minimization:
Energy minimization is a fundamental step in molecular modeling because it refines a molecule’s structure, making it more stable and biologically relevant (meaning it will behave similarly in real-world biological processes and interactions) before further computational analysis. Although it does not guarantee finding the global energy minimum, it serves as a crucial “first layer of repair” that eliminates obvious errors and structural inconsistencies. Many molecular models begin in artificially high-energy states due to rough initial placements of atoms or structural approximations. Without minimization, these starting structures may contain severe steric clashes (atoms positioned too closely together), unnatural bond lengths, and etc, all of which are physically unrealistic and biologically irrelevant. If left uncorrected, these issues can cause errors in later molecular docking simulations, leading to misleading results. Even though energy minimization may only bring the molecule to a local minimum rather than the absolute lowest energy state (global minimum), it still provides a structure that is significantly more stable and biologically meaningful than an unminimized one. In essence, energy minimization does not aim to find the perfect solution but rather to ensure that the system is in a reasonable and workable state. Without it, the risk of inaccurate simulations increases dramatically, making downstream computational studies unreliable. In this way, energy minimization follows a “something is better than nothing” principle, while it may not always yield the globally optimal conformation, it is far better to work with a stable, realistic structure than to proceed with an unrefined, error-prone model. Therefore, even though more sophisticated sampling methods are often needed to find the true lowest-energy conformation, energy minimization remains an essential first step in ensuring accurate and meaningful molecular simulations, playing a critical role in eliminating major structural issues, improving stability, and providing a solid foundation for further computational studies, being a fundamental step in improving their accuracy and predictive power.
2.6.7. Overcoming Limitations:
To overcome the limitations of energy minimization, molecular dynamics (MD) simulations are widely used to help molecules explore a broader range of conformations and identify more stable structures, working towards the global minimum. This technique allows the overcoming of energy barriers that may trap the molecule in a local minimum and prevent it from reaching lower-energy, more biologically relevant states, eventually the global minimum.
Molecular dynamics (MD) is a computational technique that simulates the physical movements of atoms and molecules over time by applying Newton’s laws of motion. Unlike energy minimization, which makes small stepwise adjustments, molecular dynamics treats atoms as particles that are constantly in motion, responding to forces such as bond stretching, angle bending, electrostatic interactions, and van der Waals forces. The simulation begins with an initial molecular structure placed in an environment that mimics real-world conditions (e.g., solvated in water, at physiological temperature and pressure). Molecular dynamics simulations progress in small time steps (on the order of femtoseconds, 10⁻¹⁵ seconds), where the velocity (the speed) and position of each atom are updated at each step based on the forces acting on it. Over time, this allows the molecule to explore different conformations naturally, similar to how a real molecule would move in solution due to thermal fluctuations. Molecular dynamics captures the natural motion of molecules, instead of a step-by-step artificial modification of properties approach, which allows the molecule to overcome small energy barriers that might trap it in a local minimum, enabling the discovery of more stable structures, and eventually the global minimum. However, as a grade 10 student, conducting molecular dynamics simulations is not feasible due to the significant resources required. These simulations demand access to powerful computers or supercomputers to process the vast amounts of data generated and to run the necessary algorithms efficiently. Additionally, the software programs needed for molecular dynamics simulations often come with expensive licensing fees, which are difficult to obtain without access to resources typically available at universities or research institutions. Furthermore, the data generated by these simulations can be enormous, requiring substantial storage capacity. Without access to high-performance computing resources and the appropriate software, running molecular dynamics simulations is impractical. Although the underlying concepts may be understandable, the lack of these essential tools makes it challenging to carry out such simulations at the high school level.
2.7. Molecular Docking Simulations - Predicting Ligand-Target Protein Interactions
Molecular docking is a crucial computational technique used to predict how bioactive compounds from Ayurvedic herbs interact with specific target proteins, enzymes, or receptors. It helps estimate the binding affinity between a ligand (the 3D structure of bioactive compound) and its target protein, which is key to determining its therapeutic potential. A stronger binding, indicated by a more negative docking score, suggests a more stable and effective interaction, increasing the likelihood of the compound exerting a biological effect. This process allows researchers to prioritize promising compounds for further experimental testing, providing valuable insights into their efficacy as potential drug candidates.
A docking score is a numerical value representing the predicted binding affinity of a ligand when interacting with a target protein. Binding affinity refers to the strength and stability of the interaction between a ligand and a protein and is a critical factor in determining how effectively a ligand can bind to a protein’s active site, influencing its potential as a drug candidate. Binding affinity depends on the binding energy of the interaction, which is the energy released when a ligand binds to a protein, measured in kilocalories per mole (kcal/mol). The release of energy during binding stabilizes the ligand-protein system, reducing the likelihood of dissociation (breaking of ligand-protein system). Consequently, the more energy released during this process, the more stable the interaction and the higher the binding affinity. Binding energy is expressed as a negative value because energy is released during the binding process, and a more negative binding energy (or lower docking score) indicates a stronger interaction between the ligand and the protein, as greater energy release corresponds to increased stability and a stronger bond between the two molecules. A more significant energy release makes the system harder to separate, enhancing stability and leading to a higher binding affinity. In molecular docking, ligands interact with binding pockets on the surface of target proteins, which are specific regions, often within the active site, where the ligand can attach and form stable interactions. A tightly bound ligand remains in the binding pocket for longer, increasing its likelihood of exerting a biological effect.
To perform molecular docking simulations, this study uses CB-Dock, an automated docking tool designed to predict ligand-protein interactions. CB-Dock follows a structured approach in three key steps. First, it identifies binding pockets on the target protein by analyzing its three-dimensional structure. A binding pocket is a specific region on a protein where a ligand can attach and interact. These pockets are typically located within the protein’s active site, where most biochemical reactions occur. In molecular docking, CB-Dock automatically identifies binding pockets and predicts which ligand fits best. The better a ligand fits into a binding pocket, the stronger the interaction, leading to a lower docking score and potentially greater biological activity. Next, it performs docking simulations, where the ligand is computationally inserted into these binding pockets. Finally, CB-Dock calculates the binding affinity of the ligand-protein complex, based on how much energy is released during the docking stimulation, assigning a docking score to each interaction, backed by the binding energy of each interaction.
Molecular docking provides a computational approach for evaluating the potential efficacy of Ayurvedic herbs before laboratory and clinical testing. Molecular docking is crucial for understanding how molecules, such as drugs and bioactive compounds, interact with specific targets like proteins or enzymes, providing insights into their potential efficacy through binding affinity. With an accuracy rate of 85.9%, CB-Dock proves to be a reliable tool for molecular docking that focuses on protein-ligand docking with an emphasis on the precise prediction of binding sites. It uses a fast and efficient docking algorithm to predict interactions with high accuracy, making it a popular choice for researchers working with protein-ligand complexes. As a preclinical testing tool, CB-Dock sets a solid foundation for further experimental validation, as well as in vitro (laboratory-based) and in vivo (animal or human-based) studies.
From the range of scores provided, the highest binding affinity score (labeled “molecular docking score” below) is commonly used, instead of average score for several important reasons. First, it reflects the strongest interaction between an active compound in an herb or a drug, and its target protein or receptor. This is crucial because the most potent interaction is likely to be the most biologically relevant and effective, making it a key indicator of a compound's potential to influence the biological activity of the target. Additionally, focusing on the highest binding affinity ensures researchers are considering the "best-case" or most favorable interaction, which provides a more conservative and reliable estimate of the compound's potential efficacy. This is particularly important when a compound may have multiple binding modes (specific orientation and interaction pattern of a molecule when it binds to its target protein or receptor), due to which the average score can be skewed by outliers, which may obscure the strongest potential interaction. By focusing on the highest score, researchers highlight the compound's best fit with the target, which is of primary interest in drug development or herbal medicine analysis. The highest binding affinity score allows for a more direct comparison of each compound's maximum possible efficacy. In conclusion, using the highest binding affinity score helps ensure that the most potent interactions are taken into account, leading to more informed decisions about which compounds to pursue for further research and potential therapeutic applications.
2.7.1. Attached below is the molecular docking results of bioactive compounds in ayurvedic herbs to target proteins of adverse drug reactions
Abdominal Pain - Cyclooxygenase-2 (COX-2)- Triphala
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Chebulinic acid |
|
-7.3 kcal/mol |
|
Ellagic acid |
|
-7.2 kcal/mol |
|
Proanthocyanidins |
|
-7.7 kcal/mol |
|
Gallic Acid |
|
-5.4 kcal/mol |
|
Chebulagic Acid |
|
-7.5 kcal/mol |
|
Quercetin |
|
-6.9 kcal/mol |
|
Ascorbic Acid |
|
-5.2 kcal/mol |
|
Emblicanin A |
|
-7.4 kcal/mol |
|
Emblicanin B |
|
-7.5 kcal/mol |
|
Linoleic acid |
|
-4.7 kcal/mol |
|
Oleic acid |
|
-4.3 kcal/mol |
Bloating - Guanylate cyclase C (GC-C) - Ajwain
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Scores |
|
Thymol |
|
-6 kcal/mol |
|
Carvacrol |
|
-6.4 kcal/mol |
|
Para-Cymene (p-Cymene) |
|
-5.7 kcal/mol |
|
Gamma-Terpinene (γ-Terpinene) |
|
-5.7 kcal/mol |
|
Alpha-Pinene (α-Pinene) |
|
-5.6 kcal/mol |
|
Beta-Pinene (β-Pinene) |
|
-5.6 kcal/mol |
|
Alpha-Terpinene (α-Terpinene) |
|
-5.7 kcal/mol |
|
Limonene |
|
-5.5 kcal/mol |
|
Fenchone |
|
-5.5 kcal/mol |
|
Borneol |
|
-5.2 kcal/mol |
|
Myrcene |
|
-4.7 kcal/mol |
|
Sabinene |
|
-5.4 kcal/mol |
Constipation - Guanylate cyclase C (GC-C) - Triphala
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Chebulinic acid |
|
-8.6 kcal/mol |
|
Ellagic acid |
|
-8.4 kcal/mol |
|
Proanthocyanidins |
|
-8.3 kcal/mol |
|
Gallic Acid |
|
-5.9 kcal/mol |
|
Chebulagic Acid |
|
-8.2 kcal/mol |
|
Quercetin |
|
-8.2 kcal/mol |
|
Ascorbic Acid |
|
-5.2 kcal/mol |
|
Emblicanin A |
|
-8.3 kcal/mol |
|
Emblicanin B |
|
-8.5 kcal/mol |
|
Linoleic acid |
|
-5.4 kcal/mol |
|
Oleic acid |
|
-5.2 kcal/mol |
Decreased appetite - Ghrelin receptor (GHS-R1a) - Chitrak
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Plumbagin |
|
-6.6 kcal/mol |
|
Gallic Acid |
|
-5.9 kcal/mol |
|
Caffeic Acid |
|
-6.4 kcal/mol |
|
Quercetin |
|
-8 kcal/mol |
|
Kaempferol |
|
-7.7 kcal/mol |
|
Betulinic Acid |
|
-8.4 kcal/mol |
|
Oleanolic Acid |
|
-8.6 kcal/mol |
|
α-Pinene |
|
-6.7 kcal/mol |
|
β-Caryophyllene |
|
-7.3 kcal/mol |
|
β-Sitosterol |
|
-8.1 kcal/mol |
Diarrhea - 5-HT3 receptor (serotonin receptor 3) - Bael
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Aegeline |
|
-6.6 kcal/mol |
|
Quercetin |
