Impact of Mycorrhizal Fungi on Water Uptake in Common Ivy within Closed Mesocosms
Grade 11
Presentation
Hypothesis
Research question: How does the addition of varying mycorrhizal fungi concentrations (0.00g, 2.00g, 3.00g, 4.00g, 5.00g) affect the soil moisture levels of common ivy (Hedera helix) in a closed mesocosm over 14 days?
Hypothesis: The addition of varying concentration of mycorrhizal fungi (0.00g, 2.00g, 3.00g, 4.00g, 5.00g) will significantly affect the soil moisture levels of Hedera helix (common ivy) in a closed mesocosm system over a 14-day period. Specifically, it is expected that higher concentrations of mycorrhizal fungi will result in increased soil moisture retention compared to the control group (0.00g). Additionally, a dose-dependent relationship is expected, with intermediate concentration (3.00g and 4.00g) producing optimal moisture retention in the soil, while lower and higher concentration may produce negligible results.
Research
Introduction
Mycorrhizal Fungi
Sustainability refers to the productive performance of a system over time. It implies successful use of resources over time for agriculture to satisfy human needs while maintaining or enhancing the quality of the environment and conserving natural resources (Liu et al., 2022). With a faster-growing global market compared to agrochemicals (Querejeta et al., 2016), it is becoming more difficult to reduce the use of agricultural practices and their harmful impact on wildlife, which contributes to the acceleration of climate change (Querejeta et al., 2016). Microbial inoculants are beneficial microorganisms or mixtures of microorganisms applied to either soil or the plant to improve soil quality and crop productivity (Liu et al., 2022). Microbial inoculants are gaining more importance and attention in practical agriculture due to their minimal hazardous influence on the environment, soil, plant, and human health (Liu et al., 2022). Mycorrhizal fungi are an example of microbial inoculants, originating from the term "mycorrhiza," which describes the symbiotic relationship between plant roots and fungi (Masse et al., 1981). Mycorrhiza results from mutualism between the fungi and the roots of terrestrial plants (Masse et al., 1981). Arbuscular mycorrhizal fungi are the most common mycorrhizal association in most plants and agricultural crops (Liu et al., 2022). Arbuscular mycorrhiza produces large thick-walled resting spores called extrametricular chlamydospores which can survive a range of soil conditions and germinate when conditions are favorable (Beaulieu, 2024). The germ tubes penetrate the host root and form an appressorium on the root surface, allowing penetration into the root cortex (Gubbs, n.d.). The fungi exist by taking sugars from plants in exchange for moisture and nutrients gathered from the soil by the fungal strains (Mycorrhizal Fungi I RHS, n.d.).
Common Ivy (Hedera helix)
Common Ivy (Hedera helix) is an evergreen perennial. With its hardy, fast-growing abilities, the plant is native to Europe and Western Asia (Beaulieu, 2024). It is grown primarily for its evergreen leaves but will eventually bear insignificant greenish flowers (Beaulieu, 2024). Ivy plants are highly adaptable and capable of thriving in low light and nutrient-limited conditions, marking their efficiency in utilizing available resources, making it a well-fit candidate for studying the effects of mycorrhizal fungi on plant transpiration (Gubbs, n.d.). Hedera helix has a vast network of roots that allow for effective water uptake. Furthermore, the plant demonstrates steady transpiration rates under stable conditions, and ivy serves as an effective option for examining water dynamics within closed ecosystems (Beaulieu, 2024). Common ivy is characterized by its extensive and highly adaptable root system. Its roots not only anchor the plant but also play an important role in its ability to thrive in diverse environmental conditions (Allen et al., 2021). Ivy's roots exhibit significant surface area and branching, which enhances their capacity to absorb water and nutrients, making them an ideal partner for symbiotic interactions with the observed fungal inoculant (Miller, 2020). Mycorrhizal fungi adhere to and penetrate the cortical cells of the roots, forming specialized structures such as arbuscules and vesicles that facilitate resource exchange (Taylor et al., 2014). In this mutualistic relationship, the fungi increase the absorption surface area of the plant's roots through their hyphal networks, thus enabling more efficient water uptake and nutrient acquisition (Brown & Thompson, 2021).
