The Future of Healthcare - Precision Medicine
Helli Chalishajar
Grade 7
Presentation
Problem
PROBLEM
Every year, many people don't recover to their best condition as quickly or as efficiently due to them needing to use a common one-size-fits-all treatment. However, with precision medicine, we have the opportunity to change this and give patients treatments tailored to them. Thus, In the landscape of modern healthcare, precision medicine stands out as a promising frontier, offering tailored treatments based on individual genetic, environmental, and lifestyle factors. However, despite its growing prominence, there remains a need to understand the concept of precision medicine, understand its mechanics, particularly in cancer treatment, and assess its feasibility within today's economic constraints. As of this, throughout my project, I will be researching the following questions:
- What is precision medicine, and how does it differ from traditional approaches to healthcare?
- How does precision medicine function as a treatment for cancer?
- What are the key components and processes involved in delivering precision medicine? specifically for cancer patients?
- Is precision medicine feasible in today's economy?
HYPOTHESIS
I hypothesize that through targeted therapies based on genetic profiling, precision medicine in cancer treatment will demonstrate improved patient response rates compared to conventional treatments. This improvement will be evidenced by higher rates of remission, lower rates of recurrence, and overall higher survival rates among patients undergoing precision medicine interventions. Moreover, I propose that with further comprehensive research and rigorous testing, precision medicine has the potential to evolve into a truly revolutionary approach, capable of not only addressing cancer but also serving as a long-term solution to combat the disease. As precision medicine continues to advance, it may also fundamentally alter our perception and management of diseases beyond cancer. By unlocking intricate genetic pathways and leveraging personalized treatment strategies, precision medicine could pave the way for a great shift in healthcare, ushering in a new era of targeted therapies and individualized care. This transformative potential extends beyond cancer, suggesting that precision medicine may allow breakthroughs in understanding and treating countless of other diseases. Ultimately, I believe that precision medicine holds the promise to not only revolutionize cancer care but also have the potential to help unlock a new area of research in the medical community to cure other common diseases and have a tremendous impact on the healthcare landscape.
Method
I used a thorough and clear methodological approach in my precision medicine research. I conducted an elaborate analysis to gather insights into various aspects of this field. I meticulously reviewed clinical trials to collect data on the efficiency and safety of precision medicine approaches, gathering information from databases like ClinicalTrials.gov, NCBI and PubMed. Additionally, I did a deep dive into the understanding of the molecular mechanisms underlining precision medicine, focusing on genetics, DNA, RNA, NGS and RNA sequencing. To capture diverse perspectives on precision medicine, I searched through trusted websites. Furthermore, I analyzed diagrams to visualize complicated biological processes. Some key areas of exploration I did included understanding precision medicine principles, explaining its mechanisms of action, navigating precision oncology, and investigating emerging immunotherapy approaches such as Tumor-infiltrating lymphocytes (TILs) and Chimeric Antigen Receptor T-cell therapy (CAR T-cells). By gathering information from these varied sources, my project aims to share the potential of precision medicine to transform cancer care and potentially revolutionize the medical field.
Research
WHAT IS PRECISION MEDICINE - ABSTRACT
Precision medicine represents a groundbreaking advancement within the medical field. It involves tailoring treatments to specific subpopulations that share common sensitivities or issues related to particular diseases or exhibit similar responses to specific drugs. The concept of tailoring treatments to individual patient characteristics does not mean creating new unique drugs or medical devices for each patient. Instead, it involves classifying individuals into smaller categories based on their unique susceptibilities to certain diseases, the biology and prognosis of those diseases, or their responses to particular treatments.
In precision medicine, diagnostic testing is often used for selecting the best and most appropriate therapies based on the context of a patient's genetic content or other molecular or cellular analysis.
As the field continues to evolve, precision medicine has the potential to revolutionize healthcare, bringing in a new era of personalized and more effective medical intervention.
GOAL OF PRECISION MEDICINE
The main aim of Precision medicine is to give each individual/group their own customized healthcare plan. Furthermore, the goal is to shift from the idea of a one-size-fits-all approach to taking into account each individual’s unique genetic makeup, environment, and lifestyle to provide a more personalized treatment. In turn, this could result in better health outcomes and cost savings in the long run.
“The right therapy, to the right patient, at the right time.”
HOW IS PRECISION MEDICINE DIFFERENT FROM TRADITIONAL MEDICINE?
The primary distinction between traditional and precision medicine lies in their approach to addressing an individual's health and requirements.
Traditional medicine is commonly used across various age groups and individuals. Physicians use broad-ranging therapies and diagnostic techniques to address common health issues faced by patients. However, traditional medicine overlooks the significance of an individual's genetic makeup, which significantly impacts overall health and well-being.