|
-6.4 kcal/mol |
|
Kaempferol |
|
-6.5 kcal/mol |
|
Rutin |
|
-7.4 kcal/mol |
|
Apigenin |
|
-6.6 kcal/mol |
|
Luteolin |
|
-6.5 kcal/mol |
|
Oleanolic Acid |
|
-7.9 kcal/mol |
|
Ursolic Acid |
|
-8.3 kcal/mol |
|
Aegle Marmelos |
|
-6.6 kcal/mol |
|
Gallic Acid |
|
-4.6 kcal/mol |
|
Caffeic Acid |
|
-5 kcal/mol |
|
Ellagic Acid |
|
-6 kcal/mol |
|
Ferulic Acid |
|
-5.2 kcal/mol |
|
α-Pinene |
|
-4.9 kcal/mol |
|
β-Caryophyllene |
|
-6.5 kcal/mol |
|
Limonene |
|
-5 kcal/mol |
|
Myrcene |
|
-4.7 kcal/mol |
Heartburn - H+/K+ ATPase - Amla
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Scores |
|
Ascorbic Acid |
|
-5.8 kcal/mol |
|
Emblicanin A |
|
-9.2 kcal/mol |
|
Emblicanin B |
|
-9.2 kcal/mol |
|
Gallic Acid |
|
-6.3 kcal/mol |
|
Ellagic Acid |
|
-8.4 kcal/mol |
|
Quercetin |
|
-8.2 kcal/mol |
|
Linoleic acid |
|
-5.3 kcal/mol |
|
Oleic acid |
|
-5.3 kcal/mol |
Cough - Transient Receptor Potential Cation Channel Subfamily V Member 1 (TRPV1) - Tulsi
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Apigenin |
|
-8.2 kcal/mol |
|
Luteolin |
|
-8.5 kcal/mol |
|
Rutin |
|
-9.2 kcal/mol |
|
Quercetin |
|
-8.2 kcal/mol |
|
Eugenol |
|
-6.2 kcal/mol |
|
Rosmarinic Acid |
|
-8.2 kcal/mol |
|
Linalool |
|
-5.8 kcal/mol |
|
Cineole |
|
-6.3 kcal/mol |
|
Camphor |
|
-6 kcal/mol |
|
Oleanolic Acid |
|
-7.7 kcal/mol |
|
Ursolic Acid |
|
-7.8 kcal/mol |
|
Stigmasterol |
|
-8.7 kcal/mol |
Difficulty breathing - Beta-2 Adrenergic Receptor (β2AR) - Pippali
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Piperine |
|
-9.6 kcal/mol |
|
Piperlongumine |
|
-7.9 kcal/mol |
|
β-Caryophyllene |
|
-7.1 kcal/mol |
|
Myristicin |
|
-7.1 kcal/mol |
|
Eugenol |
|
-7.1 kcal/mol |
|
Chavicine |
|
-8.9 kcal/mol |
|
Piperamide |
|
-7.8 kcal/mol |
|
Quercetin |
|
-9.8 kcal/mol |
|
Rutin |
|
-10.6 kcal/mol |
|
Diosgenin |
|
-9.1 kcal/mol |
|
Stigmasterol |
|
-9.6 kcal/mol |
|
Campesterol |
|
-9.2 kcal/mol |
Pneumonitis - Tumor Necrosis Factor-alpha (TNF-α) Receptor - Haridra
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Curcumin |
|
-6.6 kcal/mol |
|
Demethoxycurcumin |
|
-6.2 kcal/mol |
|
Bisdemethoxycurcumin |
|
-6.5 kcal/mol |
|
Ar-turmerone |
|
-6.3 kcal/mol |
|
Zingiberene |
|
-6.3 kcal/mol |
|
Alpha-curcumene |
|
-6.4 kcal/mol |
|
Beta-sesquiphellandrene |
|
-6 kcal/mol |
|
Eugenol |
|
-5.3 kcal/mol |
|
Gallic acid |
|
-5.6 kcal/mol |
|
Ellagic acid |
|
-7.4 kcal/mol |
|
Catechin |
|
-7.5 kcal/mol |
|
Epicatechin |
|
-6.9 kcal/mol |
Angioedema (swollen tissue) - Bradykinin Receptor (B2 receptor) - Turmeric
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Curcumin |
|
-6.5 kcal/mol |
|
Demethoxycurcumin |
|
-7.3 kcal/mol |
|
Bisdemethoxycurcumin |
|
-7.8 kcal/mol |
|
Turmerone |
|
-7.3 kcal/mol |
|
Zingiberene |
|
-7.6 kcal/mol |
|
Beta-sesquiphellandrene |
|
-7.6 kcal/mol |
|
Alpha-curcumene |
|
-7.3 kcal/mol |
|
Eugenol |
|
6.1 kcal/mol |
|
Quercetin |
|
-7.6 kcal/mol |
|
Kaempferol |
|
-7.7 kcal/mol |
|
Gallic Acid |
|
-6 kcal/mol |
|
Ellagic Acid |
|
-8.1 kcal/mol |
|
Catechin |
|
-7.5 kcal/mol |
|
Epicatechin |
|
-7.1 kcal/mol |
Arrhythmia (irregular heartbeat) - Sodium Channels (Nav1.5) - Arjuna
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Arjunetin |
|
-10.8 kcal/mol |
|
Arjungenin |
|
-9.9 kcal/mol |
|
Quercetin |
|
-8.1 kcal/mol |
|
Kaempferol |
|
-8.2 kcal/mol |
|
Myricetin |
|
-8 kcal/mol |
|
Rutin |
|
-9.2 kcal/mol |
|
Arjunolic Acid |
|
-9.3 kcal/mol |
|
Gallic Acid |
|
-5.6 kcal/mol |
|
β-Sitosterol |
|
-8.6 kcal/mol |
|
Stigmasterol |
|
-9.6 kcal/mol |
|
Curcumin |
|
-8 kcal/mol |
|
Demethoxycurcumin |
|
-8.3 kcal/mol |
|
Bisdemethoxycurcumin |
|
-8.2 kcal/mol |
|
Turmerone |
|
-7.6 kcal/mol |
|
Zingiberene |
|
-7.9 kcal/mol |
Increased risk of blood clots - Factor Xa - Bibhitaki
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Procyanidin B1 |
|
-9.3 kcal/mol |
|
Procyanidin B2 |
|
-9.2 kcal/mol |
|
Prodelphinidin B3 |
|
-8.6 kcal/mol |
|
Gallic Acid |
|
-6.5 kcal/mol |
|
Chebulagic Acid |
|
-9.6 kcal/mol |
|
Ellagic Acid |
|
-7.3 kcal/mol |
|
Bellericoside |
|
-8.1 kcal/mol |
|
Bellericagenin |
|
-7.6 kcal/mol |
Chest pain - Troponin I - Arjuna
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Arjunetin |
|
-6.6 kcal/mol |
|
Arjungenin |
|
-6 kcal/mol |
|
Quercetin |
|
-6 kcal/mol |
|
Kaempferol |
|
-6 kcal/mol |
|
Myricetin |
|
-6.1 kcal/mol |
|
Rutin |
|
-6.8 kcal/mol |
|
Arjunolic Acid |
|
-6.7 kcal/mol |
|
Gallic Acid |
|
-4.4 kcal/mol |
|
β-Sitosterol |
|
-6.5 kcal/mol |
|
Stigmasterol |
|
-7 kcal/mol |
|
Curcumin |
|
-5.5 kcal/mol |
|
Demethoxycurcumin |
|
-5.5 kcal/mol |
|
Bisdemethoxycurcumin |
|
-6.2 kcal/mol |
|
Turmerone |
|
-6.2 kcal/mol |
|
Zingiberene |
|
-5 kcal/mol |
Rapid heartbeat (tachycardia) - β1-adrenergic receptor - Arjuna
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Arjunetin |
|
-8.6 kcal/mol |
|
Arjungenin |
|
-8.7 kcal/mol |
|
Quercetin |
|
-9.4 kcal/mol |
|
Kaempferol |
|
-9.3 kcal/mol |
|
Myricetin |
|
-9.2 kcal/mol |
|
Rutin |
|
-7.9 kcal/mol |
|
Arjunolic Acid |
|
-8.8 kcal/mol |
|
Gallic Acid |
|
-6.2 kcal/mol |
|
β-Sitosterol |
|
-9.5 kcal/mol |
|
Stigmasterol |
|
-9.9 kcal/mol |
|
Curcumin |
|
-9.2 kcal/mol |
|
Demethoxycurcumin |
|
-9 kcal/mol |
|
Bisdemethoxycurcumin |
|
-9 kcal/mol |
|
Turmerone |
|
-7.8 kcal/mol |
|
Zingiberene |
|
-7.8 kcal/mol |
High blood pressure (Hypertension) - Angiotensin-converting enzyme (ACE) - Arjuna
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Arjunetin |
|
-9.7 kcal/mol |
|
Arjungenin |
|
-10 kcal/mol |
|
Quercetin |
|
-8.9 kcal/mol |
|
Kaempferol |
|
-8.6 kcal/mol |
|
Myricetin |
|
-8.7 kcal/mol |
|
Rutin |
|
-10.5 kcal/mol |
|
Arjunolic Acid |
|
-9.5 kcal/mol |
|
Gallic Acid |
|
-6.1 kcal/mol |
|
β-Sitosterol |
|
-9.1 kcal/mol |
|
Stigmasterol |
|
-9.6 kcal/mol |
|
Curcumin |
|
-9 kcal/mol |
|
Demethoxycurcumin |
|
-9.3 kcal/mol |
|
Bisdemethoxycurcumin |
|
-9 kcal/mol |
|
Turmerone |
|
-7 kcal/mol |
|
Zingiberene |
|
-6.5 kcal/mol |
Low blood pressure (Hypotension) - Alpha-1 Adrenergic Receptor - Ashwagandha
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Withaferin A |
|
-8 kcal/mol |
|
Withanolide D |
|
-9.4 kcal/mol |
|
Withanolide E |
|
-8.6 kcal/mol |
|
Somniferine |
|
-8.8 kcal/mol |
|
Anaferine |
|
-6.7 kcal/mol |
|
Isopelletierine |
|
-5.5 kcal/mol |
|
Linoleic acid |
|
-6.1 kcal/mol |
|
Oleic acid |
|
-5.9 kcal/mol |
|
Stigmasterol |
|
-9 kcal/mol |
|
Beta-sitosterol |
|
-8.4 kcal/mol |
Low white cell count (leukopenia) - Granulocyte colony-stimulating factor (G-CSF) - Guduchi
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Berberine |
|
-8.8 kcal/mol |
|
Tinosporaside |
|
-8.9 kcal/mol |
|
Tinosporin |
|
-8.9 kcal/mol |
|
Tinocordiside |
|
-8.6 kcal/mol |
|
Tinocordifolin |
|
-7.5 kcal/mol |
|
Columbin |
|
-9 kcal/mol |
|
Cordifolioside |
|
-7.6 kcal/mol |
|
Magnoflorine |
|
-8.1 kcal/mol |
|
Tinosporoside |
|
-8.4 kcal/mol |
|
Sitosterol |
|
-8.9 kcal/mol |
|
Stigmasterol |
|
-9.2 kcal/mol |
|
Campesterol |
|
-8.9 kcal/mol |
|
Germacrene D |
|
-6.9 kcal/mol |
|
Beta-caryophyllene |
|
-7.1 kcal/mol |
|
Gallic acid |
|
-6.2 kcal/mol |
|
Caffeic acid |
|
-6.3 kcal/mol |
|
Chlorogenic acid |
|
-8.3 kcal/mol |
|
Octadecanoic acid |
|
-5.7 kcal/mol |
|
Hexadecanoic acid |
|
-5.6 kcal/mol |
Blurred Vision - Vascular endothelial growth factor - Amla
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Ascorbic Acid |
|
-5.3 kcal/mol |
|
Emblicanin A |
|
-9.5 kcal/mol |
|
Emblicanin B |
|
-9.7 kcal/mol |
|
Gallic Acid |
|
-5.7 kcal/mol |
|
Ellagic Acid |
|
-8.5 kcal/mol |
|
Quercetin |
|
-8.7 kcal/mol |
|
Linoleic acid |
|
-5.9 kcal/mol |
|
Oleic acid |
|
-5.5 kcal/mol |
Fatigue - A2A adenosine receptor - Ashwagandha
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Withaferin A |
|
-10 kcal/mol |
|
Withanolide D |
|
-10.8 kcal/mol |
|
Withanolide E |
|
-9.6 kcal/mol |
|
Somniferine |
|
-9.1 kcal/mol |
|
Anaferine |
|
-6.9 kcal/mol |
|
Isopelletierine |
|
-5.2 kcal/mol |
|
Linoleic acid |
|
-7 kcal/mol |
|
Oleic acid |
|
-6.5 kcal/mol |
|
Stigmasterol |
|
-10 kcal/mol |
|
Beta-sitosterol |
|
-9.6 kcal/mol |
Hair loss - 5-alpha reductase enzyme - Bhringraj
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Ecliptasaponin A |
|
-8.3 kcal/mol |
|
Ecliptasaponin D |
|
-11.3 kcal/mol |
|
demethylwedelolactone |
|
-9.4 kcal/mol |
|
Wedelolactone |
|
-9.5 kcal/mol |
|
Luteolin |
|
-9.1 kcal/mol |
|
Apigenin |
|
-9.6 kcal/mol |
|
Quercetin |
|
-9.6 kcal/mol |
|
Rutin |
|
-11 kcal/mol |
|
Kaempferol |
|
-9.5 kcal/mol |
|
β-Sitosterol |
|
-10.3 kcal/mol |
|
Caffeic acid |
|
-6.9 kcal/mol |
|
Chlorogenic acid |
|
-9.6 kcal/mol |
Joint pain - Tumor Necrosis Factor-alpha (TNF-alpha) - Shallaki
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Boswellic acid |
|
-7.4 kcal/mol |
|
β-Boswellic acid |
|
-7.4 kcal/mol |
|
Alpha-pinene |
|
-4.7 kcal/mol |
|
Limonene |
|
-5.7 kcal/mol |
|
Beta-myrcene |
|
-4.7 kcal/mol |
|
Borneol |
|
-4.4 kcal/mol |
|
Acetyl boswellic acid |
|
-7.3 kcal/mol |
|
Keto boswellic acid |
|
-7.4 kcal/mol |
|
Quercetin |
|
-7.1 kcal/mol |
|
Kaempferol |
|
-6.8 kcal/mol |
|
Rutin |
|
-6.9 kcal/mol |
|
Gallic acid |
|
-5.6 kcal/mol |
|
Caffeic acid |
|
-6.3 kcal/mol |
|
Chlorogenic acid |
|
-6.8 kcal/mol |
Muscle cramps - Ryanodine receptor 1 (RyR1) - Nirgundi
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Vitexin |
|
-6.9 kcal/mol |
|
Negundoside |
|
-7.6 kcal/mol |
|
Matairesinol |
|
-7.8 kcal/mol |
|
Luteolin |
|
-8.3 kcal/mol |
|
Apigenin |
|
-8 kcal/mol |
|
Quercetin |
|
-8.2 kcal/mol |
|
Rutin |
|
-8.1 kcal/mol |
|
Beta-sitosterol |
|
-7.7 kcal/mol |
|
Limonene |
|
-5.9 kcal/mol |
|
Alpha-pinene |
|
-5.5 kcal/mol |
|
Eucalyptol |
|
-4.9 kcal/mol |
|
Camphor |
|
-4.8 kcal/mol |
|
Caffeic acid |
|
-7 kcal/mol |
|
Gallic acid |
|
-6.2 kcal/mol |
|
Chlorogenic acid |
|
-7.8 kcal/mol |
|
Ursolic acid |
|
-7 kcal/mol |
|
Oleanolic acid |
|
-7.5 kcal/mol |
Muscle weakness - Dystrophin - Ashwagandha
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Withaferin A |
|
-8.6 kcal/mol |
|
Withanolide D |
|
-10.1 kcal/mol |
|
Withanolide E |
|
-8.6 kcal/mol |
|
Somniferine |
|
-9.8 kcal/mol |
|
Anaferine |
|
-6.2 kcal/mol |
|
Isopelletierine |
|
-4.4 kcal/mol |
|
Linoleic acid |
|
-5.5 kcal/mol |
|
Stigmasterol |
|
-7.9 kcal/mol |
|
Beta-sitosterol |
|
-7.6 kcal/mol |
Osteoporosis (bone weakening) - RANKL (Receptor Activator of Nuclear Factor Kappa-Β Ligand) - Hadjod
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Quercetin |
|
-6.5 kcal/mol |
|
Kaempferol |
|
-6.4 kcal/mol |
|
Rutin |
|
-7.8 kcal/mol |
|
β-Sitosterol |
|
-6.8 kcal/mol |
|
Gallic acid |
|
-5.6 kcal/mol |
|
Caffeic acid |
|
-5.5 kcal/mol |
|
Vitamin C |
|
-5.6 kcal/mol |
|
Beta-carotene |
|
-7.5 kcal/mol |
|
3-Keto-β-sitosterol |
|
-7 kcal/mol |
Fever - Cyclooxygenase-2 (COX-2) - Giloy
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Beta-Glucans |
|
-8.3 kcal/mol |
|
Berberine |
|
-8.5 kcal/mol |
|
Protopine |
|
-9 kcal/mol |
|
Tinosporaside |
|
-8.1 kcal/mol |
|
Tinocordiside |
|
-8.2 kcal/mol |
|
Stigmasterol |
|
-8.1 kcal/mol |
|
Beta-Sitosterol |
|
-8.1 kcal/mol |
|
Germacrene D |
|
-6.9 kcal/mol |
|
Quercetin |
|
-9.1 kcal/mol |
|
Gallic Acid |
|
-6.8 kcal/mol |
|
Hexadecanoic Acid |
|
-6.5 kcal/mol |
|
Octadecanoic Acid |
|
-6.4 kcal/mol |
Anxiety - Serotonin 5-HT1A receptor - Ashwagandha
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Withaferin A |
|
-8.6 kcal/mol |
|
Withanolide D |
|
-9.3 kcal/mol |
|
Withanolide E |
|
-9.2 kcal/mol |
|
Somniferine |
|
-9.5 kcal/mol |
|
Anaferine |
|
-6.3 kcal/mol |
|
Isopelletierine |
|
-4.9 kcal/mol |
|
Linoleic acid |
|
-5.8 kcal/mol |
|
Stigmasterol |
|
-8.6 kcal/mol |
|
Beta-sitosterol |
|
-8.9 kcal/mol |
Depression - Serotonin 5-HT1A receptor - Ashwagandha
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Withaferin A |
|
-8.6 kcal/mol |
|
Withanolide D |
|
-9.3 kcal/mol |
|
Withanolide E |
|
-9.2 kcal/mol |
|
Somniferine |
|
-9.5 kcal/mol |
|
Anaferine |
|
-6.3 kcal/mol |
|
Isopelletierine |
|
-4.9 kcal/mol |
|
Linoleic acid |
|
-5.8 kcal/mol |
|
Stigmasterol |
|
-8.6 kcal/mol |
|
Beta-sitosterol |
|
-8.9 kcal/mol |
Difficulty sleeping (Insomnia) - GABA(A) receptor - Tagara
|
Bioactive Compound |
Molecular Docking Scores |
Highest Molecular Docking Score |
|
Valerenic Acid |
|
-7 kcal/mol |
|
Valtrate |
|
-6.8 kcal/mol |
|
Isovaltrate |
|
-6.6 kcal/mol |
|
Schizandrin |
|
-5.8 kcal/mol |
|
Pinoresinol |
|
-6.9 kcal/mol |
|
Matairesinol |
|
-7.5 kcal/mol |
|
Quercetin |
|
-7.7 kcal/mol |
|
Apigenin |
|
-7.6 kcal/mol |
|
Luteolin |
|
-8 kcal/mol |
|
Borneol |
|
-5 kcal/mol |
|
Camphor |
|
-4.8 kcal/mol |
|
Beta-Sitosterol |
|
-7 kcal/mol |
|
Dammarenediol |
|
-6.9 kcal/mol |
|
Lupeol |
|
-6.8 kcal/mol |
|
Caffeic Acid |
|
-5.7 kcal/mol |
|
Chlorogenic Acid |
|
-8 kcal/mol |
|
Ferulic Acid |
|
-5.8 kcal/mol |
|
Oleanolic Acid |
|
-7.2 kcal/mol |
2.7.2. Comparing Ayurveda and Conventional Medicine
Since this is a comparative study, to compare the therapeutic potential of Ayurvedic herbs with conventional medications, docking scores of pharmaceutical drugs used to manage adverse drug reactions of treatments for chronic diseases were also analyzed. The 3D structure of the compounds in conventional drugs were obtained from Pubchem, and were processed through the same methodology of molecular docking as bioactive compounds of ayurvedic herbs, via CB Dock.