Mycorrhizal Fungi and Hedera Helix
The mycorrhizas greatly increase the absorptive area of a plant, acting as an extension to the root system. As the extremely fine threads of mycorrhizal hyphae grow to fill the soil volume of the root zone, they act as a sponge to absorb water when it becomes available (Querejeta et al., 2016). The fungal structures, including the hyphae and vesicles, are essentially microscopic water reservoirs attached to the plants, which increase the plant's ability to absorb and utilize the resource. These benefits of mycorrhizal-inoculated plants include the ability to withstand drought conditions and decrease the labor and water involved in keeping potted plants stable and alive through extremely hot temperatures (Beaulieu, 2024).
In closed mesocosms, the potential of mycorrhizal fungi to enhance plant water uptake can be observed through changes in soil moisture levels (Querejeta et al., 2016). The fungi can indirectly affect soil moisture content by increasing the plant's transpiration rate and efficiency, which serves as an indicator of the plant's overall health and water usage (Gubbs, n.d.).
Variables
Independent Variable: Concentration of mycorrhizal fungi (0.00g, 2.00g, 3.00g, 4.00g, 5.00g)
Each closed mesocosm contained different amounts of mycorrhizal fungi. 5 intervals were selected to allow accurate monitoring of the effect of the mycorrhizal fungi on plant transpiration and water retention rate, with jar containing 0.00g of fungi being the controlled group. The amounts were chosen to predict the most effective fungi concentration rate that will provide the ivy plant with most benefits.
Dependent Variable: Soil moisture levels of each closed mesocosm in the study
Soil moisture levels were measured before and after closing the mesocosms. Soil moisture levels would indicate the effectiveness of water retention of ivy plants with different concentrations of fungi. The moisture levels were measured with technology and monitored before and after the closure of the experiment.
Controlled Variables:
Table 1: Controlled Variables
| Controlled Variable | Reason for Control | Method of Control |
| Jar Volume | Different jar volumes could alter the process of condensation and mess up the experiment |
All 25 jars were bought from the same stock at Walmart. |
| Type of plant used in each jar | Different types of plants could possess different processes and levels of water absorption thus altering the results of the experiment. |
All 25 common ivy plants were bought from the same stock at Home Depot. |
| Light availability | Light intensity influences the rate of light dependent reaction of photosynthesis, therefore, it directly impacts the transpiration rates and the water absorption levels of plants. |
All mesocosms were placed on the same table in front of a big window, with approximately the same supply of sunlight. |
| Temperature | Temperature changes can alter the activity of enzymes involved in the plant growth and water retention process. |
All mesocosms were grown in the same room, with the thermostat set to 22 degrees Celsius. |
| Amount of water in each jar | The amount of water directly impacts a plants water absorption and retention rates, thus, directly affecting the purpose of the experiment. |
Same amount of water l20mL was put in each mesocosm. |
| Type of mycorrhizal fungi used in each jar | The type of mycorrhizal fungi used in each mesocosm directly affects the rate of water absorption and retention rates due to its qualities and properties. |
Fungi from the same pack was used through all 25 jars. |
| Type of soil | The type of soil can influence the dynamics of water absorption by the plant, hence influencing the absorption and retention effectiveness of the plants. |
Soil from the same pack was used throughout all twenty- five jars. |
| Length of the experiment | The length of the experiment will be controlled by keeping the duration the same (14 days) for all treatnents to ensure that any changes in soil moisture are due to mycorrhizal fungi concentration, not differences in experimental time. | The experiment lasted exactly 14 days, with the start and final measurements at 3:00pm. |
Procedure
Procedure
-
Preparation of the mesocosms
1.1 Gather all materials on the working table, ensuring that all risk assessments are observed.
1.2 Start by measuring 100 g of gravel and putting it into each jar. ensuring that it is evenly spread out and properly covers the bottom of the jar.
