Salt Solution: Do sidewalk deicer salts affect radish seed germination?
Grade 9
Presentation
Hypothesis
I hypothesize that if deicer salts are added to a water solution in increasing amounts and applied to radish seeds, then the seed’s capacity to germinate will decrease because the rise in salinity will reduce the seeds ability to absorb enough water to start the germination process. I also predict that if different deicer salts are used on radish seeds then the salt that has more seeds germinate in higher concentrations of salt solutions then it is less harmful to seeds because it is allowing the seeds to get the water necessary to start germination.
Research
What Are Salt Deicers?
Winters in Canada can make roads and sidewalks snowy, icy, and slippery. Shoveling and plowing are often used to remove snow but increasingly different substances are being used to help reduce ice buildup. Salts are one of these substances used to get rid of ice (Canada Salt Group Ltd, 2023).
Sidewalk deicers are made from salts and chemicals and work by lowering water’s freezing point (Salt Smart Collaborative, n.d.). Water molecules are made up of positive hydrogen atoms and negative oxygen atoms and when the temperature drops to 0℃ (32℉) these water molecules slow down and the positive hydrogen atoms become drawn to the negative oxygen atoms of nearby water molecules forming crystals and turns water into its solid form ice (Iowa Department of Transportation, 2016). Salt lowers water's freezing point by making it harder for water molecules to bond together to form ice at 0℃ (32℉). When salt is added to the layer of water that is on ice, the salt molecules separate into its positive and negative ions and they attract the hydrogen and oxygen atoms in the water which breaks the bonds between the ice crystals so the ice turns back into a liquid or a slush and prevents the water from refreezing and sticking to roads and sidewalks (Canada Salt Group Ltd, 2023). Different salts affect water's freezing point differently.
(Image source: Salt Smart Collaborative)
The most used salts in deicers are sodium chloride, calcium chloride, potassium chloride and magnesium chloride or some kind of mixtures of these (Melgares, 2017).
Sodium Chloride (NaCl) or rock salt is the most commonly used salt to deice roads because it is easier to get and cheaper to buy than the other salts (Minnesota Pollution Control Agency, 2022). It is made up of one positive sodium ion and one negatively charged chlorine ion (Loeffler, 2021). When these ions mix with the thin water layer on top of ice it stops the water from refreezing by getting between the hydrogen and oxygen molecules (Salt Smart Collaborative, n.d.). Interestingly, sodium chloride does not work well in really cold temperatures and is only effective at lowering water’s freezing point from 0℃ to between -6℃ to -10℃, after this point the sodium and chlorine ions start to lose their ability to hold onto the water molecules and stops being dissolved so the water is able to turn back to ice (Loeffler, 2021) (Ninja Deicer, 2024). Sodium chloride is generally mixed into a brine or with other salts and chemicals to make it work at lower temperatures. Sodium chloride is endothermic which means it needs to draw heat from water to start the melting process so if there is little water on the surface of ice it is not able to work (Loeffler, 2021) (Occidental Chemical Corporation, n.d.).
(Image souce: Loeffler, 2021)
Calcium Chloride (CaCL2) is the most used salt after sodium chloride and is often used to pretreat roads and sidewalks before a snowfall or when temperatures are very cold or it is important to melt ice fast (Occidental Chemical Corporation, n.d.) (Ninja Deicer, 2021). It is a bit more expensive than rock salt so it is often mixed with rock salt to improve its ability to melt ice (Minnesota Pollution Control Agency, 2022). Calcium chloride is made up of one positively charged calcium ion and two negatively charged chlorine ions (Loeffler, 2021). Calcium chloride acts extremely fast at starting the melting process as it is hygroscopic which means it is able to draw moisture from its surrounds and the air so it does not need a lot water on the ice to begin to dissolve and interrupt the bonds between the hydrogen and oxygen water molecules (Occidental Chemical Corporation, n.d.). Calcium chloride is also exothermic which means it releases energy when it dissolves so it gives off heat making it faster at melting ice even when it is cold (Occidental Chemical Corporation, n.d.). Calcium chloride is able to lower the freezing point of water to -32℃ due to it being exothermic, hygroscopic and having more ions that are able to attract and hold onto water molecules longer to stop them from turning into ice (Ninja Deicer, 2021) (Loeffler, 2021).
(Image source: Loeffler, 2021)
Magnesium chloride (MgCl2) is the most expensive of the chloride salts so it is usually used to treat sodium chloride to increase its ability to melt ice (Minnesota Pollution Control Agency, 2022). Magnesium chloride contains one positively charged magnesium ion and two negatively charged chloride ions. It is similar to calcium chloride in that it is hygroscopic, exothermic (but not as much as calcium chloride) and has three ions but it is often sold in its hydrated form which means it has six extra water molecules attached so this can dilute its ability to melt ice and more is often needed to be used (Occidental Chemical Corporation, n.d.). Magnesium chloride can lower the freezing point of water to around -15℃ to -18℃ and works in the way that calcium chloride does (Loeffler, 2022) (Occidental Chemical Corporation, n.d.).
(Image source Loeffler, 2021).
Potassium Chloride (KCl) is used the least as a deicer and is more often added to the other salts in ice melts (Brightly Inc., 2017). It is made up of one positive potassium ion and one negatively charged chlorine ion. It is similar to sodium chloride in that it is endothermic and has two ions so it needs water to be around in the environment to start the melting process so it is slower to work. Potassium chloride is only effective at lowering water’s freezing point to around -4℃ possibly -11℃ but after this point the potassium and chlorine ions start to lose their hold onto water molecules and water starts to become ice (Occidental Chemical Corporation, n.d.) (Melgares, 2017) (Loeffler, 2021).
(Image source Loeffler, 2021).
Since these salts are the most used in deicers, I wanted to test all four of these salts to see what effect they would have on seed germination.
Deicers And The Environment
In Canada around five to seven million tonnes of road salts are used each year to help with ice and snow on roads and sidewalks (Government of Canada, 2018). Since salts are a natural mineral and we even put it on our food, you would think that it would be pretty harmless to use but it is having a negative impact on the environment.
The salt in deicers can speed up the rusting process in the metals on vehicles, in sewers and pipes and around streets and sidewalks. Salts can get into concrete causing it to corrode and crack which can damage streets, bridges, sidewalks and buildings possibly making these structures unsafe and costs a lot to fix (Bridgestone Tires, 2021).
As the snow and ice melt the salt in it goes into the groundwater and even into lakes, rivers and ponds making them more salty. This causes a lot of problems for freshwater systems because the salt makes less oxygen in the water which aquatic plants and animals need to survive (Wasson, 2022). High salt levels also make water undrinkable for humans and animals. Salt is also harmful to land animals. It can dry out and burn animals' paws and can make them sick if they ingest the salt (Minnesota Pollution Control Agency, n.d.).
When deicer salts get into the soil and on plants it can do a lot of damage. Salt that sprays onto plants can burn the leaves which can make it easier for them to get sick and can get in the way of photosynthesis which plants need to live (Bayer & Njue, 2016). When the soil has too much salt in it it starts to dry out and water gets drawn away from the plants roots which makes them dehydrated and not able to grow and even die (Wasson, 2022). Salt can change the quality of the surface of the soil so that it is not able to hold onto water or nutrients and begins to harden and compact and even erode which makes growing plants very difficult (Bayer & Njue, 2016).