2.7.3. Attached below is the molecular docking results of conventional drugs to target proteins of adverse drug reactions
Abdominal or stomach pain - Cyclooxygenase-2 (COX-2) - Antacids
|
Modern Drug |
Molecular Docking Scores |
|
Calcium carbonate |
|
Bloating - Guanylate cyclase C (GC-C) - Simethicone and Antacids
|
Modern Drug |
Molecular Docking Scores |
|
Simethicone |
|
|
Modern Drug |
Molecular Docking Scores |
|
Calcium carbonate |
|
Constipation - Guanylate cyclase C (GC-C)
|
Modern Drug |
Molecular Docking Scores |
|
Magnesium citrate |
|
|
Lactitol |
|
Decreased Appetite - Ghrelin receptor (GHS-R1a) - Appetite stimulants and steroids
|
Modern Drug |
Molecular Docking Scores |
|
Megesterol acetate |
|
|
Medroxyprogesterone acetate |
|
|
Prednisone |
|
|
Dexamethasone |
|
Diarrhea - 5-HT3 receptor (serotonin receptor 3)
|
Modern Drug |
Molecular Docking Scores |
|
Loperamide |
|
Heartburn - H+/K+ ATPase
|
Modern Drug |
Molecular Docking Scores |
|
Calcium carbonate |
|
|
Cimetidine |
|
|
Famotidine |
|
|
Nizatidine |
|
|
Esomeprazole |
|
Cough - Transient Receptor Potential Cation Channel Subfamily V Member 1 (TRPV1)
|
Modern Drug |
Molecular Docking Scores |
|
Pholcodine |
|
|
Codeine |
|
|
Dextromethorphan |
|
Difficulty breathing - Beta-2 Adrenergic Receptor (β2AR)
|
Modern Drug |
Molecular Docking Scores |
|
Salbutamol |
|
|
Formoterol |
|
|
Ipratropium |
|
|
Tiotropium |
|
|
Aclidinium |
|
|
Glycopyrronium |
|
|
Theophylline |
|
Pneumonitis - Tumor Necrosis Factor-alpha (TNF-α) Receptor
|
Modern Drug |
Molecular Docking Scores |
|
Prednisone |
|
|
Mycophenolate |
|
|
Azathioprine |
|
|
Prednisolone |
|
|
Methylprednisolone |
|
|
Betamethasone |
|
|
Dexamethasone |
|
|
Triamcinolone |
|
|
Hydrocortisone |
|
Angioedema (swollen tissue) - Bradykinin Receptor (B2 receptor)
|
Modern Drug |
Molecular Docking Scores |
|
Loratadine |
|
|
Cetirizine |
|
|
Diphenhydramine |
|
|
Fexofenadine |
|
|
Prednisone |
|
Arrhythmia (irregular heartbeat) - Sodium Channels (Nav1.5)
|
Modern Drug |
Molecular Docking Scores |
|
Amiodarone |
|
|
Flecainide |
|
|
Sotalol |
|
Increased risk of blood clots - Factor Xa
|
Modern Drug |
Molecular Docking Scores |
|
Apixaban |
|
|
Betrixaban |
|
|
Dabigatran |
|
|
Edoxaban |
|
|
Rivaroxaban |
|
|
Warfarin |
|
Chest pain - Troponin I
|
Modern Drug |
Molecular Docking Scores |
|
Nitroglycerin |
|
|
Aspirin |
|
Rapid heartbeat (tachycardia) - β1-adrenergic receptor
|
Modern Drug |
Molecular Docking Scores |
|
Verapamil |
|
|
Diltiazem |
|
|
Metoprolol |
|
|
Nadolol |
|
|
Propranolol |
|
|
Adenosine |
|
High blood pressure (Hypertension) - Angiotensin-converting enzyme (ACE)
|
Modern Drug |
Molecular Docking Scores |
|
Chlorothiazide |
|
|
Hydrochlorothiazide |
|
|
Carvedilol |
|
Low blood pressure (Hypotension) - Alpha-1 Adrenergic Receptor
|
Modern Drug |
Molecular Docking Scores |
|
Fludrocortisone |
|
|
Midodrine |
|
Low white cell count (leukopenia) - Granulocyte colony-stimulating factor (G-CSF)
|
Modern Drug |
Molecular Docking Scores |
|
Penicillins |
|
|
Cephalosporins |
|
Blurred Vision - Vascular endothelial growth factor
|
Modern Drug |
Molecular Docking Scores |
|
Pilocarpine hydrochloride |
|
Fatigue - A2A adenosine receptor
|
Modern Drug |
Molecular Docking Scores |
|
Amitriptyline |
|
|
Desipramine |
|
|
Citalopram |
|
|
Escitalopram |
|
|
Fluoxetine |
|
|
Paroxetine |
|
|
Sertraline |
|
Hair loss - 5-alpha reductase enzyme
|
Modern Drug |
Molecular Docking Scores |
|
Finasteride |
|
|
Clobetasol propionate |
|
|
Hydrocortisone |
|
Joint pain - Tumor Necrosis Factor-alpha (TNF-alpha)
|
Modern Drug |
Molecular Docking Scores |
|
Morphine |
|
|
Tramadol |
|
|
Prednisone |
|
|
Ibuprofen |
|
|
Naproxen |
|
|
Diclofenac |
|
|
Acetaminophen |
|
|
Duloxetine |
|
|
Aspirin |
|
Muscle cramps - Ryanodine receptor 1 (RyR1)
|
Modern Drug |
Molecular Docking Scores |
|
Carisoprodol |
|
|
Aspirin |
|
|
Chlorzoxazone |
|
|
Cyclobenzaprine |
|
|
Metaxalone |
|
|
Methocarbamol |
|
|
Orphenadrine |
|
|
Tizanidine |
|
|
Ibuprofen |
|
|
Acetaminophen |
|
|
Naproxen |
|
Muscle weakness - Dystrophin
|
Modern Drug |
Molecular Docking Scores |
|
Prednisone |
|
Osteoporosis (bone weakening) - RANKL (Receptor Activator of Nuclear Factor Kappa-Β Ligand)
|
Modern Drug |
Molecular Docking Scores |
|
Alendronate |
|
|
Ibandronate |
|
|
Risedronate |
|
Fever - Cyclooxygenase-2 (COX-2)
|
Modern Drug |
Molecular Docking Scores |
|
Acetaminophen |
|
|
Ibuprofen |
|
|
Aspirin |
|
Anxiety - Serotonin 5-HT1A receptor
|
Modern Drug |
Molecular Docking Scores |
|
Clonazepam |
|
|
Alprazolam |
|
|
Lorazepam |
|
|
Bromazepam |
|
|
Oxazepam |
|
|
Chlordiazepoxide |
|
|
Clorazepate |
|
|
Diazepam |
|
Depression - Serotonin 5-HT1A receptor
|
Modern Drug |
Molecular Docking Scores |
|
Fluoxetine |
|
|
Paroxetine |
|
|
Fluvoxamine |
|
|
Citalopram |
|
|
Escitalopram |
|
|
Sertraline |
|
|
Venlafaxine |
|
|
Duloxetine |
|
|
Levomilnacipran |
|
|
Desvenlafaxine |
|
Difficulty sleeping (Insomnia) - GABA(A) receptor
|
Modern Drug |
Molecular Docking Scores |
|
Lorazepam |
|
|
Nitrazepam |
|
|
Oxazepam |
|
|
Temazepam |
|
|
Triazolam |
|
|
Flurazepam |
|
2.7.4. Results
Molecular docking scores of bioactive compounds present in Ayurvedic herbs often surpass those of conventional drugs, highlighting their potential as superior therapeutic agents. These strong interactions suggest that Ayurvedic compounds can effectively inhibit, activate, or modulate target proteins, potentially outperforming synthetic drugs in specificity and efficacy. Moreover, the natural origin and synergistic properties of these compounds reduce the likelihood of off-target effects, making them not only potent but also safer alternatives for therapeutic use. Such findings underscore the promise of bioactive compounds in managing adverse drug reactions of chronic disease treatments and redefining the role of natural products in healthcare.
2.8. Bioavailability Assessment
Bioavailability is a crucial factor in preclinical drug development, as it determines how much of an administered drug successfully reaches the systemic circulation (bloodstream). This metric is essential for evaluating the efficiency of drug absorption, as a drug must reach its target site in sufficient concentrations to produce a therapeutic effect.
To determine the exact bioavailability of a drug, experimental studies in animals or humans are required. These studies involve administering the drug through different routes and measuring how much of the drug appears in the bloodstream over time.
2.8.1. Definition of Oral Bioavailability
Oral bioavailability refers to the fraction (%) of an orally administered drug that enters the systemic circulation in an active form. Since orally administered drugs must pass through the digestive system before reaching the bloodstream, their bioavailability is often lower than drugs administered via intravenous (IV) injection, which delivers the drug directly into circulation.
2.8.2. Categories of Bioavailability
Bioavailability is categorized into two main types:
- Relative Bioavailability: This compares the bioavailability of two different formulations of the same drug. For example, comparing the bioavailability of a tablet versus a liquid formulation of the same drug. This is useful for evaluating different drug delivery systems or formulations to determine which one provides the best absorption.
- Absolute Bioavailability: This measures how much of a drug administered via a non-intravenous route (e.g., oral) reaches the bloodstream compared to the same drug given intravenously (IV), which is injected directly into the bloodstream. Since IV administration delivers 100% of the drug directly into circulation, oral bioavailability is expressed as a percentage of the IV dose. For example, if a drug has 50% absolute bioavailability, only half of the orally administered dose enters the bloodstream.
2.8.3. Which category for bioavailability is relative in this Research
This study aims to explore Ayurvedic herbs as alternatives to conventional oral drugs used for managing adverse drug reactions (ADRs) from chronic disease treatments. Since a drug’s therapeutic potential depends not only on its ability to bind to a target protein but also on its absorption in the body, it is crucial to assess the oral bioavailability of these herbs. Even if an Ayurvedic herb contains bioactive compounds that show strong binding to a target protein in molecular docking studies, it will not be effective unless the body can absorb and transport it to the intended site of action. Therefore, determining absolute bioavailability is a critical factor in evaluating the potential of these herbs as therapeutic agents.
2.8.3.1. How is Absolute Bioavailability Determined?
Formula for Absolute Bioavailability (F_abs):
F_abs = AUC_oral × Dose_IV / AUC_IV × Dose_oral
Where:
AUC_oral = Area under the concentration-time curve (AUC) for the non-IV route (e.g., oral administration).
AUC_IV = Area under the concentration-time curve for IV administration.
Dose_oral = Dose administered via a non-IV route.
Dose_IV = Dose administered via IV.
Absolute Bioavailability Ratings:
High (>85%) – Indicates that a large portion of the drug dose reaches systemic circulation; considered highly efficient.
Moderate (50% - 80%) – Indicates decent absorption.
Low (<50%) – Indicates poor absorption, possibly requiring higher doses or alternative non-oral administration methods (e.g., injections).
2.8.4. Understanding the Area Under the Curve (AUC)
Area Under the Curve (AUC) reflects exposure to a drug after administration, how much drug reaches a person’s bloodstream in a given period of time after a dose is given. The “curve” refers to a graph of drug concentration (amount in the blood) on the y-axis versus time on the x-axis.
- Higher AUC means greater drug exposure, thus has a higher chance of having a therapeutic effect.
- Lower AUC means less drug reaches circulation, therefore has a lower chance of having a therapeutic effect.
When a drug is administered, its concentration in the blood initially increases as it is absorbed, then peaks, and finally declines as it is broken down and eliminated from the body. The total area under this curve (AUC) represents the total drug exposure over time experienced by the body.
2.8.5. How Area Under the Curve (AUC) is Measured
To determine AUC, blood samples are collected at different time points after drug administration, to determine the drug concentration at different time periods.
Our blood is primarily composed of red blood cells (carriers of nutrients and oxygen), white blood cells (also called leukocytes, are a crucial part of the immune system, protecting the body against infection and disease by identifying and destroying harmful invaders), platelets (tiny cell fragments in your blood that are crucial for forming blood clots to stop bleeding when a blood vessel is damaged), and plasma (liquid portion of the blood, where platelets, red and white cells float).
Blood composition: Whole blood = Red blood cells + White blood cells + platelets + Plasma
Since plasma (the liquid component of blood, where red and white cells float) is the medium in which drugs are transported, it is used for measuring drug concentration, the procedure involves drawing a whole blood sample from the patient, then separating red and white blood cells from plasma, the plasma is withdrawn and analyzed for drug concentration, while the platelets, red and white cells are discarded. This analysis of drug concentration in plasma is done at different time points from when the drug was first administered, until the drug concentration becomes negligible (essentially 0).