1.3 Measure 600 g of potting mix and use a small garden shovel to accurately place the soil inside of the jar
1.4 Dig a rough hole in the middle of the jar through the soil to later put the common ivy plant inside of.
1.5 Take one common ivy plant per each jar and accurately take it out of its original container by lightly pulling on the thickest stem of the plant. Make sure to flip the container upside down to ensure that the roots are not going to get ripped off during this process.
1.6 Place the plant inside of the previously dug out hole, stabilizing the plant while using your hands to cover the roots with soil from all sides.
1.7 After completing the potting process for all 25 jars. Split all the jars into five groups: control (0.00g), 2.00g, 3.00g, 4.00g, 5.00g. Labeling each group's jars with the according labels indicating the amount of fungi to be placed in each. Place the experimental groups jars (2.00g, 3.00g, 4.00g, 5.00g) on the working table for easy access while placing the controlled group jars (0.00g) in a different location to ensure that the experimental and control groups do not mix.
-
Preparation of the mycorrhizal fungi
2.1 Prepare the 20 mason jar lids by splitting them in four groups. You should be left with 4 groups of lids each containing 5 lids. Each group will be used according to each jar group in the experimental groups mentioned above (2.00g, 3.00g, 4.00g, 5.00g).
2.2 Take the 2.00g jar lid group first and start by placing the first lid on the balance scale and pressing "tare". Ensure that the scale weight turns to 0.00g after pressing the button while having the jar lid on top.
2.3 Carefully start to pour the mycorrhizal fungi from the pack onto the lid on top of the scale until the weight turns to the desired amount.
2.4 Once the weighing process is completed for all five lids from the same group, put them in front of the according jar group, ensuring that the jar labels match with the amount of fungi you put in the lids.
2.5 Start by pouring mass from each lid into the according jar, ensuring that the fungi is evenly distributed throughout the topsoil. This process can be done easily by rotating the jar as the fungi from the lid is being slowly poured inside.
2.6 After all the fungi from the lid have been relocated inside of the jar. Put on the gardening gloves and place your hand inside of the jar to mix the fungi. Make sure to carefully hold the plant stem and leaves with your other hand to avoid breaking or disturbing the plant.
2.7 Take one finger and carefully mix the fungi by moving your finger back and forth throughout the perimeter of the jar, visually approximating that the fungi is able to reach the roots of the plant.
2.8 Repeat steps 2.2-2.6 for all other experimental groups (3.00g, 4.00g, 5.00g). Make sure to leave the controlled group without any fungi.
-
Preparation for sealing of the mesocosms.
3.1 Take all the 25 jars and pour 120 mL of distilled water from an already filled beaker inside each, ensuring that the water is evenly distributed throughout all of the topsoil.
3.2 If there is any dry soil stuck on the sides of the jar, wipe it off with a paper towel, to ensure clear visibility from the outside to observe the mesocosms after closure.
4. Measuring soil moisture before sealing.
4.1 Prepare the soil moisture sensor by downloading the "Flower Care" app from AppStore or Google Play Store.
4.2 Turn on the sensor by holding down on the "On/Off' button located at the top of the instrument.
4.3 Connect the device to App by finding the instrument on the "nearby devices" option located at the top right.
4.4 After pairing the device to your phone, place the sensor in each jar ensuring that the blades of the instrument are fully submerged into the soil.
4.5 The soil moisture percentage % will be shown on your phone screen. Record the data for the jar in the observation table.
4.6 Complete steps 4.4-4.5 for the rest of the jars. Record the data for all 25 jars.
-
Final sealing of the mesocosms.
5.1 Cut out a small piece of plastic wrap, enough to cover the top of the jar. Make enough covers for 25 jars.
5.2 Place the wrap on top of the jar, fully covering the open space, ensuring that no air gets in through possibly small ruptures in the plastic wrap.