Salt also affects seeds. Seed germination is one of the stages of a plant's growth where it goes from a seed into a plant or seedling. In order for a seed to start to germinate it needs to take in water and oxygen through the seed coat. This makes the cells in the embryo begin to enlarge, when this happens the seed's coat will break open and then the roots/radical will begin to emerge. After the roots emerge from the seed the shoot will come afterward, the shoot contains the leaves and stem of the plant (Bennett, 2021). Salt reduces the seed's ability to absorb the water it needs to start the germination process so the seed is slower to develop or the seed might not be able to get enough water to germinate at all (Garden and Lawn, 2018). If seeds are not able to germinate then we won't have plants and crops which are so important for food and clean air.
There does seem to be some differences in the environmental impact between the different salts but the information is conflicting. There does seem to be some agreement that sodium chloride is the most harmful to plants and animals (Canada Salt Group Ltd., 2022). Magnesium chloride and calcium chloride are thought to be less toxic to plants and soil because they don’t release as much chloride into the environments as sodium chloride and potassium chloride because less is needed to melt ice (Melgares, 2017) (Snow & Ice Salt & Chemicals Unlimited, LLC., 2018) but information about concrete and animals is mixed. The information about potassium chloride is mixed, some suggest it is safe for animals and plants, other sources say it is harmful to plants and I found one article that stated that “applying 1 pound of potassium chloride to soil is equivalent to applying 1 gallon of bleach” (Viana, 2021).
Overall, the chloride salts no matter which one will cause damage to the environment if overused (Melgares, 2017). The Canadian Ecofiscal Commission estimates that the environmental and physical damage of road salt use costs around $4.8 billion dollars each year (Frank, 2017).
Radish seeds
To explore how these salts affect seed germination, I chose to use radish seeds for my experiment because they are fast germinators, easy to grow, were recommended for science fair projects (Sweetman, 2018) (Spider Farmer, 2023) and mostly because they were used in the experiment which I modeled my investigation on (Kearney, 2022). I did think about using grass seed but they seemed very thin, long and fine so I didn’t think it would be easy to count out and put into bags and it seems to be grown in soil rather than plastic bags and had a longer