Plasma composition: Plasma = Whole blood - (Red blood cells + White blood cells + platelets)
2.8.5.1. Calculating Area Under the Curve (AUC) Using the Linear Trapezoidal Method
The most versatile and commonly used method for calculating the Area Under the Curve (AUC) is the Linear Trapezoidal Method, the standard method required by the Food and Drug Administration (FDA). This method uses linear interpolation between data points to calculate the Area Under the Curve (AUC). Linear interpolation happens if the two known points are given by the coordinates (x0, y0) and (x1,y1), the linear interpolation is the straight line between these points. This method further calculates the area under this line segment as a trapezoid.
Formula for AUC of one time interval:
AUC = ½(C_1 + C_2)(t_2 - t_1) can also be written as AUC = (c_1 + c_2)(t_2 - t_1)/2
The two expressions are the same because multiplication is associative, meaning changing the grouping of factors in a multiplication problem doesn't change the product. In other words, (a*b)c = a*(b*c). In ½(C_1 + C_2)(t_2 - t_1), the grouping first pairs ½(C_1 + C_2) meaning (C_1 + C_2) is multiplied by ½ before being multiplied to (t_2 - t_1). On the other hand, in (c_1 + c_2)(t_2 - t_1)/2, the grouping first calculates (c_1 + c_2)(t_2 - t_1), then multiplies by ½, which results in division by two. This reordering of groups, made possible by the associative property of multiplication, ensures that both expressions yield the same final value.
Where:
C_1, C_2 = Drug concentrations at time points T1 and T2.
t_2 - t_1 = Time interval between measurements.
Concept:
- The first term ½ (C_1 + C_2), calculates the average drug concentration over the time interval.
- The second term (t_2 - t_1) represents the duration of that interval.
- Multiplying these gives the area under the curve for that segment.
Summing the AUC values for all time intervals gives the total AUC, which we need for our absolute bioavailability equation, and which reflects total drug exposure.
- Total AUC = Sum of AUC of all time intervals.
2.8.6 Why AUC is Important for Bioavailability Assessment
AUC provides raw data on drug absorption, but absolute bioavailability (F_abs) interprets this data by comparing oral to IV Area Under the Curve (AUC) values. Since IV administration gives the highest possible Area Under the Curve (AUC) value, comparing oral Area Under the Curve (AUC) to IV Area Under the Curve (AUC) helps set a benchmark, and on the basis of that assesses a drug’s potential for oral absorption.
- If F_abs > 50%, oral administration is considered effective.
- If F_abs < 50%, IV administration may be preferred, or strategies to improve oral absorption may be necessary.
2.8.7. Limitations in Determining AUC and Absolute Bioavailability in This Study
Although measuring Area Under the Curve (AUC) and absolute bioavailability would provide valuable insights into Ayurvedic herb absorption and its efficiency as well as feasibility as an oral drug, these experiments require blood sampling in humans or animals, which is not feasible due to ethical concerns and experimental resources constraints.
Research
2.9. Alternative Approach to Determine Oral Bioavailability of Ayurvedic Herbs: Lipinski’s Rule of Five
Since calculating Area Under the Curve (AUC) and absolute bioavailability through experiments are beyond the scope of this study, Lipinski’s Rule of Five will be used as an alternative method to predict the oral bioavailability of Ayurvedic herbs.
2.9.1. How the Drug Reaches Its Target After Oral Administration:
After oral administration, the drug undergoes a series of steps before it reaches its target site in the body. When a drug is ingested, it first enters the gastrointestinal (GI) tract,where it must dissolve into the aqueous environment of the stomach and intestines to be absorbed. This step is crucial because only dissolved drug molecules can be absorbed through the intestinal lining into the bloodstream. Hydrophilic (water-soluble) drugs have an advantage here because they can easily dissolve in the aqueous fluids of the stomach and intestines, facilitating absorption. Once the drug is dissolved in the gastrointestinal fluids, it must cross the intestinal epithelial cells (the cells that line the inner surface of the intestine, forming a barrier that protects the body from harmful substances while allowing for the absorption of nutrients from food) to enter the bloodstream. Here, the drug’s lipophilicity plays a crucial role. Lipophilic drugs can easily diffuse across the lipid bilayers of the intestinal cells by passive diffusion, a process where molecules move from an area of higher concentration (in the lumen of the intestines) to an area of lower concentration (the bloodstream). Once absorbed into the bloodstream, the drug is transported throughout the body. In the bloodstream, hydrophilic drugs are often more easily distributed because they can remain in the aqueous environment of the plasma. They are typically carried in the blood’s water-based solution and can reach a variety of tissues that are well-supplied with blood flow. One of the key characteristics of lipophilic drugs is their ability to cross biological membranes, which are of lipid or fat-like nature, including the blood-brain barrier (a protective layer of cells and tissue that surrounds the brain's blood vessels) or cell membranes (separates the interior of the cell from the outside environment). This allows lipophilic drugs to reach intracellular (within cells) targets, such as receptors or enzymes within cells. When the drug reaches its target site, it binds to specific molecules, usually receptors, enzymes, or proteins, on the surface of or within cells (intracellular). This interaction is highly specific, similar to a key fitting into a lock. The drug’s binding to its target initiates a biological response that causes the desired therapeutic effect. For example, a drug may bind to a receptor on the surface of a cell and activate an enzyme, receptor, or protein, that leads to a therapeutic outcome, such as pain relief, reduced inflammation, or regulation of blood pressure.
The equilibrium between hydrophilic and lipophilic characteristics is vital for a drug’s overall effectiveness. Hydrophilic drugs tend to have better solubility in water, making them easier to dissolve in the gastrointestinal tract and the blood, but they can encounter difficulty in crossing lipid membranes to reach intracellular targets. In contrast, lipophilic drugs are more adept at crossing lipid biological membranes to target intracellular sites, though they may face challenges in dissolving within the gastrointestinal tract and distributing effectively through the water-based bloodstream.
2.9.2. Polar Functional Groups and Molecular Interactions
Polar functional groups play a crucial role in determining the solubility, absorption, and distribution of a drug. These groups arise due to differences in electronegativity between atoms within a molecule, which influences how electrons are distributed in a chemical bond. Electronegativity is a chemical property that describes the tendency of an atom to attract negatively charged electrons towards itself.
When two bonded atoms have a significant difference in electronegativity, the electrons are pulled closer to the more electronegative atom rather than being evenly shared. This unequal charge distribution results in the formation of partial charges, where the more electronegative atom acquires a partial negative charge (δ⁻) due to the higher electron density, while the less electronegative atom develops a partial positive charge (δ⁺) due to electron deficiency. Higher electron density refers to an increased concentration of electrons around an atom, making it more negatively charged, whereas electron deficiency refers to a lower concentration of electrons, making the atom more positively charged.
As a result, the molecule forms polar functional groups within itself, meaning it has a distinct separation of charge, creating a dipole—a molecule with one partially positive and one partially negative end. The presence of polar functional groups influences how these molecules interact with their surroundings, particularly with other polar molecules like water. This significantly affects the solubility and absorption of a drug.
Polar functional groups enhance hydrophilicity (affinity for water or aqueous environments), enabling a molecule to dissolve in water-based environments such as blood and cellular fluids. This property is crucial for drug transport in the body and contributes to a drug’s oral bioavailability. Conversely, the absence of polar bonds within functional groups leads to lipophilicity (affinity for fat or lipid environments), enhancing the drug’s ability to cross lipid membranes to reach its target.
The balance between hydrophilic and lipophilic properties is critical for an oral drug’s overall success, as determined by Lipinski's Rule of Five. Hydrophilic drugs generally have better water solubility, making them easier to dissolve in the gastrointestinal tract, but they may struggle to cross lipid membranes to reach intracellular targets. Lipophilic drugs, on the other hand, are more capable of crossing biological membranes to reach intracellular targets, but they may face challenges with dissolution in the gastrointestinal tract and systemic distribution in the aqueous bloodstream.
2.9.3. Lipinski’s Rule of Five:
Lipinski’s Rule of Five is a widely used guideline in drug discovery and development that helps evaluate a compound’s potential as an orally active drug in humans. Introduced by Pfizer scientist Christopher Lipinski in 1997, this rule provides a framework for assessing the "drug-likeness" of small molecules based on their chemical properties. The rule is named the "Rule of Five" because its criteria involve numbers that are multiples of five. It states that a compound is more likely to have poor absorption in the body if it violates any of the following conditions: (1) a molecular weight exceeding 500 g/mol, (2) more than 5 hydrogen bond donors, (3) more than 10 hydrogen bond acceptors, and (4) a calculated logP value greater than 5. These parameters help ensure an optimal balance between lipophilicity (fat solubility) and hydrophilicity (water solubility), which is essential for drug absorption, distribution, and overall effectiveness. For this study, I applied Lipinski's Rule of Five to the bioactive compounds with the highest binding affinity to the target protein.
2.9.3.1. Molecular Weight Less than 500 g/mol
The size of a molecule significantly affects its ability to cross biological membranes and reach its target site in the body. Cell membranes, composed primarily of lipid bilayers, act as selective barriers that regulate the passage of molecules based on their size and solubility. Smaller molecules generally exhibit a balance between hydrophilicity and lipophilicity, just enough polar functional groups to facilitate water solubility while maintaining sufficient fat solubility to diffuse across biological membranes efficiently. They can diffuse, which is the movement of particles from higher to lower concentrations, in lipid-rich environments such as cell membranes, so they can reach their target. However, polar functional groups within the molecule introduce hydrophilic (water-soluble) properties, which allow it to dissolve in the aqueous environment of the gastrointestinal tract and blood, so they can transport throughout the body.
Optimal Size for Targeting: Smaller molecules also tend to exhibit better receptor binding due to their flexibility and ability to fit within active sites of biological targets, such as enzymes or receptors. This enhances their therapeutic effectiveness by improving binding affinity and interaction strength with their intended targets.
2.9.3.2 Optimal Lipophilicity: LogP Less than or Equal to 5
Lipophilicity refers to a molecule’s affinity for lipids or fat-like environments, enabling the molecule to dissolve in lipids, which influences its ability to cross biological membranes (e.g. cell membrane). The LogP value quantifies a compound's lipophilicity. Passive diffusion, the primary mechanism by which most small drug molecules traverse cell membranes, is the process to move from an area of higher concentration to one of lower concentration. This process is influenced by the molecule’s lipophilicity, allowing it to pass through the lipid bilayer efficiently.
- Too Lipophilic (LogP > 5): Indicates poor solubility in aqueous environments like blood or gastrointestinal fluids, and high ability to cross the lipid bilayers of cells.
- Too Hydrophilic (LogP < 0): High solubility in water and blood, leading to great distribution in the body, but limited ability to cross the lipid bilayers of cells, restricting the drug from reaching intracellular targets (within cells).
- Optimal Range (0 < LogP ≤ 5): Ensures the molecule is soluble enough in water for distribution in the body through bloodstream and absorption in the gastrointestinal tract. Also, allows the molecule to partition into and cross lipid bilayers efficiently via passive diffusion, allowing the drug to reach intracellular (within cells) targets. The optimal logP range ensures that the molecule can efficiently transition between these two phases of hydrophilicity and lipophilicity.
2.9.3.3. Hydrogen Bond Donors (≤5) and Acceptors (≤10)
Hydrogen bonding is a key factor that influences solubility and membrane permeability. Hydrogen bonds form due to the difference in electronegativity between atoms within a molecule, affecting how electrons are distributed in a chemical bond.
Electronegativity describes an atom’s tendency to attract electrons toward itself. When two bonded atoms have a significant difference in electronegativity, the more electronegative atom attracts electrons more strongly, creating partial charges within the molecule. This leads to the formation of polar functional groups, where the more electronegative atom carries a partial negative charge (δ⁻), while the less electronegative atom carries a partial positive charge (δ⁺). These polar bonds create dipoles within molecules, which influence how the molecule interacts with its surroundings, particularly with water and lipid membranes. The presence of these polar bonds form hydrogen bonds which enhances hydrophilicity (affinity for water or aqueous environments).
Balancing Hydrogen Bonding and Membrane Permeability: Molecules with too many hydrogen bond donors and acceptors tend to have high water solubility but struggle to penetrate lipid bilayers, reducing oral bioavailability. By limiting hydrogen bond donors to ≤5 and acceptors to ≤10, the molecule retains sufficient lipophilicity for effective membrane permeability while maintaining enough hydrophilicity for solubility in water-based environments like blood.
2.9.5. Analysis of Results
The analysis of the bioactive compounds from the Ayurvedic herbs, based on Lipinski's Rule of 5, revealed that at least one bioactive compound, of an ayurveda herb, which has high binding affinity scores to their target proteins passed this benchmark. These compounds exhibited favorable characteristics for oral bioavailability, with molecular weights under 500 daltons, minimal hydrogen bond donors and acceptors, and acceptable logP values. This suggests that ayurvedic herbs have a high potential for effective oral absorption, supporting their viability as potential treatments for the adverse drug reactions of chronic disease treatments.