5.3 Tightly fixate the plastic wrap onto the jar with a rubber band, ensuring that it is perfectly tight and will allow for the plastic wrap to stay in place for the desired period of experimental procedure time.
5.4 Repeat steps 5.2-5.3 for all 25 jars.
5.5 Place all jars on the observation table, ensuring that the groups and jars are properly organized in sections for easy qualitative observations.
5.6 Ensure that the observation table with the jars is receiving a sufficient amount of sunlight from a window, and the sunlight is approximately distributed evenly among all jars.
Observations
Qualitative Data
- The experimental groups containing 4.00g and 5.00g of mycorrhizal fungi in their soil, produced more condensation throughout the experiment. This was observed by the amount of water droplets on the sides and top of the jar.
- All of the plants remained healthy and green throughout the length of the experiment
-
Leaf Color
- Control group plants exhibited a consistent vibrant green color throughout the study
- Group 2 (2.00g) showed minor yellowing on the edges of the leaves by Day 14.
- Group 3 (3.00g) had the healthiest leaf color, remaining dark green with no visible discoloration.
- Group 4 (4.00g) displayed slight browning on leaf tips by Day 14
- Group 5 (5.00g) had pale green leaves by Day 10
-
Fungal Visual Growth Patterns
- No visible fungal growth was observed in the control group.
- Group 2 and 3 showed even fungal growth distributed through the soil
- Group 5 had dense, clumpy fungal growth near the plant base, suggesting uneven distribution.
-
Plant stability
- Groups 3 and 4 plants were the most stable and upright during the experiment.
-
Condensation Patterns
- Groups 4 and 5 showed the most visual condensation patterns on the top and sides of the jar as seen by large droplets of water in comparison to other groups.
Quantitative Data
Raw Data
Table 3: Soil moisture level records before sealing and after opening the mesocosms.
|
Group (Control/Fungi amount) |
Jar# |
Soil moisture before closing (±1%) |
Soil moisture after opening (±1%) |
|
Control |
1 |
23 |
35 |
|
2 |
23 |
31 |
|
|
3 |
23 |
31 |
|
|
4 |
23 |
33 |
|
|
5 |
23 |
35 |
|
|
2.00g |
1 |
23 |
56 |
|
2 |
23 |
40 |
|
|
3 |
23 |
45 |
|
|
4 |
23 |
57 |
|
|
5 |
23 |
40 |
|
|
3.00g |
1 |
23 |
51 |
|
2 |
23 |
60 |
|
|
3 |
23 |
56 |
|
|
4 |
23 |
51 |
|
|
5 |
23 |
60 |
|
|
4.00g |
1 |
23 |
47 |
|
2 |
23 |
65 |
|
3 |
23 |
69 |
|
|
4 |
23 |
62 |
|
|
5 |
23 |
50 |
|
|
5.00g |
1 |
23 |
43 |
|
2 |
23 |
35 |
|
|
3 |
23 |
44 |
|
|
4 |
23 |
37 |
|
|
5 |
23 |
34 |
Analysis
Data Processing
-
Paired t-test
To determine whether there are significant differences in soil moisture levels between the groups (control and different fungi amounts), a paired t-test for each jar group before and after mesocosms were opened, and an independent t-test for comparison between post-treatment moisture levels between control vs. experimental groups, will be performed.
- Calculating the difference for each jar, between the before and after opening, soil moisture levels.