germination time. Radish seeds are from the Brassicaceae family which includes mustard and cabbage and its genus name Raphanus means “quickly appearing” which is accurate since radish seeds can germinate in three days or less and are ready to harvest in 4 weeks from when planted (West Coast Seeds, 2020) (Ferrer, 2012). Radish seeds have an 80% germination rate and are considered easy to grow and can be grown year round (West Coast Seeds, 2020).
There was not much information about how many people are using deicers around their homes but from my experiences walking my dog I feel more and more people are using salts to control ice and snow. From what I found out about salt effects on the environment, I feel that my experiment is important because it will give people information about what salt is doing to the environment and maybe get people thinking about less harmful ways to keep sidewalks and roads safe.
Variables
Independent Variable:
- Salt type used: Sodium Chloride, Potassium Chloride, Magnesium Chloride, Calcium Chloride.
- Concentration of salt in 8 oz of distilled water: ¼ tsp; ½ tsp; ¾ tsp; 1 tsp; 1¼ tsp; 1½ tsp; 1¾ tsp; and 2 tsp.
Dependent Variable: number of radish seeds that germinate.
Control Groups: tap water and distilled water with no salts added.
Procedure
- Take ten spray bottles and label them A through J and put the type of salt used on it (Sodium Chloride).
- Place 10 Ziplock Bags down on a table and label each bag with the salt type used and the letter of the solution to be used in each one (A through J).
- Unzip the bags and put a paper napkin in each one.
- Label ten plastic cups A through J and the salt type used and then fill the plastic cups with the following solutions:
- Solution A: 8 oz. tap water only
- Solution B: 8 oz. distilled water only
- Solution C: 8 oz. distilled water with ¼ teaspoon of sodium chloride. Stir to dissolve salt.
- Solution D: 8 oz. distilled water with ½ teaspoon of sodium chloride. Stir to dissolve salt.
- Solution E: 8 oz. distilled water with ¾ teaspoon of sodium chloride. Stir to dissolve salt.
- Solution F: 8 oz. distilled water with 1 teaspoon of sodium chloride. Stir to dissolve salt.
- Solution G: 8 oz. distilled water with 1¼ teaspoon of sodium chloride. Stir to dissolve salt.
- Solution H: 8 oz. distilled water with 1½ teaspoon of sodium chloride. Stir to dissolve salt.
- Solution I: 8 oz. distilled water with 1¾ teaspoon of sodium chloride. Stir to dissolve salt.
- Solution J: 8 oz. distilled water with 2 teaspoons of sodium chloride. Stir to dissolve salt.