Abdominal Pain - Cyclooxygenase-2 (COX-2)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Proanthocyanidins (-7.7 kcal/mol) |
Molecular Weight: 594.5 g/mol Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 13 LogP: 2 |
No doesn’t fit the criteria. |
|
Chebulagic Acid (-7.5 kcal/mol) |
Molecular Weight: 954.7 g/mol LogP: 0.4 Hydrogen Bond Donor Count: 13 Hydrogen Bond Acceptor Count: 27 |
No doesn’t fit the criteria. |
|
Emblicanin B (-7.5 kcal/mol) |
Molecular Weight: 780.5 g/mol LogP: 1.3 Hydrogen Bond Donor Count: 12 Hydrogen Bond Acceptor Count: 22 |
No doesn’t fit the criteria. |
|
Chebulinic acid (-7.3 kcal/mol) |
Molecular Weight: 956.7 g/mol LogP: 0.7 Hydrogen Bond Donor Count: 13 Hydrogen Bond Acceptor Count: 27 |
No doesn’t fit the criteria. |
|
Ellagic acid (-7.2 kcal/mol) |
Molecular Weight: 302.19 g/mol LogP: 1.1 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 8 |
Yes it does! |
|
Emblicanin A (-7.4 kcal/mol) |
Molecular Weight: 782.5 g/mol LogP: 1.6 Hydrogen Bond Donor Count: 12 Hydrogen Bond Acceptor Count: 22 |
No doesn’t fit the criteria. |
Bloating - Guanylate cyclase C (GC-C)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Thymol (-6 kcal/mol) |
Molecular Weight: 150.22 g/mol LogP: 3.3 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
Yes it does! |
|
Carvacrol (-6.4 kcal/mol) |
Molecular Weight: 150.22 g/mol LogP: 3.1 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
Yes it does! |
Constipation - Guanylate cyclase C (GC-C)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Chebulinic acid (-8.6 kcal/mol) |
Molecular Weight: 956.7 g/mol LogP: 0.7 Hydrogen Bond Donor Count: 13 Hydrogen Bond Acceptor Count: 27 |
No doesn’t fit the criteria. |
|
Ellagic acid (-8.4 kcal/mol) |
Molecular Weight: 302.19 g/mol LogP: 1.1 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 8 |
Yes it does! |
|
Proanthocyanidins (-8.3 kcal/mol) |
Molecular Weight: 594.5 g/mol Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 13 LogP: 2 |
No doesn’t fit the criteria. |
|
Chebulagic Acid (-8.2 kcal/mol) |
Molecular Weight: 954.7 g/mol LogP: 0.4 Hydrogen Bond Donor Count: 13 Hydrogen Bond Acceptor Count: 27 |
No doesn’t fit the criteria. |
|
Quercetin (-8.2 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
|
Emblicanin A (-8.3 kcal/mol) |
Molecular Weight: 782.5 g/mol LogP: 1.6 Hydrogen Bond Donor Count: 12 Hydrogen Bond Acceptor Count: 22 |
No doesn’t fit the criteria. |
|
Emblicanin B (-8.5 kcal/mol) |
Molecular Weight: 780.5 g/mol LogP: 1.3 Hydrogen Bond Donor Count: 12 Hydrogen Bond Acceptor Count: 22 |
No doesn’t fit the criteria. |
Decreased appetite - Ghrelin receptor (GHS-R1a)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Quercetin (-8 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
|
Betulinic Acid (-8.4 kcal/mol) |
Molecular Weight: 456.7 g/mol LogP: 8.2 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 3 |
No doesn’t fit the criteria. |
|
Oleanolic Acid (-8.6 kcal/mol) |
Molecular Weight: 456.7 g/mol LogP: 7.5 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 3 |
No doesn’t fit the criteria. |
|
β-Sitosterol (-8.1 kcal/mol) |
Molecular Weight: 414.7 g/mol LogP: 9.3 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
Diarrhea - 5-HT3 receptor (serotonin receptor 3)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Ursolic Acid (-8.3 kcal/mol) |
Molecular Weight: 456.7 g/mol LogP: 7.3 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 3 |
No doesn’t fit the criteria. |
|
Oleanolic Acid (-7.9 kcal/mol) |
Molecular Weight: 456.7 g/mol LogP: 7.5 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 3 |
No doesn’t fit the criteria. |
|
Rutin (-7.4 kcal/mol) |
Molecular Weight: 610.5 g/mol LogP: -1.3 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 16 |
No doesn’t fit the criteria. |
|
Aegeline (-6.6 kcal/mol) |
Molecular Weight: 297.3 g/mol LogP: 2.4 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 3 |
Yes it does! |
|
Apigenin (-6.6 kcal/mol) |
Molecular Weight: 270.24 g/mol LogP: 1.7 Hydrogen Bond Donor Count: 3 Hydrogen Bond Acceptor Count: 5 |
Yes it does! |
|
Aegle Marmelos (-6.6 kcal/mol) |
Molecular Weight: 365.5 g/mol LogP: 4.5 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 3 |
Yes it does! |
Heartburn - H+/K+ ATPase
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Emblicanin A (-9.2 kcal/mol) |
Molecular Weight: 782.5 g/mol LogP: 1.6 Hydrogen Bond Donor Count: 12 Hydrogen Bond Acceptor Count: 22 |
No doesn’t fit the criteria. |
|
Emblicanin B (-9.2 kcal/mol) |
Molecular Weight: 780.5 g/mol LogP: 1.3 Hydrogen Bond Donor Count: 12 Hydrogen Bond Acceptor Count: 22 |
No doesn’t fit the criteria. |
|
Ellagic Acid (-8.4 kcal/mol) |
Molecular Weight: 302.19 g/mol LogP: 1.1 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 8 |
Yes it does! |
|
Quercetin (-8.2 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
Cough - Transient Receptor Potential Cation Channel Subfamily V Member 1 (TRPV1)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Rutin (-9.2 kcal/mol) |
Molecular Weight: 610.5 g/mol LogP: -1.3 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 16 |
No doesn’t fit the criteria. |
|
Stigmasterol (-8.7 kcal/mol) |
Molecular Weight: 412.7 g/mol LogP: 8.6 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
|
Luteolin (-8.5 kcal/mol) |
Molecular Weight: 286.24 g/mol LogP: 1.4 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Apigenin (-8.2 kcal/mol) |
Molecular Weight: 270.24 g/mol LogP: 1.7 Hydrogen Bond Donor Count: 3 Hydrogen Bond Acceptor Count: 5 |
Yes it does! |
|
Quercetin (-8.2 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
|
Rosmarinic Acid (-8.2 kcal/mol) |
Molecular Weight: 360.3 g/mol LogP: 2.4 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 8 |
Yes it does! |
Difficulty breathing - Beta-2 Adrenergic Receptor (β2AR)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Rutin (-10.6 kcal/mol) |
Molecular Weight: 610.5 g/mol LogP: -1.3 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 16 |
No doesn’t fit the criteria. |
|
Quercetin (-9.8 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
|
Piperine (-9.6 kcal/mol) |
Molecular Weight: 285.34 g/mol LogP: 3.5 Hydrogen Bond Donor Count: 0 Hydrogen Bond Acceptor Count: 3 |
Yes it does! |
|
Stigmasterol (-9.6 kcal/mol) |
Molecular Weight: 412.7 g/mol LogP: 8.6 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
|
Campesterol (-9.2 kcal/mol) |
Molecular Weight: 400.7 g/mol LogP: 8.8 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
|
Diosgenin (-9.1 kcal/mol) |
Molecular Weight: 414.6 g/mol LogP: 5.7 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 3 |
Yes it does! |
|
Chavicine (-8.9 kcal/mol) |
Molecular Weight: 285.34 g/mol LogP: 3.5 Hydrogen Bond Donor Count: 0 Hydrogen Bond Acceptor Count: 3 |
Yes it does! |
Pneumonitis - Tumor Necrosis Factor-alpha (TNF-α) Receptor
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Catechin (-7.5 kcal/mol) |
Molecular Weight: 290.27 g/mol LogP: 0.4 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Ellagic acid (-7.4 kcal/mol) |
Molecular Weight: 302.19 g/mol LogP: 1.1 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 8 |
Yes it does! |
Angioedema (swollen tissue) - Bradykinin Receptor (B2 receptor)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Ellagic Acid (-8.1 kcal/mol) |
Molecular Weight: 302.19 g/mol LogP: 1.1 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 8 |
Yes it does! |
|
Bisdemethoxycurcumin (-7.8 kcal/mol) |
Molecular Weight: 308.3 g/mol LogP: 3.3 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 4 |
Yes it does! |
|
Kaempferol (-7.7 kcal/mol) |
Molecular Weight: 286.24 g/mol LogP: 1.9 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
Arrhythmia (irregular heartbeat) - Sodium Channels (Nav1.5)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Arjunetin (-10.8 kcal/mol) |
Molecular Weight: 650.8 g/mol LogP: 3.4 Hydrogen Bond Donor Count: 7 Hydrogen Bond Acceptor Count: 10 |
No doesn’t fit the criteria. |
|
Arjungenin (-9.9 kcal/mol) |
Molecular Weight: 504.7 g/mol LogP: 4.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Stigmasterol (-9.6 kcal/mol) |
Molecular Weight: 412.7 g/mol LogP: 8.6 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
|
Arjunolic Acid (-9.3 kcal/mol) |
Molecular Weight: 488.7 g/mol LogP: 5.8 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 5 |
Yes it does! |
|
Rutin (-9.2 kcal/mol) |
Molecular Weight: 610.5 g/mol LogP: -1.3 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 16 |
No doesn’t fit the criteria. |
Increased risk of blood clots - Factor Xa
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Chebulagic Acid (-9.6 kcal/mol) |
Molecular Weight: 954.7 g/mol LogP: 0.4 Hydrogen Bond Donor Count: 13 Hydrogen Bond Acceptor Count: 27 |
No doesn’t fit the criteria. |
|
Procyanidin B1 (-9.3 kcal/mol) |
Molecular Weight: 578.5 g/mol LogP: 2.4 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 12 |
No doesn’t fit the criteria. |
|
Procyanidin B2 (-9.2 kcal/mol) |
Molecular Weight: 578.5 g/mol LogP: 2.4 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 12 |
No doesn’t fit the criteria. |
|
Prodelphinidin B3 (-8.6 kcal/mol) |
Molecular Weight: 610.5 g/mol LogP: 1.7 Hydrogen Bond Donor Count: 12 Hydrogen Bond Acceptor Count: 14 |
|
|
Ellagic Acid (-7.3 kcal/mol) |
Molecular Weight: 302.19 g/mol LogP: 1.1 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 8 |
Yes it does! |
Chest pain - Troponin I
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Stigmasterol (-7 kcal/mol) |
Molecular Weight: 412.7 g/mol LogP: 8.6 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
|
Rutin (-6.8 kcal/mol) |
Molecular Weight: 610.5 g/mol LogP: -1.3 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 16 |
No doesn’t fit the criteria. |
|
Arjunolic Acid (-6.7 kcal/mol) |
Molecular Weight: 488.7 g/mol LogP: 5.8 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 5 |
Yes it does! |
Rapid heartbeat (tachycardia) - β1-adrenergic receptor
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Stigmasterol (-9.9 kcal/mol) |
Molecular Weight: 412.7 g/mol LogP: 8.6 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
|
β-Sitosterol (-9.5 kcal/mol) |
Molecular Weight: 414.7 g/mol LogP: 9.3 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
|
Quercetin (-9.4 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
|
Kaempferol (-9.3 kcal/mol) |
Molecular Weight: 286.24 g/mol LogP: 1.9 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Myricetin (-9.2 kcal/mol) |
Molecular Weight: 318.23 g/mol LogP: 1.2 Hydrogen Bond Donor Count: 6 Hydrogen Bond Acceptor Count: 8 |
No doesn’t fit the criteria. |
|
Curcumin (-9.2 kcal/mol) |
Molecular Weight: 368.4 g/mol LogP: 3.2 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
High blood pressure (Hypertension) - Angiotensin-converting enzyme (ACE)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Rutin (-10.5 kcal/mol) |
Molecular Weight: 610.5 g/mol LogP: -1.3 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 16 |
No doesn’t fit the criteria. |
|
Arjungenin (-10 kcal/mol) |
Molecular Weight: 504.7 g/mol LogP: 4.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Arjunetin (-9.7 kcal/mol) |
Molecular Weight: 650.8 g/mol LogP: 3.4 Hydrogen Bond Donor Count: 7 Hydrogen Bond Acceptor Count: 10 |
No doesn’t fit the criteria. |
|
Arjunolic Acid (-9.5 kcal/mol) |
Molecular Weight: 488.7 g/mol LogP: 5.8 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 5 |
Yes it does! |
Low blood pressure (Hypotension) - Alpha-1 Adrenergic Receptor
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Withanolide D (-9.4 kcal/mol) |
Molecular Weight: 470.6 g/mol LogP: 3.1 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
Low white cell count (leukopenia) - Granulocyte colony-stimulating factor (G-CSF)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Stigmasterol (-9.2 kcal/mol) |
Molecular Weight: 412.7 g/mol LogP: 8.6 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
|
Columbin (-9 kcal/mol) |
Molecular Weight: 358.4 g/mol LogP: 2.2 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
Blurred Vision - Vascular endothelial growth factor
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Emblicanin B (-9.7 kcal/mol) |
Molecular Weight: 780.5 g/mol LogP: 1.3 Hydrogen Bond Donor Count: 12 Hydrogen Bond Acceptor Count: 22 |
No doesn’t fit the criteria. |
|
Emblicanin A (-9.5 kcal/mol) |
Molecular Weight: 782.5 g/mol LogP: 1.6 Hydrogen Bond Donor Count: 12 Hydrogen Bond Acceptor Count: 22 |
No doesn’t fit the criteria. |
|
Quercetin (-8.7 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
|
Ellagic Acid (-8.5 kcal/mol) |
Molecular Weight: 302.19 g/mol LogP: 1.1 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 8 |
Yes it does! |
Fatigue - A2A adenosine receptor
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Withanolide D (-10.8 kcal/mol) |
Molecular Weight: 470.6 g/mol LogP: 3.1 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Withaferin A (-10 kcal/mol) |
Molecular Weight: 470.6 g/mol LogP: 3.8 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
Hair loss - 5-alpha reductase enzyme
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Ecliptasaponin D (-11.3 kcal/mol) |
Molecular Weight: 634.8 g/mol LogP: 5.3 Hydrogen Bond Donor Count: 6 Hydrogen Bond Acceptor Count: 9 |
No doesn’t fit the criteria. |
|
Rutin (-11 kcal/mol) |
Molecular Weight: 610.5 g/mol LogP: -1.3 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 16 |
No doesn’t fit the criteria. |
|
β-Sitosterol (-10.3 kcal/mol) |
Molecular Weight: 414.7 g/mol LogP: 9.3 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
|
Apigenin (-9.6 kcal/mol) |
Molecular Weight: 270.24 g/mol LogP: 1.7 Hydrogen Bond Donor Count: 3 Hydrogen Bond Acceptor Count: 5 |
Yes it does! |
|
Quercetin (-9.6 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
Joint pain - Tumor Necrosis Factor-alpha (TNF-alpha)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Boswellic acid (-7.4 kcal/mol) |
Molecular Weight: 456.7 g/mol LogP: 8.4 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 3 |
No doesn’t fit the criteria. |
|
β-Boswellic acid (-7.4 kcal/mol) |
Molecular Weight: 456.7 g/mol LogP: 8.3 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 3 |
No doesn’t fit the criteria. |
|
Keto boswellic acid (-7.4 kcal/mol) |
Molecular Weight: 470.7 g/mol LogP: 7.2 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 4 |
Yes it does! |
|
Acetyl boswellic acid (-7.3 kcal/mol) |
Molecular Weight: 498.7 g/mol LogP: 8.3 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 4 |
No doesn’t fit the criteria. |
|
Quercetin (-7.1 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
Muscle cramps - Ryanodine receptor 1 (RyR1)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Luteolin (-8.3 kcal/mol) |
Molecular Weight: 286.24 g/mol LogP: 1.4 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Quercetin (-8.2 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
|
Rutin (-8.1 kcal/mol) |
Molecular Weight: 610.5 g/mol LogP: -1.3 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 16 |
No doesn’t fit the criteria. |
|
Apigenin (-8 kcal/mol) |
Molecular Weight: 270.24 g/mol LogP: 1.7 Hydrogen Bond Donor Count: 3 Hydrogen Bond Acceptor Count: 5 |
Yes it does! |
Muscle weakness - Dystrophin
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Withanolide D (-10.1 kcal/mol) |
Molecular Weight: 470.6 g/mol LogP: 3.1 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Somniferine (-9.8 kcal/mol) |
Molecular Weight: 608.7 g/mol LogP: 2.6 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 9 |
No doesn’t fit the criteria. |
Osteoporosis (bone weakening) - RANKL (Receptor Activator of Nuclear Factor Kappa-Β Ligand)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Rutin (-7.8 kcal/mol) |
Molecular Weight: 610.5 g/mol LogP: -1.3 Hydrogen Bond Donor Count: 10 Hydrogen Bond Acceptor Count: 16 |
No doesn’t fit the criteria. |
|
Beta-carotene (-7.5 kcal/mol) |
Molecular Weight: 536.9 g/mol LogP: 13.5 Hydrogen Bond Donor Count: 0 Hydrogen Bond Acceptor Count: 0 |
No doesn’t fit the criteria. |
|
3-Keto-β-sitosterol (-7 kcal/mol) |
Molecular Weight: 428.7 g/mol LogP: 8.3 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 2 |
No doesn’t fit the criteria. |
|
β-Sitosterol (-6.8 kcal/mol) |
Molecular Weight: 414.7 g/mol LogP: 9.3 Hydrogen Bond Donor Count: 1 Hydrogen Bond Acceptor Count: 1 |
No doesn’t fit the criteria. |
|
Quercetin (-6.5 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
|
Kaempferol (-6.4 kcal/mol) |
Molecular Weight: 286.24 g/mol LogP: 1.9 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
Fever - Cyclooxygenase-2 (COX-2)
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Quercetin (-9.1 kcal/mol) |
Molecular Weight: 302.23 g/mol LogP: 1.5 Hydrogen Bond Donor Count: 5 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
|
Protopine (-9 kcal/mol) |
Molecular Weight: 353.4 g/mol LogP: 2.8 Hydrogen Bond Donor Count: 0 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
Anxiety - Serotonin 5-HT1A receptor
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Somniferine (-9.5 kcal/mol) |
Molecular Weight: 608.7 g/mol LogP: 2.6 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 9 |
No doesn’t fit the criteria. |
|
Withanolide D (-9.3 kcal/mol) |
Molecular Weight: 470.6 g/mol LogP: 3.1 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Withanolide E (-9.2 kcal/mol) |
Molecular Weight: 486.6 g/mol LogP: 1.7 Hydrogen Bond Donor Count: 3 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
Depression - Serotonin 5-HT1A receptor
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Somniferine (-9.5 kcal/mol) |
Molecular Weight: 608.7 g/mol LogP: 2.6 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 9 |
No doesn’t fit the criteria. |
|
Withanolide D (-9.3 kcal/mol) |
Molecular Weight: 470.6 g/mol LogP: 3.1 Hydrogen Bond Donor Count: 2 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Withanolide E (-9.2 kcal/mol) |
Molecular Weight: 486.6 g/mol LogP: 1.7 Hydrogen Bond Donor Count: 3 Hydrogen Bond Acceptor Count: 7 |
Yes it does! |
Difficulty sleeping (Insomnia) - GABA(A) receptor
|
Highest Compound |
Lipinski’s rule of 5 |
Fits the criteria? |
|
Luteolin (-8 kcal/mol) |
Molecular Weight: 286.24 g/mol LogP: 1.4 Hydrogen Bond Donor Count: 4 Hydrogen Bond Acceptor Count: 6 |
Yes it does! |
|
Chlorogenic Acid (-8 kcal/mol) |
Molecular Weight: 354.31 g/mol LogP: -0.4 Hydrogen Bond Donor Count: 6 Hydrogen Bond Acceptor Count: 9 |
No doesn’t fit the criteria. |
Data
3. Final Result of This Study
Attached below is the table summarizing the results of this study. In the table, through molecular docking, the bioactive compounds with the highest (most negative) binding affinity scores for each herb are compared directly to the conventional drugs with the highest (most negative) binding affinity scores, to compare both of their therapeutic potential. We can clearly see that the bioactive compounds have mostly higher or similar binding affinity scores to conventional drugs, and even the low scores are not now enough to be considered ineffective, they are usually very close, emphasizing on their potential to replace conventional drugs without having additional adverse drug reactions.