Formula 1: Calculating the difference
Diference (%) = After opening soil moisture - before opening soil moisture
Difference (%) = 35 - 23 = 12
Table 4: Difference in soil moisture per each jar.
|
Group (Control/Fungi amount) |
Jar# |
Soil moisture before closing (±1%) |
Soil moisture after opening (±1%) |
Difference in soil moisture (after-before) (±1%) |
|
Control |
1 |
23 |
35 |
12 |
|
2 |
23 |
31 |
8 |
|
|
3 |
23 |
31 |
8 |
|
|
4 |
23 |
33 |
10 |
|
|
5 |
23 |
35 |
12 |
|
|
2.00g |
1 |
23 |
56 |
33 |
|
2 |
23 |
40 |
17 |
|
|
3 |
23 |
45 |
22 |
|
|
4 |
23 |
57 |
34 |
|
|
5 |
23 |
40 |
17 |
|
|
3.00g |
1 |
23 |
51 |
28 |
|
2 |
23 |
60 |
37 |
|
|
3 |
23 |
56 |
33 |
|
|
4 |
23 |
51 |
26 |
|
|
5 |
23 |
60 |
37 |
|
|
4.00g |
1 |
23 |
47 |
24 |
|
2 |
23 |
65 |
42 |
|
|
3 |
23 |
69 |
46 |
|
|
4 |
23 |
62 |
39 |
|
|
5 |
23 |
50 |
27 |
|
|
5.00g |
1 |
23 |
43 |
20 |
|
2 |
23 |
35 |
12 |
|
|
3 |
23 |
44 |
21 |
|
|
4 |
23 |
37 |
14 |
|
|
5 |
23 |
34 |
11 |
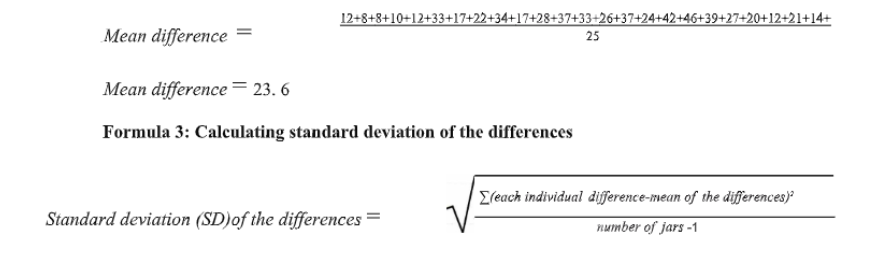
2. Calculate the Mean and Standard Deviation of the Differences
Formula 2: Calculating the mean difference
Mean difference = (sum of all differences) / (number of jars)
Control group:
- (12 - 22)2 = 100
- (8 - 22)2 = 196
- (8 - 22)2 = 196
- (10 - 22)2 = 144
- (12 - 22)2 = 100
Sum for Control group: 100 + 196 + 196 + 144 + 100 = 736
2.00 g group:
- (33 - 22)2 = 121
- (17 - 22)2 = 25
- (22 - 22)2 = 0
- (34 - 22)2 = 144
- (17 - 22)2 = 25
Sum for 2.00: 121 + 25 + 0 + 144 + 25 = 315
3.00 g group:
- (28 - 22)2 = 36
- (37 - 22)2 = 225
- (33 - 22)2 = 121
- (26 - 22)2 = 16
- (37 - 22)2 = 225
Sum for 3.00: 36 + 225 + 121 + 16 + 225 = 623
4.00 g group:
- (24 - 22)2 = 4
- (42 - 22)2 = 400
- (46 - 22)2 = 576
- (39 - 22)2 = 289
- (27 - 22)2 = 25
Sum for 4.00: 4+ 400 + 576 + 289 + 25 = 1294
5.00 g group:
- (20 - 22)2 = 4
- (12 - 22)2 = 100
- (21 - 22)2 = 1
- (14 - 22)2 = 64
- (11 - 22)2 = 121
Sum for 5.00: 4 + 100 + 1 + 64 + 121 = 290
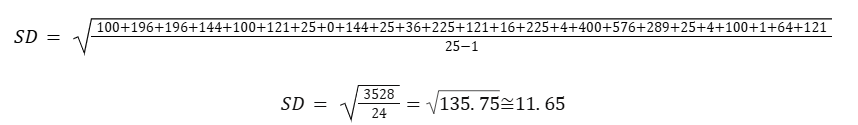
3. Calculate the t-statistic

4. Compare to the t-distribution Table
To find the critical t-value, we need to refer to the t-distribution table. For 24 degrees of freedom (df= n - 1 = 25 - 1 = 24), at a significant level of 0.05 (two-tailed), the critical t-value is approximately 2.064
5. Determine whether to reject the null hypothesis
H0= there is no significant difference in soil moisture levels before and after the fungi treatment.