- Pour one tablespoon of solution on the napkin in the bag with the same label, making sure it soaks the whole napkin.
- Put 40 seeds on the napkin of each bag. Make sure the seeds are scattered over the napkin. Zip up the bags
- Place all 10 bags at room temperature out of direct sunlight.
- Pour the remaining solutions into the similarly labeled spray bottles using a funnel. Clean the funnel after each use.
- Observe the bags daily for two weeks and record the number of seeds that have germinated in each and any other changes in the seeds.
- If the napkins in the bags seem to be drying out, spray the right solution into the bag to moisten seeds. If they seem too wet drain out excess water.
- Repeat this process for Calcium Chloride, Potassium Chloride and Magnesium Chloride.
- After the trial wash and dry the cups and spray bottles so they can be reused.

Observations
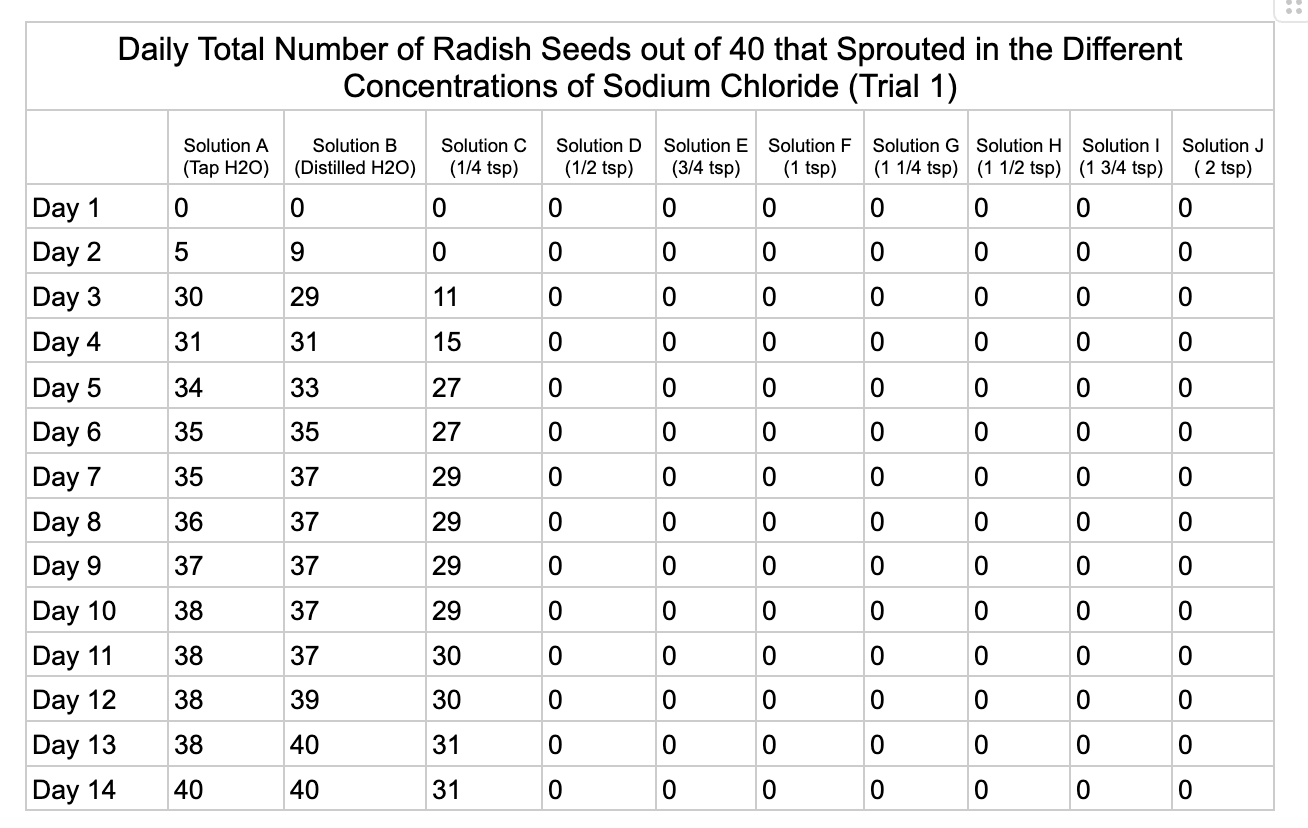
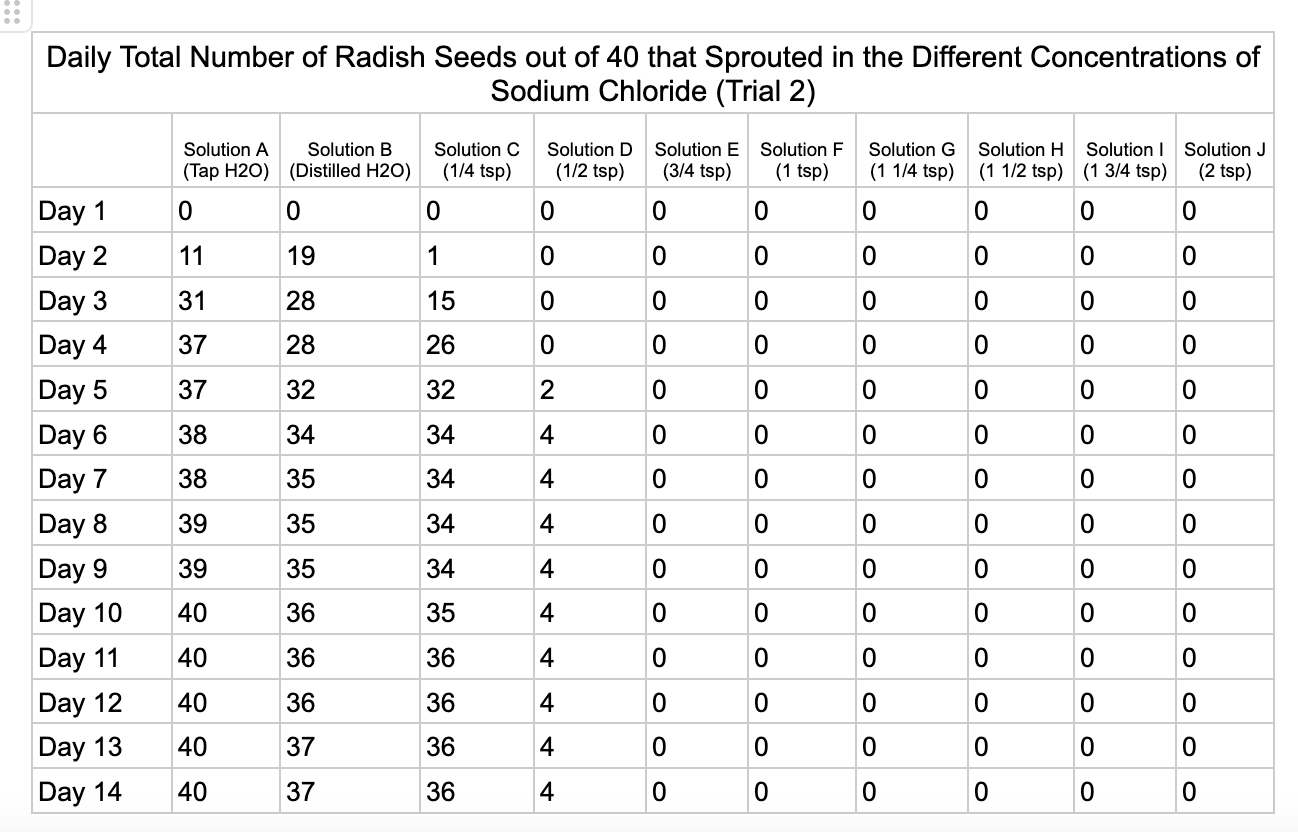
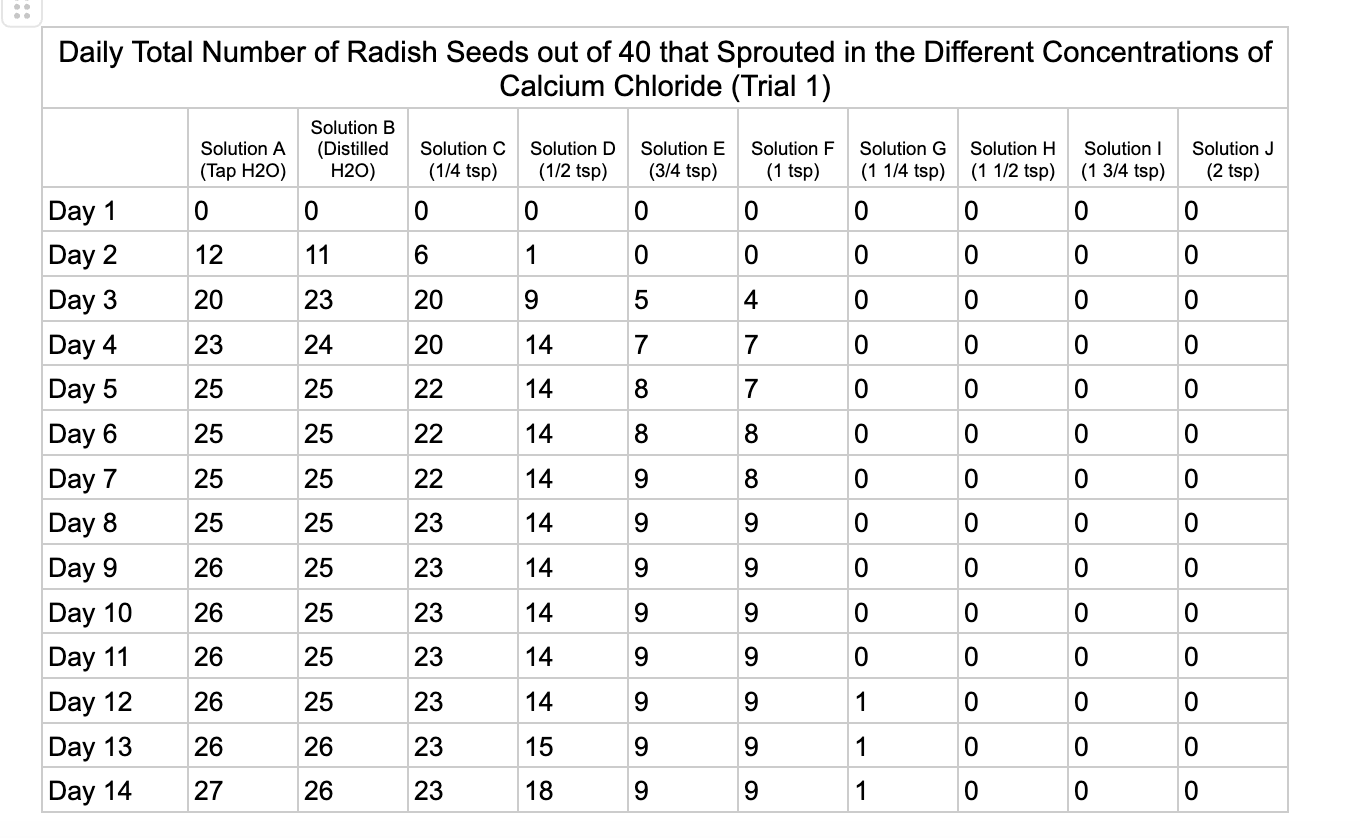
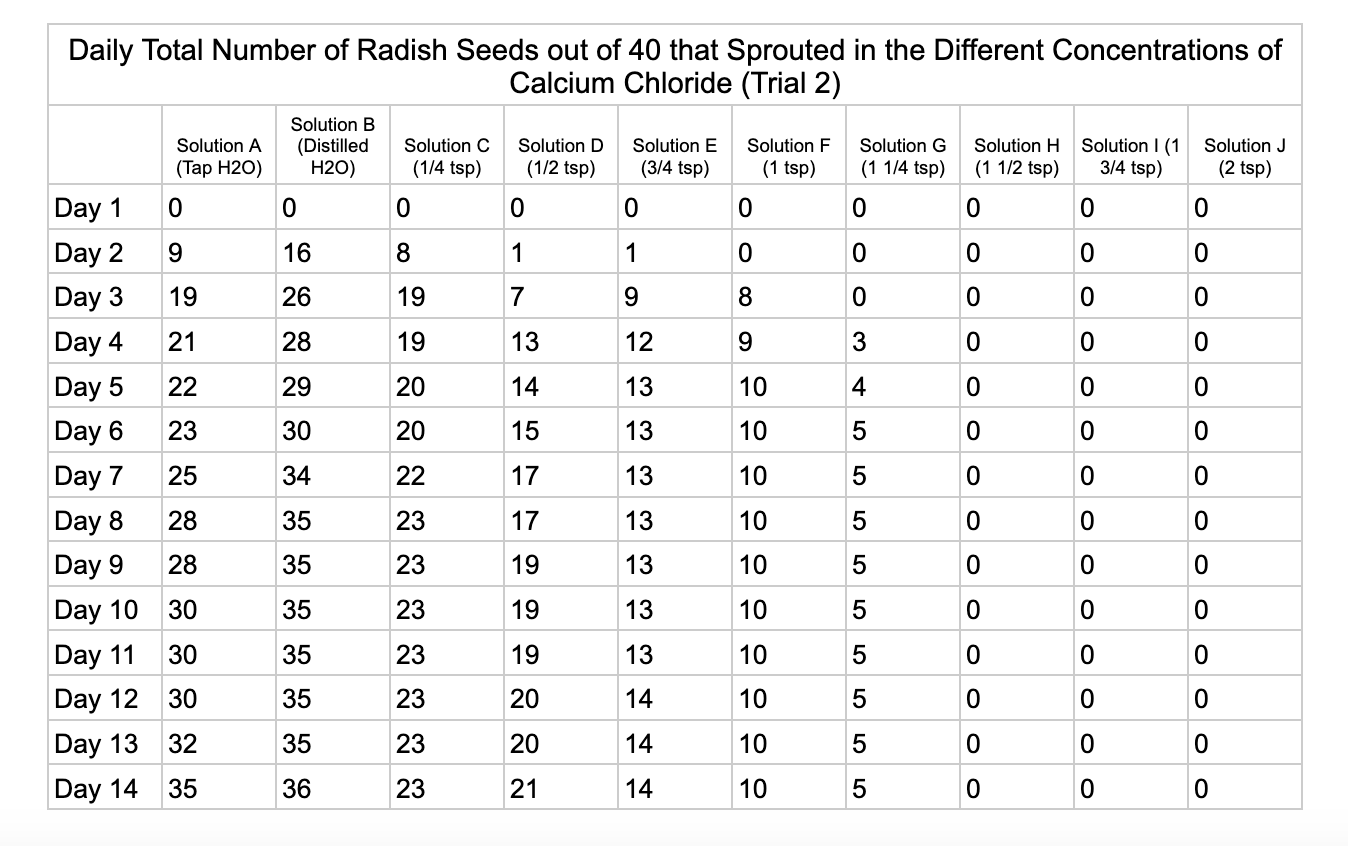
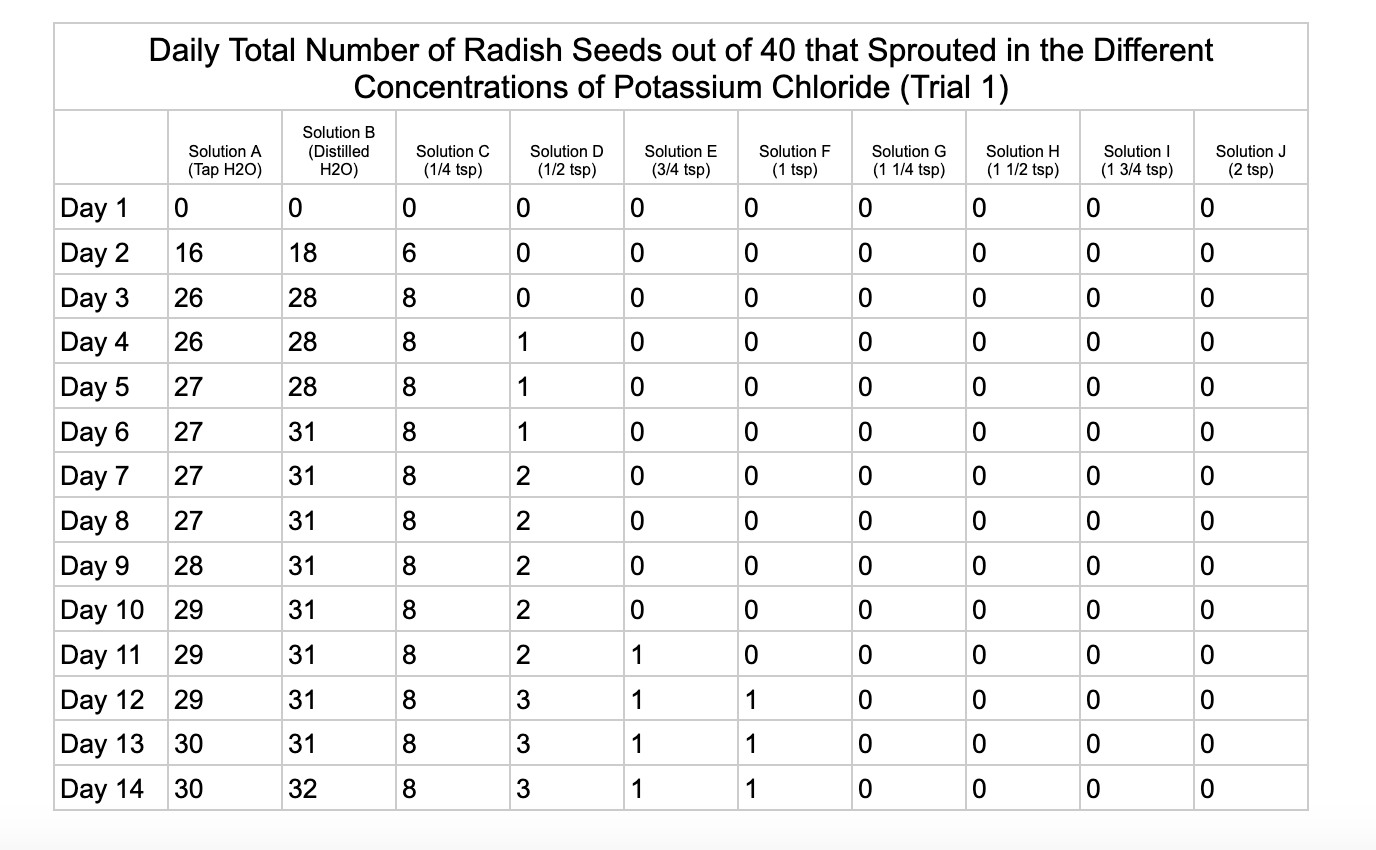
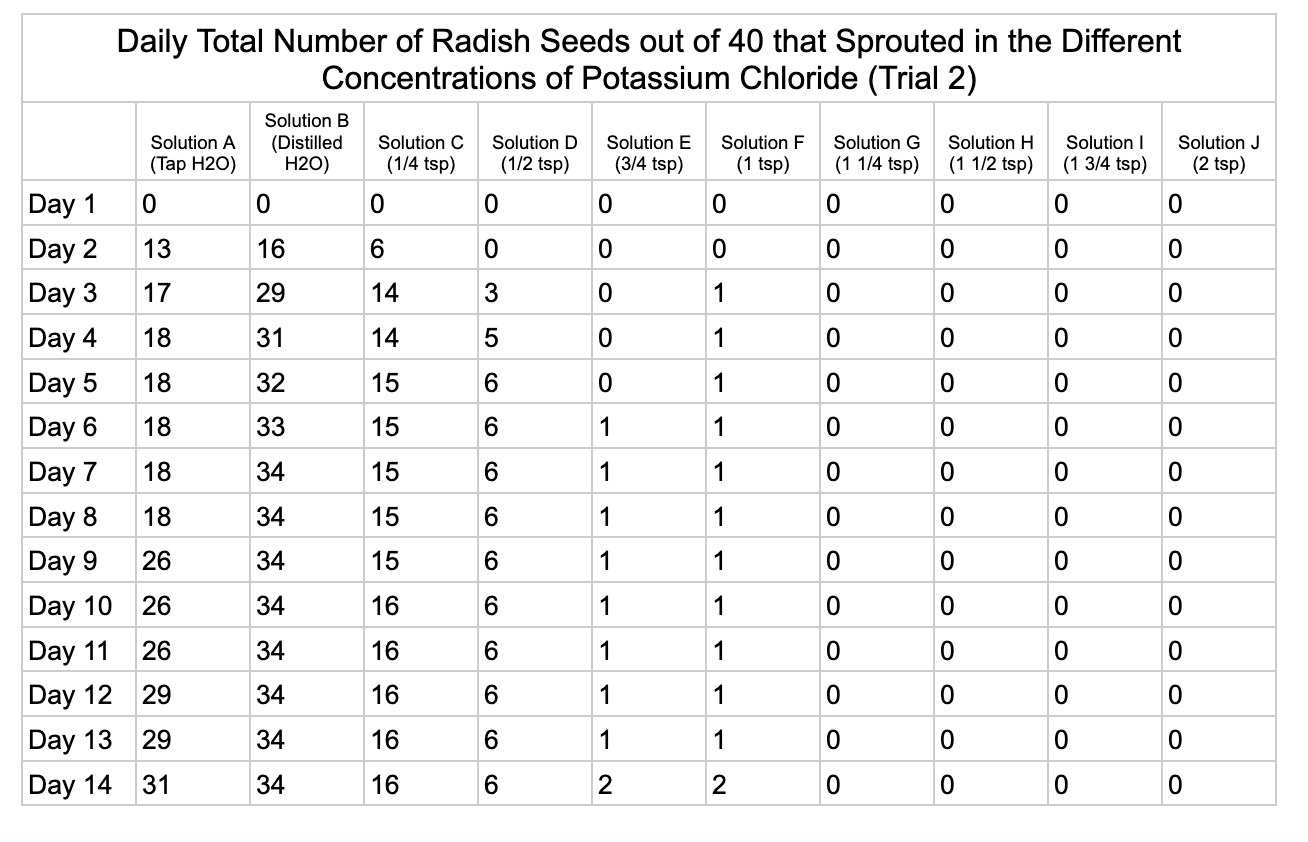
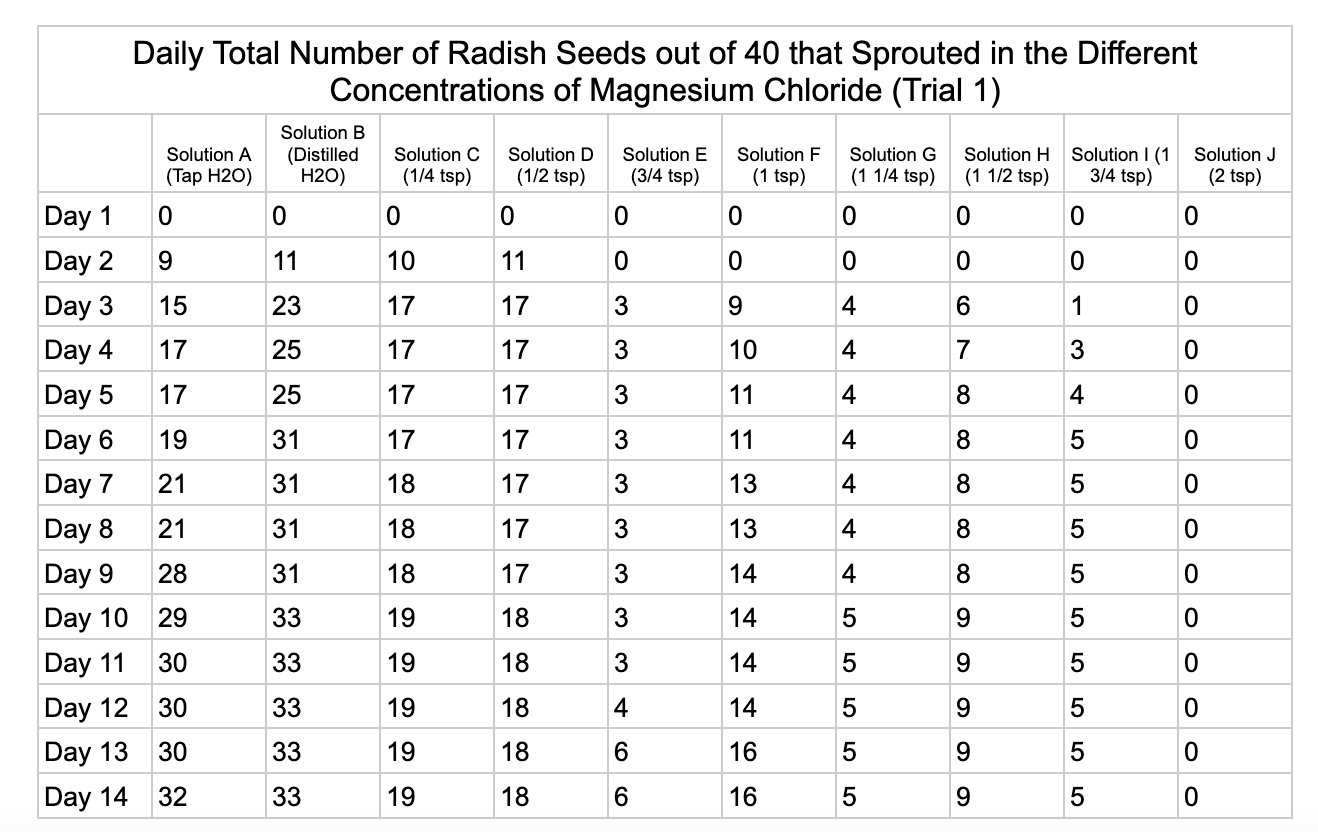
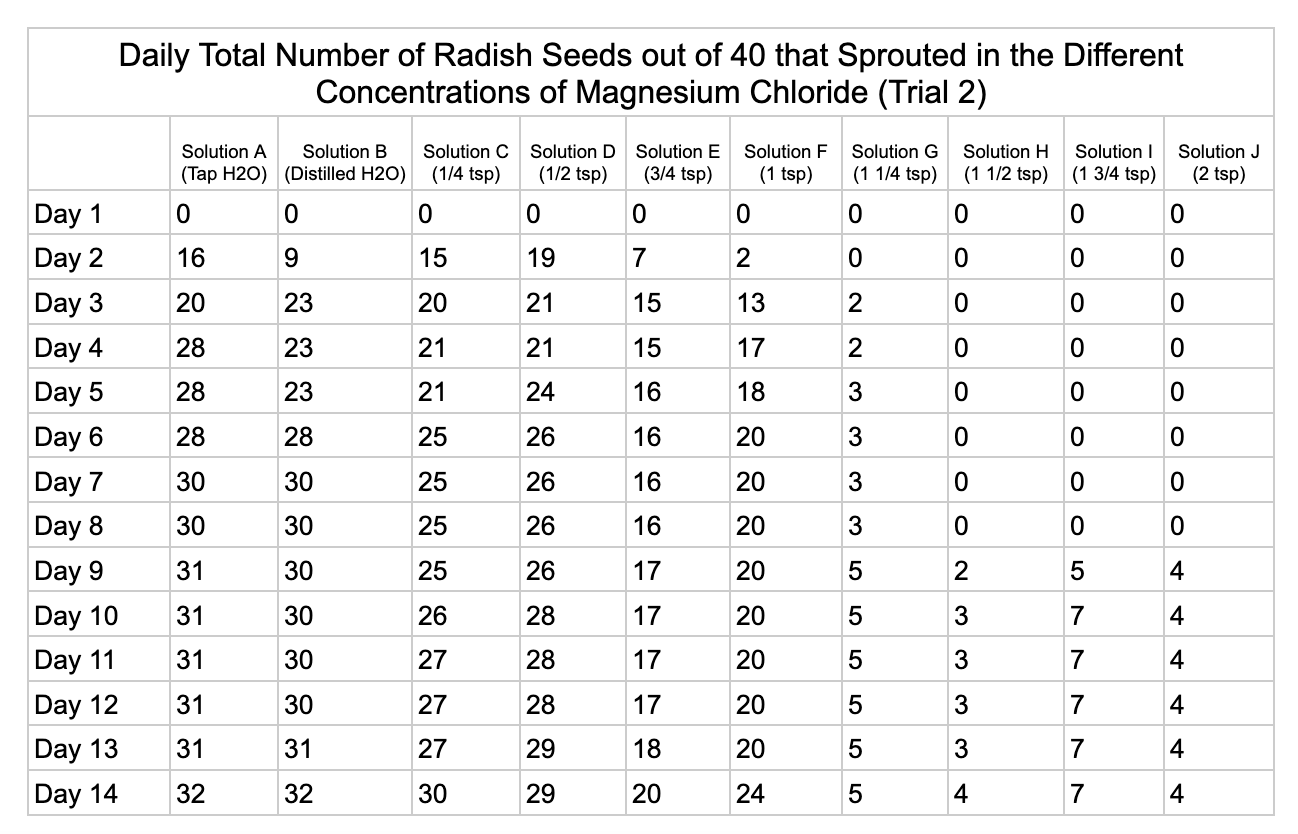
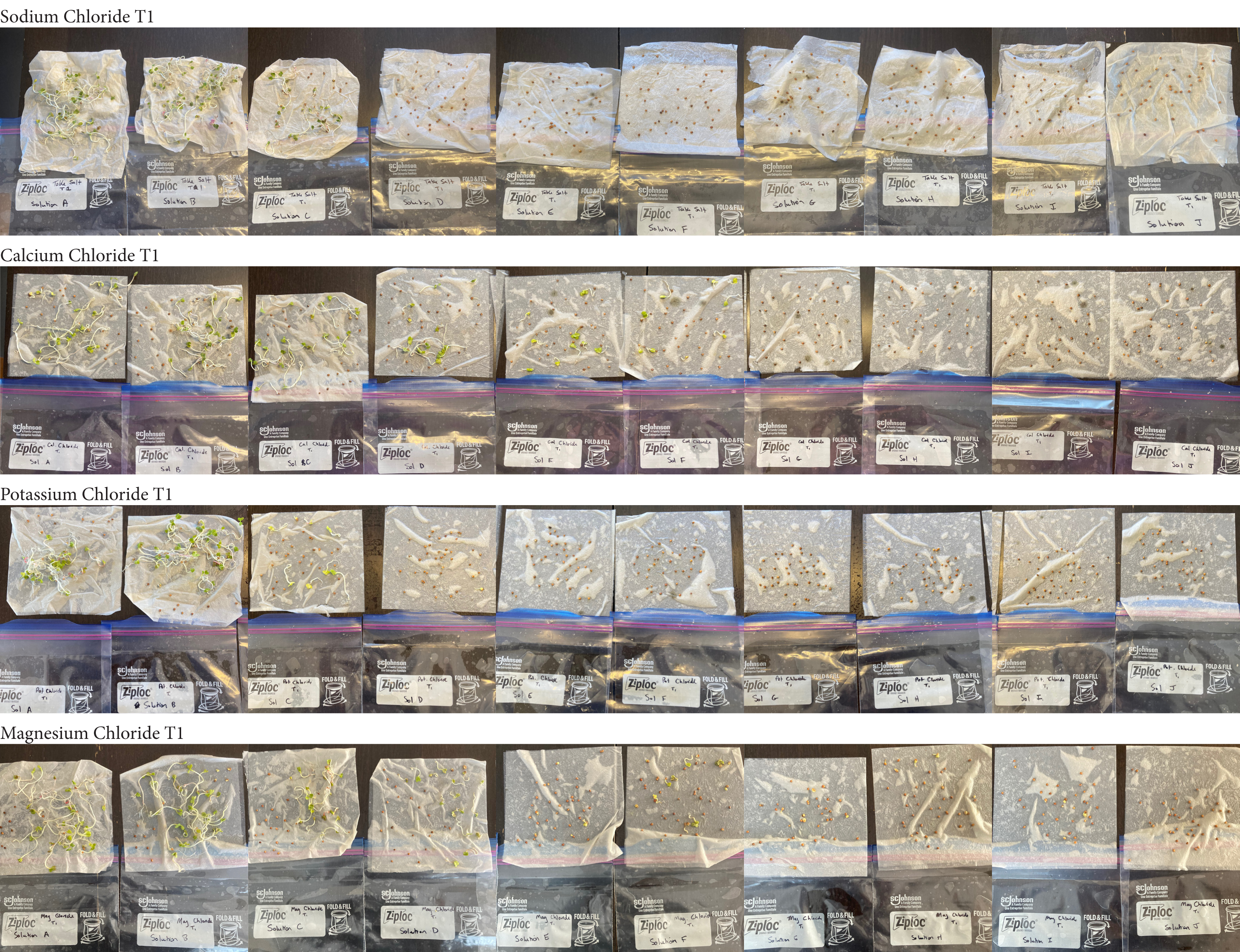


Analysis
These were my results for sodium chloride, calcium chloride, potassium chloride and magnesium chloride.