The bioactive compounds of ayurveda herbs, with the highest binding affinity scores were also examined through Lipinski’s Rule of Five, to ensure that they are orally bioavailable, and can effectively reach their target proteins within the body, when consumed. The bioactive compounds, with the highest binding affinity score, after passing the requirements of Lipinski’s Rule of Five, were compared with conventional drugs, so there is a side by side comparison of the bioactive compounds with high binding affinity, which are also orally bioavailable to the conventional drugs used. The Lipinski’s Rule of Five wasn’t applied to conventional drugs, because they are oral drugs already being widely used, which means they are already orally bioavailable, that’s why they are effective. The ones highlighted in green, symbolize higher binding affinity compared to the other.
Of the various binding affinity scores provided via molecular docking, the highest binding affinity score is commonly used when comparing Ayurvedic herbs with conventional medicines in molecular docking for several important reasons. First, it reflects the strongest interaction between an active compound in an herb or a drug, and its target protein or receptor. This is crucial because the most potent interaction is likely to be the most biologically relevant and effective, making it a key indicator of a compound's potential to influence the biological activity of the target. Additionally, focusing on the highest binding affinity ensures researchers are considering the "best-case" or most favorable interaction, which provides a more conservative and reliable estimate of the compound's potential efficacy. This is particularly important when a compound may have multiple binding modes (specific orientations and interaction patterns of a molecule when it binds to its target protein or receptor) in its interactions. The average score can be skewed by weaker interactions or outliers, by focusing on the highest score, researchers avoid the risk of outliers and highlight the compound's best fit with the target, which is of primary interest in drug development and herbal medicine analysis. When comparing Ayurvedic herbs with conventional medicines, using the highest binding affinity score allows for a more direct comparison of each compound's maximum possible efficacy, ensuring that the analysis focuses on the most promising candidates, regardless of weaker interactions. Ultimately, using the highest binding affinity score helps ensure that the most potent interactions are taken into account, leading to more informed decisions about which compounds to pursue for further research and potential therapeutic applications.
Abdominal or stomach pain - Cyclooxygenase-2 (COX-2)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Triphala - Ellagic Acid - -7.2 kcal/mol |
Calcium carbonate - -3.5 kcal/mol |
Bloating - Guanylate cyclase C (GC-C)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Ajwain - Carvacrol - -6.4 kcal/mol |
Simethicone - -3.5 kcal/mol |
Constipation - Guanylate cyclase C (GC-C)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Triphala - Ellagic acid - -8.4 kcal/mol |
Lactitol - -6.1 kcal/mol |
Decreased appetite - Ghrelin receptor (GHS-R1a)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Chitrak - Quercetin - -8 kcal/mol |
Prednisone - -8.2 kcal/mol |
Diarrhea - 5-HT3 receptor (serotonin receptor 3)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Bael - Aegeline - -6.6 kcal/mol Apigenin - -6.6 kcal/mol Aegle Marmelos - -6.6 kcal/mol |
Loperamide - -7.9 kcal/mol |
Heartburn - H+/K+ ATPase
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Amla - Ellagic Acid - -8.4 kcal/mol |
Esomeprazole - -7.6 kcal/mol |
Cough - Transient Receptor Potential Cation Channel Subfamily V Member 1 (TRPV1)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Tulsi - Luteolin - -8.5 kcal/mol |
Pholcodine - -8.7 kcal/mol |
Difficulty breathing - Beta-2 Adrenergic Receptor (β2AR)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Pippali - Quercetin - -9.8 kcal/mol |
Aclidinium - -9.4 kcal/mol |
Pneumonitis - Tumor Necrosis Factor-alpha (TNF-α) Receptor
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Haridra - Catechin - -7.5 kcal/mol |
Methylprednisolone - -7 kcal/mol |
Angioedema (swollen tissue) - Bradykinin Receptor (B2 receptor)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Turmeric - Ellagic Acid - -8.1 kcal/mol |
Fexofenadine - -8 kcal/mol |
Arrhythmia (irregular heartbeat) - Sodium Channels (Nav1.5)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Arjuna - Arjungenin - -9.9 kcal/mol |
Flecainide - -8.1 kcal/mol |
Increased risk of blood clots - Factor Xa
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Bibhitaki - Ellagic Acid - -7.3 kcal/mol |
Rivaroxaban - -9.7 kcal/mol |
Chest pain - Troponin I
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Arjuna - Arjunolic Acid - -6.7 kcal/mol |
Aspirin - -4.5 kcal/mol |
Rapid heartbeat (tachycardia) - β1-adrenergic receptor
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Arjuna - Quercetin - -9.4 kcal/mol |
Nadolol - -8.8 kcal/mol |
High blood pressure (Hypertension) - Angiotensin-converting enzyme (ACE)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Arjuna - Arjungenin - -10 kcal/mol |
Carvedilol - -7.7 kcal/mol |
Low blood pressure (Hypotension) - Alpha-1 Adrenergic Receptor
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Ashwagandha - Withanolide D - -9.4 kcal/mol |
Fludrocortisone - -7.7 kcal/mol |
Low white cell count (leukopenia) - Granulocyte colony-stimulating factor (G-CSF)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Guduchi - Columbin - -9 kcal/mol |
Penicillins - -7 kcal/mol |
Blurred Vision - Vascular endothelial growth factor
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Amla - Quercetin - -8.7 kcal/mol |
Pilocarpine hydrochloride - -5.7 kcal/mol |
Fatigue - A2A adenosine receptor
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Ashwagandha - Withanolide D - -10.8 kcal/mol |
Citalopram - -9.5 kcal/mol Escitalopram - -9.5 kcal/mol |
Hair loss - 5-alpha reductase enzyme
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Bhringraj - Apigenin - -9.6 kcal/mol Bhringraj - Quercetin - -9.6 kcal/mol |
Hydrocortisone - -10.2 kcal/mol |
Joint pain - Tumor Necrosis Factor-alpha (TNF-alpha)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Shallaki - Keto boswellic acid - -7.4 kcal/mol |
Naproxen - 7.7 kcal/mol |
Muscle cramps - Ryanodine receptor 1 (RyR1)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Nirgundi - Luteolin - -8.3 kcal/mol |
Naproxen - -7.4 kcal/mol |
Muscle weakness - Dystrophin
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Ashwagandha - Withanolide D - -10.1 kcal/mol |
Prednisone - -8.8 kcal/mol |
Osteoporosis (bone weakening) - RANKL (Receptor Activator of Nuclear Factor Kappa-Β Ligand)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Hadjod - Quercetin - -6.5 kcal/mol |
Ibandronate - -5 kcal/mol Risedronate - -5 kcal/mol |
Fever - Cyclooxygenase-2 (COX-2)
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Giloy - Quercetin - -9.1 kcal/mol |
Ibuprofen - -7.1 kcal/mol |
Anxiety - Serotonin 5-HT1A receptor
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Ashwagandha - Withanolide D - -9.3 kcal/mol |
Alprazolam - -8.1 kcal/mol |
Depression - Serotonin 5-HT1A receptor
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Ashwagandha - Withanolide D - -9.3 kcal/mol |
Paroxetine - -7.8 kcal/mol |
Difficulty sleeping (Insomnia) - GABA(A) receptor
|
Ayurveda Herb - Bioactive Compound |
Conventional Medicine |
|
Tagara - Luteolin - -8 kcal/mol |
Temazepam - -7.4 kcal/mol |
4. Limitations
While the primary limitations and key constraints associated with the methodologies employed in this study are discussed in detail within their respective sections, the following provides a broad overview of the overarching limitations of this research as a whole, without focusing extensively on any specific computational or analytical technique.
4.1. Limitations of This Specific Study
This study is not without its limitations, which must be considered when interpreting the results. One key limitation is the study's reliance on silico methods.
4.1.1. Dependence on Computational Approaches Without Experimental Validation
One of the most significant limitations of this study is its reliance on computational tools, particularly molecular docking simulations and Lipinski’s Rule of Five, for evaluating the therapeutic potential of bioactive compounds in Ayurvedic herbs. While these methods provide valuable preliminary insights into drug-target interactions, they remain predictive models rather than definitive evidence of real-world efficacy. Without in vitro (cell culture-based) and in vivo (animal-based) experimental validation, this study is unable to determine whether the binding predictions from molecular docking translate into actual therapeutic effects within living organisms. To establish more concrete evidence, laboratory-based experiments must be conducted, including enzyme assays, pharmacokinetic profiling, and whole-organism studies, before these compounds can be seriously considered for therapeutic application.
4.1.1.1. Enzyme Assays
Enzyme assays are laboratory techniques used to measure the activity of enzymes, which are proteins that act as biological catalysts in the body. A catalyst is something that speeds up a chemical reaction without being used up in the process. Enzymes are essential for life because they control nearly every biochemical reaction in cells, such as breaking down food, generating energy, and detoxifying harmful substances.
Enzyme assays are particularly important in drug research because many adverse drug reactions (ADRs) occur when a treatment accidentally interferes with an enzyme’s normal function. For example, if a drug inhibits (blocks) an enzyme that is needed to break down toxins, harmful substances may build up in the body, leading to side effects. Conversely, if a compound activates an enzyme too much, it may cause an overreaction in the body that leads to disease. Studying how Ayurvedic compounds interact with enzymes can help determine whether they have therapeutic benefits through modulating enzyme activity.
To perform an enzyme assay, scientists first isolate the enzyme they want to study. This can be done by extracting it from cells or using a genetically engineered (whose genetic material has been altered using technology) version of the enzyme produced in a laboratory. The enzyme is then mixed with a substrate, which is the molecule that the enzyme normally acts on. In a normal reaction, the enzyme speeds up the transformation of the substrate into a product (formed after the enzyme has catalyzed the reaction). If a test compound is added and it slows down the reaction, the compound is likely an enzyme inhibitor. If it speeds up the reaction, it is likely an enzyme activator. Scientists measure the speed of the reaction using techniques like spectrophotometry (which measures changes in light absorption), fluorometry (which detects fluorescent signals from the reaction), or colorimetric assays (which track color changes in the solution). The results help researchers determine how strongly the Ayurvedic compound interacts with the enzyme, which can provide insight into its potential medicinal effects.
4.1.1.2. Pharmacokinetic Profiling
Pharmacokinetics is the study of how a drug or compound moves through the body after it is taken. It is often summarized by the acronym ADME, which stands for Absorption, Distribution, Metabolism, and Excretion. Understanding pharmacokinetics is essential because even if a compound shows great therapeutic potential in laboratory tests, it may be useless in real life if it is not properly absorbed, distributed, metabolized, or excreted.
- Absorption: This refers to how a compound enters the bloodstream after being consumed. For example, some drugs are quickly absorbed in the stomach, while others require processing in the intestines.
- Distribution: Once absorbed, the compound must travel to the right part of the body.
- Metabolism: This refers to how the body breaks down the compound, usually in the liver, through enzyme activity. Some drugs are rapidly destroyed, making them ineffective, while others are converted into active forms that work even better.
- Excretion: Finally, the compound must be removed from the body, usually through urine or feces. If a compound is not excreted efficiently, it may accumulate in tissues and become toxic over time.