H1= there is a significant difference in soil moisture levels before and after the fungi treatment.
If the absolute value of the calculated t-statristic is greater than the critical t-value, we reject the null hypothesis.
If the absolute value of the t-statistic is less than the critical t-value, we fail to reject the
null hypothesis.
The calculated t-statistic is 55.93 , which is much greater than the critical t-value of 2.064
Result:
Since the calculated t-statistic (55.91) is far greater than the critical t-value (2.064), we reject the
null hypothesis. This suggests that there is a statistically significant difference in soil moisture
levels between the control group and the experimental groups with varying amounts of fungi.
2.1 Independent t-test
To dete1mine whether tbere are significant differences in two different groups (control group vs.
Experimental group with fungi), an independent-test will be performed. It will determine
whether the fungi treatment itselfresults in a significant difference in soil moisture between the
Control group and experimental group.
- Define the groups.
Use two groups at a time (ex., Control group vs. 2.00g Fungi group)
2. Null and Alternative Hypotheses.
H0= There is no significant difference between the means of the two groups.
H1= There is a significant difference between the means of the two groups.
3. Calculate the t-statistic

4. Compare the t-statistic with the critical t-value
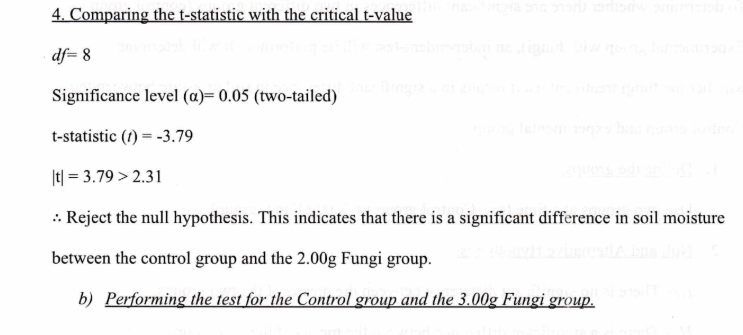



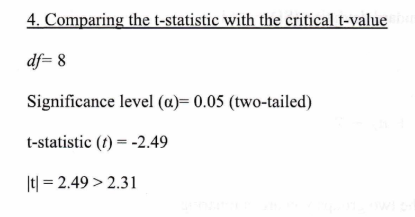
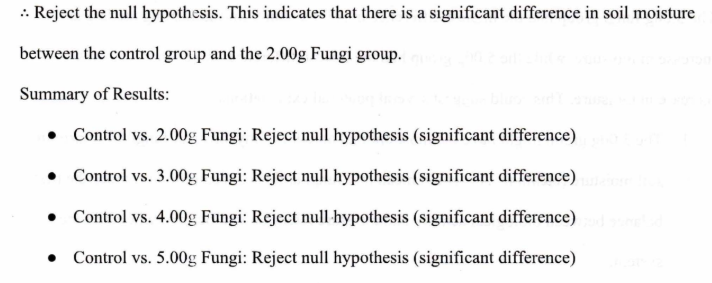
Conclusion
Conclusion
The statistical analysis demonstrates a significant difference in soil moisture levels before and after the fungi treatment across all experimental groups.
Both paired t-test and independent t-test reveal that the application of mycorrhizal fungi to the soil consistently led to increased soil moisture compared to the control group, which supports the hypothesis that fungi contribute to soil moisture retention.