In the sodium chloride trials the control groups of tap and distilled water had most of their seeds germinate. Tap water had 40/40 seeds germinate in each trial while distilled had 40/40 in trial 1 and 37/40 in trial 2. Solution C which had a teaspoon of table salt dissolved in 8 ounces of water also had 31/40 seeds germinate in trial one and 36/40 in trial 2. When the salt levels increased to ½ teaspoon of sodium chloride (Solution D) the number of seeds that germinated was 0 in trial 1 and 4/40 in trial 2. Between ¾ teaspoon and 2 teaspoons (Solutions E-J) no seed germination occurred over the 2 weeks.
For the calcium chloride trials the controls had less numbers of germination than the sodium chloride trial with only 27/40 in trial 1 for tap water and 35/40. Distilled had 26/40 seeds germinate in trial 1 and 36/40 in trial 2. In ¼ teaspoon of calcium chloride salt (Solution C) 23/40 seeds germinated in both trials. Solution D of ½ teaspoon calcium chloride had 18/40 seeds germinate in trial 1 and 21/40 seeds in trial 2. Solution E had 9/40 seeds germinate in trial 1 and 14/40 seeds germinate in trial 2. Solution F had 9/40 seeds germinate in trial 1 and 10/40 in trial 2 and Solution G had 1/40 seeds germinate in trial 1 and 5/40 in trial 2. Once calcium chloride reached 1½ teaspoons (Solution H) no seeds germinated.
For potassium chloride the tap water control had 30/40 seeds germinate in trial 1 and 31/40 in trial 2. The distilled water control had 32/40 seeds germinate in trial 1 and 34/40 in trial 2. Seed germination declined a lot in the potassium chloride solution of ¼ teaspoon with 8/40 seeds germinating in trial 1 and 16/40 in trial 2. Solution D only had 3/40 seeds germinate in trial and 6/40 in trial 2. Solutions E and F had similar results with 1/40 seeds germinating trial 1 and 2/40 seeds germinating in trial 2. Once the potassium chloride solution reached 1¼ teaspoons (Solution G) of salt the seeds did not germinate.
Magnesium chloride had the most seeds germinate in the different salt solutions. The controls had similar results of 32/40 and 33/40 seeds germinate in trial 1 and 32/40
seeds in trial 2. Solution C had 19/40 seeds germinate in trial 1 and 30/40 in trial 2. Solution D was similar to C with 18/40 seeds germinating in trial 1 and 29/40 in trial 2. Solution E had 6/40 seeds germinate in trial 1 and 20/40 seeds germinate in trial 2. Solution F had 16/40 in seeds germinate in trial 1 and 24/40 in trial 2. Solution G had 5/40 seeds germinate in both trials. Solution H had 9/40 seeds germinate in trial 1 and has 4/40 seeds in trial 2. Solution I had 5/40 seeds germinate in trial 1 and 7/40 in trial 2. Solution J had no seeds germinate in trial 1 and 4/40 seeds in trial 2.
.
From my results, it looks like salts do have a negative effect on seed germination and that my hypothesis is supported. When you average the the total number of seeds that germinated over both trials for the different salts you can see that for Sodium Chloride and Potassium Chloride once the salt solutions reached ½ teaspoon of salt per 8 ounces of water the number of seeds that germinated dropped really low and germination did not happen beyond ½ teaspoon of salt for Sodium Chloride and 1 teaspoon for Potassium Chloride. These findings suggest that these two salts are harmful for seeds and that is supported by my background research. Calcium Chloride had less of an effect on seed germination initially but once the solution reached beyond 1¼ teaspoons seed germination did not occur. Magnesium Chloride also had a slower decline in seed germination and even had seeds germinate in all of the solutions which does suggest that it might be less harmful to seed development than the other salts which is also supported by my background research. What I found interesting is when you look at the bags at the end of the experiment (see images below). The seeds that fully germinated in the controls and in Solution C (¼ teaspoon) and even Solution D (½ teaspoon) had long thick white roots and bright green sprouts. In solutions E though J if the seeds germinated it took a lot longer for the seeds to split open, they have smaller and thinner roots and have a yellowish color which suggests that the salts are slowing the seeds development and growth. It is also interesting to see that many of the solutions that contained salt started to develop mold but that did not happen in the controls even though I had to open and pull out the napkins so I could count any ungerminated seeds because the growing roots made it hard to see.
Conclusion
Based on the results I collected, my hypothesis was supported. When more salt was added to water the seeds' ability to germinate decreased. From my research it also looks like magnesium chloride is the least harmful to seed germination as it did have seeds germinate in all of the solutions. But what my research shows is that we need to be careful when putting salt down to melt ice as it does have a negative effect on seed growth and development.
I would like to next test how store bought deicers affect seed germination as many say environmentally safe but contain these chloride salts. It might be interesting to test new deicing methods like beat brine on seeds. If i would do this project again, I would give myself more time and try to do a new trial to see if the pattern continues.
Application
Based on my research I would suggest that if you had to use a salt to melt ice that salts like Calcium or Magnesium Chloride would be better for your lawn and garden but the best method for the environment to me would be shoveling or chipping away ice before salt is needed. If you need to use salt I would suggest that you follow the instructions to make sure you are not over salting and are using the right salt for the job so excess salt is not just sitting on sidewalks and getting into the environment and I would avoid putting snow that has salt in it on your grass or garden. There are other products being explored like beet, cheese and pickle brine but more research is needed to see how these will affect the environment in the long term. For my next study I would like to test store ice melts as they are a mixture of these chloride salts and other chemicals to see how they affect seed growth since many of them claim they are pet and environmentally friendly.
With global warming on the rise, we need to protect our water and our soil so we can grow plants and food and have clean water to drink and clean air to breathe. The use of salt on our roads and sidewalks is threatening this by getting into the soil and increasing its salinity so it dries out the ground so it can’t absorb water properly and plants can’t survive. As my research shows small amounts of salt can severely slow or stop seed germination so that new plants are not able to grow. When salt gets into fresh water it can be toxic to the plants and animals that live in and around it which can destroy these ecosystems. If there is too much salt in the groundwater many people will not be able to access clean drinking water. Once salt gets into the water it is extremely hard to remove it so we need to be more careful with our use of salt in the environment.
Sources Of Error
There are possible sources of errors that I found in my experiment. First, the salts that I used were the right formulas but might not be exactly what are used in commercial deicer products so that may have an impact on results. Another happened when counting the germinated seeds. When a seed germinates it gets rid of the coat of the seed which is the outer layer that protects the seed. This caused problems when trying to count the number of germinated seeds when it looks like one of the seeds is not germinated. I had to solve this by opening the bag and testing some of the seeds' hardness to find the shells but by touching some of the seeds it could contaminate the seeds, not letting them germinate. As I did not get any mold or rot in the control groups which I had to touch when it became hard to count I don’t think it had an impact on the experiment. It also affected counting as some that I thought were seeds were actually seed coats so the most accurate count was the final count when I had them out of the bags and could move and count the seedlings and seeds. Another source of error could be that the seeds were bought online and could have been damaged either in transit or when packaged so that might have had an impact on the results but there was not much we can do to control for that. Lastly, human errors in measuring, pouring, mixing and counting could have affected results.