On the other hand, precision medicine offers a more precise method of diagnosing and treating internal issues within the body. It avoids generalization, resulting in optimal outcomes by considering an individual's symptoms and DNA profile. This tailored approach ensures that treatments are specifically tailored to the individual's unique biological characteristics, leading to more effective outcomes.
GENOMICS
Genes are segments of DNA that act as a blueprint, guiding your body in producing proteins and performing various functions necessary for its operation.
While genes typically have slight differences among individuals, with bases occasionally being altered, added, or removed, most of these variations have no significant impact on overall health. However, certain variations can result in the production of abnormal proteins, potentially increasing the risk of specific diseases. Moreover, some gene variants can influence the effectiveness of medications within your body. Alternatively, they may create different side effects from a medication compared to those experienced by others.
DNA:
DNA, or Deoxyribonucleic acid, plays a vital role in precision medicine, allowing a personalized approach to healthcare by offering knowledge of an individual's genetic composition and its impact on disease susceptibility, response to treatment, and overall well-being. Central to the progress of precision medicine is the utilization of DNA testing and analysis, which serve as foundational tools in identifying genetic variations linked to diverse health conditions, thereby informing customized treatment strategies.
The fundamentals for the advancement of precision medicine are DNA testing and analysis. This is because they allow for the identification of genetic variants associated with various conditions and guide tailored treatment decisions.
NGS:
Next-generation sequencing (NGS) represents a cutting-edge technology utilized for the sequencing of DNA and RNA, as well as the detection of variants and mutations. This advanced method allows for the rapid sequencing of hundreds to thousands of genes or even entire genomes within a short timeframe.
NGS plays a critical role in precision medicine, particularly in the world of oncology, where its application is vital in understanding cancer
Precision medicine seeks to use genomic information to deliver tailored treatment to individuals precisely when needed. NGS allows the identification of clinically actionable mutations, guiding the design of treatment based on a person’s disease-driving molecular alterations.
By rapidly sequencing multiple genes and pinpointing disease-associated variants, NGS assists in matching patients with appropriate therapies and evaluating disease susceptibility. Moreover, it aids in patient stratification, contributing to more effective and personalized medical interventions.
This diagram represents an example of Next-Generation Sequencing
RNAseq:
RNA-Seq, or RNA sequencing, is a powerful technique that uses (NGS) to provide a comprehensive shot of the transcriptome, which is the complete set of RNA transcripts in a cell.
This technique allows the detection and quantification of RNA molecules present in a biological sample, allowing researchers to understand/study various aspects of gene expression, including alternative splicing, post-transcriptional modifications, gene fusions, mutations, and changes in gene expression under different experimental conditions or over time.
The high-resolution data generated by RNA-Seq holds vital importance in understanding the molecular basis of diseases, pinpointing potential therapeutic targets, and tailoring treatment approaches. By analyzing the RNA profiles of individual patients, researchers and healthcare practitioners can deepen their understanding of disease mechanisms, predict patient responses to specific therapies, and create more precise and effective therapeutic strategies. Furthermore, RNA-Seq data can be combined with other omics datasets, such as genomics and proteomics, to construct a comprehensive molecular portrait of an individual, further enhancing our comprehension of disease processes.
HOW DOES PRECISION MEDICINE WORK?
The process for precision medicine varies depending on the case however each case follows the fundamental principles of precision medicine.
1. Genomic Analysis:
- In precision medicine, the first and most vital step is to analyze the patient’s genetic makeup.
- This process entails sequencing the patient's DNA to detect genetic differences, alterations, or indicators that could potentially relate to specific diseases or medical conditions.
2. Biomarker Identification:
- After collecting the patient's genetic data, healthcare professionals use advanced diagnostic examinations to identify particular biomarkers.
- These biomarkers serve as crucial elements helping to confirm a diagnosis or assess the efficacy of treatment.
- Precision medicine heavily depends on recognizing biomarkers, which serve as measurable signals of normal or abnormal biological activities.
- These tests may include blood tests, imaging studies, or other types of diagnostic exams.
3. Disease Stratification:
- Subtyping Diseases: Precision medicine aims to categorize diseases into subtypes based on specific molecular characteristics.
- This allows for more targeted and effective treatments, as different subtypes may respond differently to interventions.
4. Tailored treatment plans:
- Targeted therapies: Following the outcomes of genomic analysis and precision diagnostics, treatment strategies can be customized to address specific genetic mutations or biomarkers that have been identified.
- In other words, this means that healthcare providers can administer targeted therapies precisely tailored to meet the individual needs of the patient.
- These therapies may involve medications, surgery, or other interventions that are tailored to the patient’s unique characteristics.
- These therapies are designed to interfere with specific molecules involved in the growth and progression of diseases.
- These therapies may involve medications, surgery, or other interventions that are tailored to the patient’s unique characteristics.
- In other words, this means that healthcare providers can administer targeted therapies precisely tailored to meet the individual needs of the patient.