Pharmacokinetic profiling is done using several methods. Caco-2 permeability assays test whether a compound can pass through intestinal cells to simulate absorption. Microsome stability assays use liver enzymes to study how quickly the compound is broken down. Animal studies track the compound in blood and urine over time to determine how long it stays in the body.
4.1.1.3. Whole-Organism Studies
Whole-organism studies, also known as in vivo studies, involve testing a compound in a living organism rather than isolated cells. While in vitro tests (like cytotoxicity and enzyme assays) provide important insights, they cannot fully replicate the complex interactions that occur in a real biological system.
Whole-organism studies are critical for determining:
- Real-world efficacy: Does the Ayurvedic compound actually reduce adverse drug reactions in a living body?
- Behavioral effects: Some compounds affect brain function, energy levels, or movement, which cannot be observed in a computational approach.
In these studies, animals such as mice, rats, or zebrafish are given the compound orally, by injection, or through inhalation. Researchers monitor their weight, activity levels, organ health, and biochemical markers in blood tests. Tissue samples are examined under a microscope to detect any signs of damage.
Although animal studies provide crucial data, ethical concerns have led researchers to develop alternative methods, such as organ-on-a-chip technology, which simulates human organ functions in a controlled laboratory setting.
4.1.2. Absence of Clinical Data and Human Trials
Another critical limitation of this study is the lack of human clinical trials to support the theoretical predictions made through computational analysis. Without clinical validation, there is no conclusive evidence to determine whether Ayurvedic herbs can effectively manage adverse drug reactions (ADRs) in real-world patients. Future research must focus on structured clinical trials that assess both the short-term and long-term effects of these herbal interventions.
4.2. Limitations of Ayurvedic Herbs’ Implementation in Modern Medicine
The integration of Ayurvedic herbs into modern medicine presents several challenges that hinder their widespread adoption. While Ayurvedic practices have been used for centuries, their implementation in contemporary healthcare systems is limited by factors such as the lack of standardized formulations, insufficient clinical evidence, and regulatory concerns.
4.2.1. Lack of Large-Scale Scientific Validation
One of the biggest challenges facing the integration of Ayurvedic herbs into modern medicine is the lack of large-scale, placebo-controlled, double-blind clinical trials, which are required for regulatory approval by agencies like the FDA (U.S.), EMA (Europe), and WHO. Most Ayurvedic treatments have historical and anecdotal evidence rather than rigorous scientific validation, making it difficult for physicians to recommend them as primary treatment options. Until standardized clinical studies are conducted to validate their safety, efficacy, and dosage, Ayurvedic medicines will continue to face skepticism from the global medical community.
4.2.2. Standardization and Quality Control Challenges in Herbal Medicine
Another important limitation is the inconsistency in the composition of herbal medicines, which makes reproducibility a challenge. Unlike synthetic drugs, which are manufactured under strict pharmaceutical standards with precisely defined chemical structures, Ayurvedic herbs are derived from natural plant sources, meaning their composition can vary significantly depending on geographical location, soil conditions, climate, harvest time, and processing methods.
This variability can lead to inconsistencies in potency, efficacy, and safety between different batches of the same herbal medicine. Moreover, standardized extraction and formulation protocols are not always established for many Ayurvedic herbs, leading to batch-to-batch variations in the concentration of active compounds. Contamination with pesticides, heavy metals, or microbial toxins can further compromise safety. Without rigorous quality control measures, the reproducibility and reliability of Ayurvedic treatments remain a major concern. This concern will be resolved if future research focuses on developing standardized protocols for herbal extraction, purification, and formulation to ensure consistent therapeutic outcomes.
Herbal extraction, purification, and formulation are critical steps to ensure that herbal medicines are both effective and safe. In the extraction process, solvents like alcohol or carbon dioxide are used to draw out the active compounds from plants, with careful control over factors like solvent strength and extraction time to guarantee the right ingredients are obtained. The purification step uses techniques such as High-Performance Liquid Chromatography (HPLC) or Thin-Layer Chromatography (TLC) to separate the beneficial compounds from any unwanted substances. Once purified, quality control tests are then performed to check the concentration of active ingredients and ensure the product is free from harmful contaminants. These standardized steps help maintain the reliability, safety, and therapeutic effectiveness of herbal medicines.
5. Importance and Further Implications of the Study
The study bridges the gap between traditional Ayurvedic knowledge and modern scientific validation, presenting significant opportunities to transform healthcare practices. By exploring Ayurvedic herbs as alternatives for managing adverse drug reactions (ADRs) associated with chronic disease treatments, it highlights their potential to revolutionize patient care, enhance sustainability, and inspire interdisciplinary innovation. The following sections delve into the broader importance and potential implications of this groundbreaking research.
5.1. Reduction in Polypharmacy
This study offers a pathway to reduce the reliance on multiple synthetic drugs commonly prescribed to manage chronic diseases and their associated adverse drug reactions (ADRs). By scientifically validating the therapeutic potential of Ayurvedic herbs, healthcare providers can explore safer, natural alternatives that address adverse drug reactions (ADRs) of chronic diseases without introducing additional adverse drug reactions (ADRs). Breaking the cycle of polypharmacy, where one drug’s adverse drug reactions (ADRs) necessitate the use of another, could significantly improve treatment outcomes. Reduced dependency on multiple medications also minimizes risks such as drug-drug interactions, organ damage, and cognitive impairments often observed in patients undergoing long-term pharmacological treatments.
5.2. Advancements in Herbal Drug Development
Apart from ayurvedic herbs addressing just the adverse drug reactions of chronic diseases treatments, this research inspires a shift towards further exploring and optimizing natural substances, fostering innovation and expanding the scope of herbal medicine in modern healthcare for the betterment of patients. As we saw in the study, ayurveda herbs had successful outcomes when binding to target proteins involved in fever, anxiety, depression, and insomnia.
Through this study we hypothesize that in the future, Ayurvedic herbs have the potential to replace over-the-counter drugs and medications for mental health conditions like depression, insomnia, and anxiety, offering a more natural and sustainable alternative for individuals seeking relief, providing a gentler, safer approach compared to conventional pharmaceutical drugs. Many pharmaceutical drugs used to treat mental health issues come with a range of undesirable adverse drug reactions, such as weight gain, dependency, cognitive impairment, and long-term health risks, which greatly hinders patients’ quality of life. Ayurvedic herbs, on the other hand, are typically associated with no adverse drug reactions, offering a more favorable option for individuals seeking effective treatment without the risk of harmful consequences. This could make mental health treatments safer and more accessible, especially for long-term use, without the fear of developing dependency or experiencing adverse reactions.
Over-the-counter drugs, such as acetaminophen and ibuprofen, are commonly used to treat ailments like fever, pain, and inflammation, offering a quick and effective solution for many. These medications are widely accessible, easy to use, and come in various forms, including liquids and chewables for children, making them convenient for families to manage mild health issues at home. However, over time, frequent or excessive use of over-the-counter drugs can still lead to potential risks, such as liver damage from overuse of acetaminophen or gastrointestinal issues from long-term ibuprofen use, to name a few, although all the risks are mentioned above in my the paper. Ayurvedic herbs, however, present an opportunity to replace these over-the-counter medications with more natural, sustainable alternatives, providing benefits without the risk of long-term side effects. In addition to being gentle on the body, these herbs come with no adverse drug reactions compared to synthetic medications. By using ayurvedic herbs as replacements for over-the-counter drugs, people could benefit from a safer, natural remedy that addresses symptoms without the risks of dependency or harm from long-term use. As further research continues to validate the effectiveness of herbal remedies, they could become a more widely accepted, sustainable option for managing common conditions like fever, reducing the need for over-the-counter medications while providing individuals with a safer, more natural approach to healthcare.
5.3. Global Acceptance of Ayurveda
One of the significant barriers to the integration of Ayurveda into contemporary healthcare systems is the lack of scientific validation. This study bridges that gap by providing evidence-based insights into the efficacy and safety of Ayurvedic herbs. As a result, it paves the way for global acceptance, further clinical studies, experiments, and inclusion of Ayurveda in mainstream medicine. Validated Ayurvedic treatments could gain recognition in international healthcare guidelines, fostering a deeper appreciation of traditional medicine while enhancing its credibility among medical professionals and regulatory bodies worldwide.
5.4. Patient-Centric Care
Chronic diseases are a leading cause of morbidity and mortality worldwide, and their treatment often involves long-term medication use that can lead to severe adverse drug reactions (ADRs). This study addresses a critical gap by exploring Ayurvedic herbs as safer alternatives, offering a novel approach to mitigate these adverse drug reactions (ADRs) effectively. By providing an evidence-based framework, the research emphasizes the importance of integrating natural therapies into chronic disease management, potentially transforming patient care and outcomes globally. Ayurvedic herbs with minimal to no adverse drug reactions (ADRs) can significantly enhance patients' quality of life, particularly for those managing chronic conditions over extended periods, because now they can break the "cycle of medication".
5.5. Environmental and Sustainable Practices
Encouraging the use of Ayurvedic herbs not only benefits individual patients but also supports sustainable and environmentally friendly healthcare practices. The cultivation of medicinal plants promotes biodiversity and ecological balance, offering a renewable resource for developing treatments. By integrating these herbs into healthcare systems, there is potential to create demand for sustainable farming practices and conservation efforts. This approach aligns with global goals for environmental sustainability and ensures the long-term availability of valuable medicinal resources.
5.6. Future Research Directions
This study provides a strong foundation for future interdisciplinary research and clinical trials. By validating Ayurvedic herbs through rigorous scientific computational methods, it invites further exploration into experiments, clinical trials, their molecular mechanisms, optimal dosages, and long-term effects. Moreover, this research establishes a model that can be applied to other traditional medicinal systems, encouraging a broader investigation into natural remedies and their potential contributions to modern healthcare.
5.7. Interdisciplinary Collaboration
This research exemplifies the power of interdisciplinary collaboration by combining traditional knowledge with modern scientific techniques. The integration of Ayurveda with tools such as molecular docking and Lipinski’s Rule of Five highlights a holistic approach to innovation in healthcare. Such collaborations bridge the gap between traditional and modern medicine, fostering mutual respect and understanding among diverse scientific communities. This paradigm shift could inspire similar interdisciplinary initiatives aimed at addressing complex healthcare challenges.
Conclusion
6. Conclusion
The integration of Ayurvedic herbs into modern medicine as a safer alternative for managing adverse drug reactions (ADRs) of treatment for chronic diseases offers a promising solution to the pressing issue of polypharmacy and the cycle of medication dependency. By leveraging advanced computational tools such as molecular docking and Lipinski's Rule of Five, this study establishes a robust and systematic framework for evaluating the therapeutic potential and oral bioavailability of these herbs. This dual approach not only bridges the gap between traditional Ayurvedic knowledge and contemporary scientific standards but also emphasizes the importance of evidence-based validation for gaining acceptance in global healthcare systems.
The findings of this comparative study reveal that Ayurvedic herbs, enriched with bioactive compounds possess strong binding affinities to target proteins associated with adverse drug reaction pathways, most even surpassing the binding affinities of conventional drugs used to combat adverse drug reactions of chronic disease treatments. These herbs demonstrate a unique ability to address adverse drug reactions while simultaneously avoiding the cascading adverse drug reactions often caused by synthetic drugs used to address the adverse drug reaction of treatment for chronic diseases. The bioactive compounds with the highest binding affinity were also examined for their oral bioavailability using Lipinski's Rule of Five to ensure that the herbs can reach their targets effectively to have a therapeutic impact. Their natural compatibility with the human body underscores their role as holistic and sustainable solutions that enhance patient outcomes by reducing dependency on multiple medications and breaking the debilitating cycle of medication.
This study underscores the critical challenge of polypharmacy, the simultaneous use of several medications to counteract adverse drug reactions of primary treatments, which frequently compounds health issues and diminishes patient quality of life. Ayurvedic herbs, with no adverse drug reactions of their own, present a compelling alternative by mitigating the need for additional medications and offering a safer, more sustainable pathway for managing adverse drug reactions of chronic disease treatments.
While the results are highly encouraging, the study emphasizes the necessity for further validation through in vitro (controlled experiments conducted outside of living organisms, such as in petri dishes) and in vivo (experiments conducted within living organisms) testing. Large-scale clinical trials are also crucial to determine the efficacy and safety of these treatments in real-world scenarios. Additionally, addressing challenges such as standardization, quality control, and regulatory compliance will be pivotal for the successful integration of Ayurvedic remedies into modern medical practices.
In its current state, this research not only highlights the therapeutic potential of Ayurvedic herbs in addressing the adverse drug reactions of treatments for chronic diseases, but also establishes a foundation for interdisciplinary collaboration and future studies. The findings support a harmonious coexistence of traditional and modern medicine, paving the way for integrative healthcare approaches that prioritize patient safety, efficacy, and sustainability. Ultimately, the study affirms that with continued innovation and rigorous validation, Ayurvedic herbs have the potential to revolutionize healthcare, and significantly improve the quality of life for patients worldwide.