Citations
Bayer, M., & Njue, G. (2016). The Impact of Salts on Plants and How to Reduce Plant Injury from Winter Salt Applications. UMass Extension. Retrieved October 21, 2023, from https://ag.umass.edu/landscape/fact-sheets/impact-of-salts-on-plants-how-to-reduce-plant-injury-from-winter-salt
Bennett, M. B. (2021, February 1). Germinating Seeds | Extension | West Virginia University. WVU Extension. Retrieved October 23, 2023, from https://extension.wvu.edu/lawn-gardening-pests/news/2021/02/01/germinating-seeds
Bridgestone Tires. (2021). Road Salt in Winter: Pros & Cons. Bridgestone Tires. Retrieved December 27, 2023, from https://www.bridgestonetire.ca/learn/maintenance/pros-and-cons-of-using-road-salt-in-winter/
Brightly Inc. (2017, December 3). Pros and Cons of Different De-Icers. Brightly Software. Retrieved March 1, 2024, from https://www.brightlysoftware.com/blog/pros-and-cons-of-different-de-icers
Canada Salt Group Ltd. (2022, November 30). What Are The Best Deicers? Canada Salt. Retrieved December 27, 2023, from https://canadasalt.ca/what-are-the-best-deicers/
Canadian Salt Group Ltd. (2023, August 19). How Do Deicers Work? Canada Salt. Retrieved December 27, 2023, from https://canadasalt.ca/how-do-deicers-work/
Ferrer, A. (2012, March 8). 7 Fun Facts About Radishes. WebMD. Retrieved March 1, 2024, from https://www.webmd.com/diet/features/7-healthy-facts-about-radishes
Frank, B. (2017, April 26). Road salt — a costly way to fight winter | Ecofiscal. Ecofiscal Commission. Retrieved March 1, 2024, from https://ecofiscal.ca/2017/04/26/road-salt-costly-way-fight-winter/
Garden and Lawn. (2018, August 31). Why Does Salt Affect the Germination of Seeds? YouTube. Retrieved January 3, 2024, from https://www.youtube.com/watch?v=x0n6vzmqZFM
Government of Canada. (2018). Road salts: frequently asked questions. Canada.ca. Retrieved January 8, 2024, from https://www.canada.ca/en/environment-climate-change/services/pollutants/road-salts/frequently-asked-questions.html
Iowa Department of Transportation. (2016, November 16). How Deicing Chemicals Work. YouTube. Retrieved December 27, 2023, from https://www.youtube.com/watch?v=QsNOpUQmw2k
Kearney, V. (2022, July 27). Science Project: How Does Salt Affect Seed Germination? Owlcation. Retrieved September 20, 2023, from https://owlcation.com/stem/Science-Project-How-Does-Salt-Affect-Seed-Germination
Loeffler, B. (2021, September 14). Chloride Spotlight: What is Sodium Chloride? Ice Slicer Blog. Retrieved February 28, 2024, from https://blog.iceslicer.com/chloride-spotlight-sodium-chloride
Loeffler, B. (2021, October 13). Chloride Spotlight: What is Calcium Chloride? IceSlicer. Retrieved February 28, 2024, from https://blog.iceslicer.com/chloride-spotlight-calcium-chloride
Loeffler, B. (2021, December 15). Chloride Spotlight: What is Potassium Chloride? Ice Slicer Blog. Retrieved March 1, 2024, from https://blog.iceslicer.com/chloride-spotlight-potassium-chloride
Loeffler, B. (2022, November 22). Chloride Spotlight: What is Magnesium Chloride? Ice Slicer Blog. Retrieved March 1, 2024, from https://blog.iceslicer.com/chloride-spotlight-magnesium-chloride
Melgares, P. (2017, December 18). Picking the right product is key to melting ice from sidewalks, driveways. K-State Research and Extension. Retrieved December 27, 2023, from https://www.ksre.k-state.edu/news/stories/2017/12/winter-deicing-landscapes.html
Minnesota Pollution Control Agency. (n.d.). Chloride. Minnesota Pollution Control Agency. Retrieved November 28, 2023, from https://www.pca.state.mn.us/pollutants-and-contaminants/chloride
Minnesota Pollution Control Agency. (2022, November 23). How salt works and overview of deicing chemicals - Minnesota Stormwater Manual. Minnesota Stormwater Manual. Retrieved December 28, 2023, from https://stormwater.pca.state.mn.us/index.php/How_salt_works_and_overview_of_deicing_chemicals
Minnesota Poluution Control Agency. (2022, November 23). Environmental impacts of road salt and other de-icing chemicals - Minnesota Stormwater Manual. Minnesota Stormwater Manual. Retrieved January 6, 2024, from https://stormwater.pca.state.mn.us/index.php/Environmental_impacts_of_road_salt_and_other_de-icing_chemicals
Ninja Deicer. (2021, December 13). What Is Calcium Chloride Ice melt? Ninja De-Icer. Retrieved February 28, 2024, from https://ninjadeicer.com/blogs/resources/what-is-calcium-chloride-ice-melt
Ninja Deicer. (2024, January 14). De-Icers Efficiency Overview: What Melts Ice the Fastest? Ninja De-Icer. Retrieved February 28, 2024, from https://ninjadeicer.com/blogs/resources/de-icers-efficiency-overview-what-melts-ice-the-fastest
Occidental Chemical Corporation. (n.d.). Choosing the Right Deicer. OxyChem Calcium Chloride. Retrieved December 28, 2023, from https://www.oxycalciumchloride.com/sidewalk-ice-melting/effective-ice-melting/how-to-melt-ice-effectively/choosing-the-right-deicer
Salt Smart Collaborative. (n.d.). How Does Salt Melt Snow and Ice? Salt Smart. Retrieved December 27, 2023, from https://saltsmart.org/how-does-salt-melt-snow-and-ice/
Snow & Ice Salt & Chemicals Unlimited, LLC. (2018, March 7). What is the Most Environmentally Friendly Deicer? Ice Salt: Road Salt. Retrieved February 29, 2024, from https://snowicesalt.com/environmentally-friendly-deicer/
Spider Farmer. (2023, May 22). The Ultimate Guide to Achieving Perfect Germination. Spider Farmer. Retrieved March 1, 2024, from https://spiderfarmer.eu/2023/05/22/from-seed-to-sprout-the-ultimate-guide-to-achieving-perfect-germination/
Sweetman, S. (2018, March 14). Which Seeds Will Germinate the Fastest for a Science Fair Project? Sciencing. Retrieved March 1, 2024, from https://sciencing.com/seeds-fastest-science-fair-project-6064794.html
Viana, C. (2021, August 19). Potassium Chloride mines: climate wolf in sheep's clothing. LinkedIn. Retrieved February 29, 2024, from https://www.linkedin.com/pulse/potassium-chloride-mines-climate-wolf-sheeps-clothing-carol-viana
Wasson, S. (2022, September 7). The Environmental Impact of Driveway Salt (2024). Today's Homeowner. Retrieved January 8, 2024, from https://todayshomeowner.com/eco-friendly/guides/the-environmental-impact-of-driveway-salt/
West Coast Seeds. (2020, May 18). About Radishes | Facts About Radishes – West Coast Seeds. West Coast Seeds. Retrieved March 1, 2024, from https://www.westcoastseeds.com/blogs/wcs-academy/radishes
Acknowledgement
I would like to thank my teachers and family for supporting me thorugh this project and letting me have the chance to go to CYSF. I would also like to thank my dog Watson for giving me the idea for this science fair project.