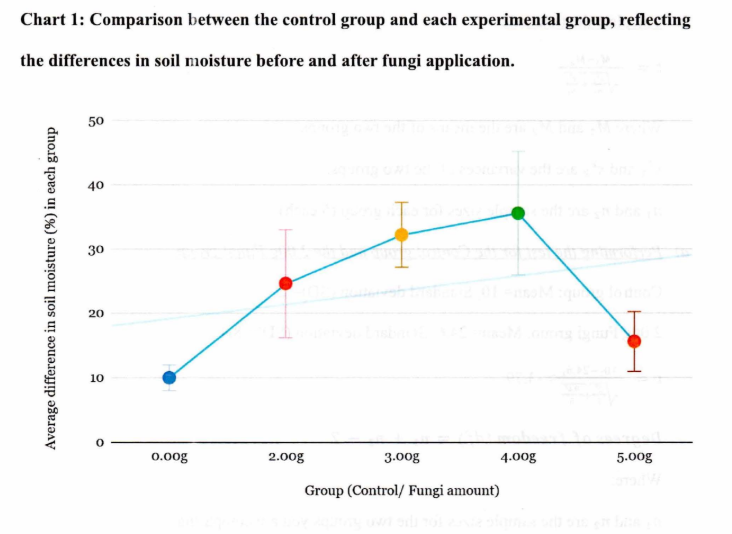
Since the paired t-test showed significant differences between before and after measurements, and the independent t-test confirmed that the differences between the control and experimental groups (across all fungi amounts) are significant, we can conclude that the fungal treatment regardless of the amount, affects soil moisture retention in a meaningful way. The results suggest that mycorrhizal fungi enhance soil moisture retention, which could have broader implications for improving soil health, especially where water retention is a concern. Based on statistical results, it can be concluded that mycorrhizal fungi play a significant role in altering soil moisture levels, and this effect is consistent across all experimental groups tested. The 3.00g fungi group had the highest difference in soil moisture, thus indicating the largest increase in moisture, while the 5.00g group had the lowest difference, indicating the lowest increase in moisture. This could suggest several potential explanations:
1. The 3.00g group might have the most optimal amount of mycorrhizal fungi for improving soil moisture retention. This could mean that fungi at this concentration provides the best balance between biological activity and moisture retention without overwhelming the system.
2. The 5.00g group may have experienced diminishing returns. At this higher concentration, the fungi could have become too abundant, potentially leading to competition for resources, overgrowth, or reduced efficiency in water absorption, which may have led to a lower increase in moisture levels.
3. There could be a saturation point beyond which adding more fungi does not significantly improve soil moisture retention. It is possible that the soil environment reached a point where the amount of fungi exceeded the soil's capacity to support them effectively, reducing the impact on moisture retention.
Application
Discussion:
The increase in soil moisture levels can be explained by plant transpiration, which is the process through which plants absorb water through their roots and release it as vapor through small pores in their leaves. When plants transpire they create a pull on water within the soil, thus, drawing more moisture into their roots. The rate of transpiration is influenced by several factors including the plant's root system, environmental conditions, and the efficiency of nutrient and water uptake. The presence of mycorrhizal fungi likely enhanced the plants' ability to absorb water and nutrients. The fungi formed a symbiotic relationship with plant roots, extending the root system and improving the plant's ability to access water and minerals from the soil. With a more efficient root system, th e plants were able to transpire more effectively leading to increased moisture levels in soil a the plant takes up more water from the surrounding environment. Additionally, the fungi increased the plant's water absorption capacity which in return allowed the plant to transpire more water and maintain higher moisture levels in the soil. The control group which lacked fun gi may have experienced slower or less efficient water uptake, leading to lower moisture retention in the soil. This explains the observed trend of increased moisture levels in soils for jars containing mycorrhizal fungi compared to the control group.
Sources Of Error
Evaluation
Strengths:
- The inclusion of five replicates per each of the five conditions contained increased statistical reliability and reduced the impact of outliers on the final results.
- The range of mycorrhizal fungi concentrations (0.00g, 2.00g, 3.00g, 4.00g, 5.00g) allows for a systematic analysis of the effects on soil moisture and transpiration rates, providing a clear gradient for identifying optimal levels.