Citations
8. Citations and Sources
Abosamak, N. R., & Shahin, M. H. (2023, July 3). Beta2 receptor agonists and antagonists. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK559069/
About chronic diseases. (2024, October 4). Chronic Disease. https://www.cdc.gov/chronic-disease/about/index.html
Ahmad, H., Khan, H., Haque, S., Ahmad, S., Srivastava, N., & Khan, A. (2023). Angiotensin-Converting Enzyme and Hypertension: A systemic analysis of various ACE inhibitors, their side effects, and bioactive peptides as a putative therapy for hypertension. Journal of the Renin-Angiotensin-Aldosterone System, 2023. https://doi.org/10.1155/2023/7890188
Akimova, E., Lanzenberger, R., & Kasper, S. (2009). The Serotonin-1A receptor in anxiety disorders. Biological Psychiatry, 66(7), 627–635. https://doi.org/10.1016/j.biopsych.2009.03.012
Andrei, I. I. (2004). Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Frontiers in Bioscience, 9(1–3), 1977. https://doi.org/10.2741/1383
Azam, Z., Sapra, L., Baghel, K., Sinha, N., Gupta, R. K., Soni, V., Saini, C., Mishra, P. K., & Srivastava, R. K. (2023). Cissus quadrangularis (Hadjod) Inhibits RANKL-Induced Osteoclastogenesis and Augments Bone Health in an Estrogen-Deficient Preclinical Model of Osteoporosis Via Modulating the Host Osteoimmune System. Cells, 12(2), 216. https://doi.org/10.3390/cells12020216
Basu, S. (2024, April 24). Hadjod: Health benefits, nutritional values, therapeutic uses, dosage and side effects. Netmeds. https://www.netmeds.com/health-library/post/hadjod-health-benefits-nutritional-values-therapeutic-uses-dosage-and-side-effects?srsltid=AfmBOopPwgibQJUHiR_8P_cCOGojIfj-ScQi-SfOgbwj8ya5hlCPfzxJ&utm
Begum, S., Lee, M. R., Gu, L. J., Hossain, M. J., Kim, H. K., & Sung, C. K. (2014). Comparative Hair restorer efficacy of medicinal herb on nude (FOxN1NU) mice. BioMed Research International, 2014, 1–9. https://doi.org/10.1155/2014/319795
Bertoncini-Silva, C., Vlad, A., Ricciarelli, R., Fassini, P. G., Suen, V. M. M., & Zingg, J. (2024). Enhancing the bioavailability and bioactivity of curcumin for disease prevention and treatment. Antioxidants, 13(3), 331. https://doi.org/10.3390/antiox13030331
Betz, D., & Fane, K. (2023, August 14). Human chorionic gonadotropin. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK532950/
Bibhitaki | Herbs | Herbal Reality. (n.d.). Herbal Reality. https://www.herbalreality.com/herb/bibhitaki/
Boskabady, M. H., Alitaneh, S., & Alavinezhad, A. (2014). Carum copticumL.: A Herbal Medicine with Various Pharmacological Effects. BioMed Research International, 2014, 1–11. https://doi.org/10.1155/2014/569087
Causes/Inheritance - Duchenne Muscular dystrophy (DMD) - Diseases | Muscular Dystrophy Association. (2024, April 5). Muscular Dystrophy Association. https://www.mda.org/disease/duchenne-muscular-dystrophy/causes-inheritance
CB-Dock: An accurate protein-ligand blind docking tool. (n.d.). http://clab.labshare.cn/cb-dock/
Comparative study of immunomodulating activity of Indian medicinal plants, lithium carbonate and glucan. (1988, October 1). PubMed. https://pubmed.ncbi.nlm.nih.gov/3236938/
COX-2 inhibitors. (2025, January 24). Cleveland Clinic. Retrieved March 16, 2025, from https://my.clevelandclinic.org/health/drugs/23119-cox-2-inhibitors
Database, A. P. S. (n.d.). AlphaFold Protein Structure Database. https://alphafold.ebi.ac.uk/
De Bem Alves, A. C., De Souza Santos, N., Santos, A. P. T., Da Panatta, G., Speck, A. E., Cunha, R. A., & Aguiar, A. S. (2024). Adenosine A2A and dopamine D2 receptor interaction controls fatigue resistance. Frontiers in Pharmacology, 15. https://doi.org/10.3389/fphar.2024.1390187
DMD gene: MedlinePlus Genetics. (n.d.). https://medlineplus.gov/genetics/gene/dmd/
Dwivedi, S., & Chopra, D. (2014). Revisiting Terminalia arjuna – An Ancient Cardiovascular Drug. Journal of Traditional and Complementary Medicine, 4(4), 224–231. https://doi.org/10.4103/2225-4110.139103
Ent, B., & Ent, B. (2025, January 13). Understanding the mechanism of bradykinin antagonists in hereditary angioedema. Penn Medicine Becker ENT & Allergy. https://www.beckerentandallergy.com/blog/bradykinin-antagonists-in-hereditary-angioedema
George, A. M., & Liu, M. (2023, July 31). Ropivacaine. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK532924/
Gottesmann, C. (2002). GABA mechanisms and sleep. Neuroscience, 111(2), 231–239. https://doi.org/10.1016/s0306-4522(02)00034-9
Government of Canada, Statistics Canada. (2023, November 29). A glimpse at the health of Canadians. Statistics Canada. https://www.statcan.gc.ca/o1/en/plus/5102-glimpse-health-canadians
Guo, S., & Rezaei, M. J. (2024). The benefits of ashwagandha (Withania somnifera) supplements on brain function and sports performance. Frontiers in Nutrition, 11. https://doi.org/10.3389/fnut.2024.1439294
Herbals, V. (2020, December 11). Triphala for constipation: Best time to take, dosage, effectiveness. HempCann Solutions. https://vediherbals.com/blogs/blog/best-way-to-take-triphala-for-constipation#:~:text=A%3A%20Triphala%20stimulates%20peristalsis%2C%20the,which%20contribute%20to%20relieving%20constipation
Jang, D., Lee, A., Shin, H., Song, H., Park, J., Kang, T., Lee, S., & Yang, S. (2021). The role of tumor necrosis factor alpha (TNF-Α) in autoimmune disease and current TNF-Α inhibitors in therapeutics. International Journal of Molecular Sciences, 22(5), 2719. https://doi.org/10.3390/ijms22052719
Jayasinghe, M., Caldera, D., Prathiraja, O., Jena, R., Coffie-Pierre, J. A., Agyei, J., Silva, M. S., Kayani, A. M. A., & Siddiqui, O. S. (2022). A comprehensive review of Bradykinin-Induced angioedema versus Histamine-Induced angioedema in the Emergency Department. Cureus. https://doi.org/10.7759/cureus.32075
Kaufman, J., DeLorenzo, C., Choudhury, S., & Parsey, R. V. (2016). The 5-HT1A receptor in Major Depressive Disorder. European Neuropsychopharmacology, 26(3), 397–410. https://doi.org/10.1016/j.euroneuro.2015.12.039
Kaur, N., Shafiq, N., Negi, H., Pandey, A., Reddy, S., Kaur, H., Chadha, N., & Malhotra, S. (2014). Terminalia arjunain Chronic Stable Angina: Systematic Review and Meta-Analysis. Cardiology Research and Practice, 2014, 1–7. https://doi.org/10.1155/2014/281483
Khalid, S., Murdoch, R., Newlands, A., Smart, K., Kelsall, A., Holt, K., Dockry, R., Woodcock, A., & Smith, J. A. (2014). Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: A double-blind randomized controlled trial. Journal of Allergy and Clinical Immunology, 134(1), 56-62.e4. https://doi.org/10.1016/j.jaci.2014.01.038
Kommu, S., Carter, C., & Whitfield, P. (2024, January 10). Adverse drug reactions. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK599521/
Liu, Y., Yang, X., Gan, J., Chen, S., Xiao, Z., & Cao, Y. (2022). CB-Dock2: improved protein–ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Research, 50(W1), W159–W164. https://doi.org/10.1093/nar/gkac394
Majeed, A., Majeed, S., Satish, G., Manjunatha, R., Rabbani, S. N., Patil, N. V. P., & Mundkur, L. (2024). A standardized Boswellia serrata extract shows improvements in knee osteoarthritis within five days-a double-blind, randomized, three-arm, parallel-group, multi-center, placebo-controlled trial. Frontiers in Pharmacology, 15. https://doi.org/10.3389/fphar.2024.1428440
Matsumoto, T., & Endo, I. (2020). RANKL as a target for the treatment of osteoporosis. Journal of Bone and Mineral Metabolism, 39(1), 91–105. https://doi.org/10.1007/s00774-020-01153-7
Merecz, K., Hirsa, M., Biniszewska, O., Fichna, J., & Tarasiuk, A. (2023). An overview of 5-HT3 receptor antagonists as a treatment option for irritable bowel syndrome with diarrhea. Expert Opinion on Pharmacotherapy, 24(10), 1189–1198. https://doi.org/10.1080/14656566.2023.2214314
Monika, S., Thirumal, M., & Kumar, P. (2023). Phytochemical and Biological review of Aegle Marmelos Linn. Future Science OA, 9(3). https://doi.org/10.2144/fsoa-2022-0068
Mukhopadhyay, S., Hoidal, J. R., & Mukherjee, T. K. (2006). Role of TNFα in pulmonary pathophysiology. Respiratory Research, 7(1). https://doi.org/10.1186/1465-9921-7-125
Muscular Dystrophy Ayurvedic Treatment and Herbal Cure. (n.d.). Pure Herbal Ayurved Clinic. https://www.pureherbalayurved.com.au/muscular-dystrophy-natural-treatment-melbourne.htm
Nashine, S., Kanodia, R., Nesburn, A. B., Soman, G., Kuppermann, B. D., & Kenney, M. C. (2019). Nutraceutical effects of Emblica officinalis in age-related macular degeneration. Aging, 11(4), 1177–1188. https://doi.org/10.18632/aging.101820
Office of Dietary Supplements - Ashwagandha: Is it helpful for stress, anxiety, or sleep? (n.d.). https://ods.od.nih.gov/factsheets/Ashwagandha-HealthProfessional/
Peterson, C. T., Denniston, K., & Chopra, D. (2017). Therapeutic Uses ofTriphalain Ayurvedic Medicine. The Journal of Alternative and Complementary Medicine, 23(8), 607–614. https://doi.org/10.1089/acm.2017.0083
PubChem. (n.d.). PubChem. PubChem. https://pubchem.ncbi.nlm.nih.gov/
Ramaswamy, S. (2018). Reflections on current Ayurveda research. Journal of Ayurveda and Integrative Medicine, 9(4), 250–251. https://doi.org/10.1016/j.jaim.2018.11.001
Roberts, K. C., Rao, D. P., Bennett, T. L., Loukine, L., & Jayaraman, G. C. (2015). Prevalence and patterns of chronic disease multimorbidity and associated determinants in Canada. Health Promotion and Chronic Disease Prevention in Canada, 35(6), 87–94. https://doi.org/10.24095/hpcdp.35.6.01
Role of Terminalia arjuna in improving cardiovascular functions : a review. (2018). Indian J Physiol Pharmacol, 62(1), 8–19. https://ijpp.com/IJPP%20archives/2018_62_1/8-19.pdf
Salisbury, B. H., Leslie, S. W., & Tadi, P. (2024, June 8). 5Α-Reductase inhibitors. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK555930/
Salve, J., Pate, S., Debnath, K., & Langade, D. (2019). Adaptogenic and anxiolytic effects of ashwagandha root extract in healthy adults: a double-blind, randomized, placebo-controlled clinical study. Cureus. https://doi.org/10.7759/cureus.6466
Saxena, K. (2024, May 18). Giloy: Benefits, uses, side effects and more. MyHealth. https://redcliffelabs.com/myhealth/health/giloy-benefits-uses-side-effects-and-more/
Sharma, A. & JMPS. (2024). The review study on Haridra medicinal plant (Curcuma longa). Journal of Medicinal Plants Studies, 63–66. https://www.plantsjournal.com/archives/2024/vol12issue3/PartA/12-2-2-212.pdf
Sharma, P. (2024, February 23). Ayurveda for muscle cramps: Proven Natural Remedies to Soothe aching legs. Netmeds. https://www.netmeds.com/health-library/post/ayurveda-for-muscle-cramps-proven-natural-remedies-to-soothe-aching-legs?srsltid=AfmBOopfnfvvdv-u9RJ5LAK8QNIaKonkHp4oJDrPWvQJXf48tnqwnOiJ
Shin, J. M., Munson, K., Vagin, O., & Sachs, G. (2008). The gastric HK-ATPase: structure, function, and inhibition. Pflügers Archiv - European Journal of Physiology, 457(3), 609–622. https://doi.org/10.1007/s00424-008-0495-4
Song, W., & Shou, W. (2012). Cardiac sodium channel NAV1.5 mutations and cardiac arrhythmia. Pediatric Cardiology, 33(6), 943–949. https://doi.org/10.1007/s00246-012-0303-y
Team, A. (n.d.). Avogadro - Free cross-platform molecular editor. Avogadro. https://avogadro.cc/
Team, Z. A. (2023, December 7). Pippali (Long pepper) Benefits, uses & precautions. Zandu Care. https://zanducare.com/blogs/exploring-ayurveda/pippali-guide-to-ayurveda?srsltid=AfmBOoryCn-xnCME2S68JVapfksjCKSCbBzalrpLK2tTOxh9e6m4UWbT
Tilak, J. C., Adhikari, S., & Devasagayam, T. P. (2004). Antioxidant properties ofPlumbago zeylanica, an Indian medicinal plant and its active ingredient, plumbagin. Redox Report, 9(4), 219–227. https://doi.org/10.1179/135100004225005976
Toolika, E., Bhat, N., & Shetty, S. (2015). A comparative clinical study on the effect of Tagara (Valeriana wallichii DC.) and Jatamansi (Nardostachys jatamansi DC.) in the management of Anidra (primary insomnia). AYU (an International Quarterly Journal of Research in Ayurveda), 36(1), 46. https://doi.org/10.4103/0974-8520.169008
Ugolev, Y., Berdichevsky, Y., Weinbaum, C., & Pick, E. (2008). Dissociation of RAC1(GDP)·RHOGDI complexes by the cooperative action of anionic liposomes containing phosphatidylinositol 3,4,5-Trisphosphate, RAC guanine nucleotide exchange factor, and GTP. Journal of Biological Chemistry, 283(32), 22257–22271. https://doi.org/10.1074/jbc.m800734200
Varnosfaderani, S. K., Hashem-Dabaghian, F., Amin, G., Bozorgi, M., Heydarirad, G., Nazem, E., Toosi, M. N., & Mosavat, S. H. (2018). Efficacy and safety of Amla ( Phyllanthus emblica L.) in non-erosive reflux disease: a double-blind, randomized, placebo-controlled clinical trial. Journal of Integrative Medicine, 16(2), 126–131. https://doi.org/10.1016/j.joim.2018.02.008
Waldman, S. A., & Camilleri, M. (2018). Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut, 67(8), 1543–1552. https://doi.org/10.1136/gutjnl-2018-316029
Wang, L., Zhou, C., Zhu, D., Wang, X., Fang, L., Zhong, J., Mao, Q., Sun, L., Gong, X., Xia, J., Lian, B., & Xie, P. (2016). Serotonin-1A receptor alterations in depression: a meta-analysis of molecular imaging studies. BMC Psychiatry, 16(1). https://doi.org/10.1186/s12888-016-1025-0
Xiang, T., Liao, J., Cai, Y., Fan, M., Li, C., Zhang, X., Li, H., Chen, Y., & Pan, J. (2023). Impairment of GABA inhibition in insomnia disorders: Evidence from the peripheral blood system. Frontiers in Psychiatry, 14. https://doi.org/10.3389/fpsyt.2023.1134434
Yetman, D. (2020, March 27). Does Ayurvedic medicine effectively treat cough, sore throat, and other cold symptoms? Healthline. https://www.healthline.com/health/ayurvedic-medicine-for-cough
Acknowledgement
7. Acknowledgement
I would like to thank the many people who have helped me throughout the completion of this research paper, all of whom played an essential role in its success. Firstly, I would like to thank my parents for their unwavering support throughout this journey. Their encouragement helped me push through, even when things seemed overwhelming, and their thoughtfulness, like packing my lunch when I forgot to because I was working too late. Without their constant love and support, I would not have been able to finish this paper. I would also like to thank my English teacher, Ms. Qureshi, for her time and effort in proofreading my work, providing valuable feedback, and ensuring that my ideas were clearly expressed. Additionally, I want to express my gratitude to my math teacher, Mr. Cameroux, for his patience and guidance when I struggled to understand the equations involved in my research. I want to acknowledge the significant contributions of everyone involved in helping me with this project. Your support, encouragement, and assistance have made this paper possible, and I am deeply grateful to each of you. Thank you all so much!