- Strict controls on variables like jar volume, plant type, light availability, temperature, water amount, soil type, and fungi source ensure that changes in soil moisture are solely attributed to the concentration of fungi. This enhances the reliability of the final results.
- Utilizing a soil moisture sensor to ensure accurate and consistent measurements, minimizing human error compared to manual methods.
- Closed system design of the mesocosm eliminates the interference of external factors such as changes in environmental conditions, thus making it easier to isolate the effects of the fungi on plants.
- The method could be easily replicated or scaled up for future research, including different plants or longer durations, making it a versatile and scalable experimental framework.
Weaknesses:
Table 5: Weaknesses of the study
|
Weakness |
Explanation |
Modification and explanation |
|
Limited duration |
Limited duration of only a 14-day period may not have fully captured the long-term effects of mycorrhizal fungi on plant growth and soil moisture dynamics. Fungal colonization often takes time, so the results might not reflect the fungi's full potential benefits. |
Extend the timeline to at least 4-6 weeks. Longer time allows for observing fungi colonization and potential long-term benefits on plant health. |
|
Small jar size |
Small jar size might restrict root growth and fungal colonization. |
Use larger jars or containers which could provide roots and fungi more space to grow, therefore, better simulating natural conditions. |
|
Uneven fungi colonization |
Uneven colonization could lead to inconsistent results |
Mix fungi evenly into the soil before planting. This ensures that each plant gets equal exposure to the fungi. |
|
Plant species |
Results may not generalize to other plant species due to Ivy being a manageable species with consistent growth characteristics. |
Add a secondary/multiple plant species to the experiment. Testing with a fast-growing plant like radish or wheat allows for broader conclusions and provides results appropriate for a more general group of plants. |
|
Data Collection Tools |
Potential for sensor calibration errors or measurement inconsistencies |
Regularly recalibrate sensors and cross-check measurements. This will prevent inaccuracies from impacting results. |
|
Relevance and Application |
Lack of external validity due to artificial conditions of a closed system. |
Complement closed-system study with an open-system field test. Apply similar fungi treatments in outdoor conditions to compare outcomes for a better evaluation. |
Limitations:
Table 6: Limitations of the study
|
Limitation |
Explanation |
Modification and explanation |
|
Species-specific results |
Testing with ivy alone means the results are specific to this plant species and may not generalize to others. Different plants interact differently with myc01Thizal fungi |
Include multiple plant species in the experiment (e.g., beans, grasses, local native species) alongside ivy. Testing multiple species ensures broader applicability of findings, as different plants may interact with mycorrhizal fungi in unique ways. |
|
Small sample size |
Five jars limit statistical power. Any anomalies in one jar could disproportionately affect overall results. Reduced ability to identify subtle trends or differences between groups. |
Increase the number of jars per group (e.g., 10 or more). A larger sample size reduces the impact of outliers and provides more reliable and statistically significant results. |
|
Simplified Ecosystem |
Excluding additional soil organisms like bacteria or other fungi limits the complexity of interactions that occur in natural ecosystems. The study may oversimplify the role of mycorrhizal fungi as they usually operate within a larger microbial network. |
Add other organisms like decomposer fungi (without breaching closed-ecosystem rules) or additional abiotic elements. This adjustment more closely mimics natural ecosystems, allowing results to be more representative of real-world interactions. |
Citations


Acknowledgement
Acknowledgements:
I would like to express my sincere gratitude to all those who have supported me throughout this research project. I am particularly grateful to my old Biology teachers-,Mr. Andrews and Ms. Foisy, for their invaluable and insightful feedback. Additionally, I would like to acknowledge my Science Fair School Coordinator, Ms. O'Keefe, who kindly guided me through my first in-person science fair participation process. Finally, I am deeply appreciative of my family, especially, my mom and dad, for their unwavering support and for constantly running missions to buy and carry the jars and plants required for the experiment.
Thank you for your contributions to making this work possible.