5. Predictive Modeling + Integration of clinical & Molecular Data:
- Predictive Analytics: Precision medicine relies on advanced data analytics and computational modeling to anticipate the potential responses of individuals to specific treatments.
- Predictive modeling serves as a valuable tool for healthcare professionals, assisting them in identifying the most effective and least harmful treatments tailored to each patient's unique requirements.
- Comprehensive Analysis: Precision medicine involves the integration of clinical data (patient history, symptoms, etc.) with molecular data (genomic, proteomic, metabolomic, etc.).
- This aids in creating the best course of action
6. Ongoing Monitoring:
- Doctors will continue to monitor the patient’s progress and do tests and assessments to adjust their treatment plan as needed to achieve the best possible outcomes
- Precision medicine is not a one-time event, but rather an ongoing process.
PATIENT STRATIFICATION:
Patients are stratified into different groups in precision medicine based on the molecular and genetic characteristics of their diseases. This stratification allows healthcare professionals to tailor treatments to specific subtypes, maximizing the effectiveness of treatments. The specific groups can vary depending on the disease, however below are some of the most common ways:
1. Genetic Mutations:
- Patients may be stratified based on specific genetic mutations associated with their disease.
- Ex. in cancer, tumors with certain mutations may be categorized into distinct groups for targeted therapies.
2. Biomarker Expression:
- Stratification based on the expression of specific biomarkers, such as proteins or other molecules, is common.
- Ex. Breast cancer patients may be stratified based on the expression of hormone receptors (e.g., estrogen or progesterone receptors).
3. Molecular Subtypes:
- Diseases, particularly cancers, may have different molecular subtypes based on unique molecular characteristics.
- Ex. Breast cancer can be stratified into subtypes like HER2-positive, triple-negative, or luminal based on the expression of certain markers.
4. Metabolic Profiling:
- Some diseases are stratified based on metabolic profiles.
- Metabolomic data, which involves studying the small molecules involved in cellular processes, can be used to categorize patients into different groups with varied responses to treatments.
5. Immune System Characteristics:
- Patients may be stratified based on the characteristics of their immune system.
- This could involve assessing the presence or absence of specific immune markers that influence responses to immunotherapeutic interventions.
6. Response to Previous Treatments:
- Patients may be stratified based on their responses to previous treatments.
- This approach helps identify specific subgroups that are more likely to respond to certain therapies, guiding future treatment decisions.
7. Disease Stage:
- Stratification based on the stage or severity of the disease is a common approach.
- helps tailor treatments to the specific needs of patients at different stages, optimizing the likelihood of success.
8. Genomic Profiling:
- Whole-genome or whole-exome sequencing can reveal a comprehensive genomic profile of a patient's disease.
- Stratification based on this detailed genomic information can guide treatment decisions
9. Risk Factors and Predispositions:
- Stratification may also involve assessing patients for specific risk factors or predispositions that influence disease development.
- This approach allows for personalized preventive strategies and early interventions.
10. Epigenetic Modifications:
- Epigenetic changes, such as DNA methylation or histone modifications, can influence gene expression.
- Stratification based on epigenetic profiles may reveal subgroups with distinct treatment responses.
Patient stratification is crucial as it allows different individuals to fit into a specific category tailored for them rather than being placed in one large category where they may not receive the most vital treatment required for them.
BIOMARKERS:
WHAT ARE THEY & HOW DO THEY WORK
Biomarkers represent distinct biological indicators, whether cellular, biochemical, or molecular, that reliably measure a process, event, or condition within tissues, cells, or fluids. They serve to detect early changes in a patient's health, offering an objective measure of cellular or organismal activity at a specific moment.
- While some biomarkers come from routine doctor visits, such as blood pressure or body weight measurements, others come from laboratory analyses of blood, urine, or tissues.
- Molecular or cellular changes are often observed by examining genes or proteins.
MAIN BIOMARKERS IN PRECISION MEDICINE
Precision medicine relies on various types of biomarkers to tailor medical treatments to individual patients. These biomarkers can provide information about a person's genetic makeup, molecular characteristics, and other factors that influence their response to specific treatments.
Main Biomarkers used in Precision medicine:
1. Genetic Biomarkers:
- Biomarkers in genomics involve the study of an individual's genes and their interactions with each other and the environment.
- Single Nucleotide Polymorphisms (SNPs): Variations in a single nucleotide base in the DNA sequence, which can impact traits and disease susceptibility/disease risks and drug responses.
- Copy Number Variations (CNVs): Changes in the number of copies of a particular gene, associated with diseases and drug responses.
- Gene Mutations: Specific alterations in the DNA sequence of a gene that may be linked to diseases or drug responses.
2. Protein Biomarkers:
- Biomarkers in proteomics focus on the study of proteins and their functions.
- Protein Expression Levels: Measurement of the quantity of specific proteins, which can indicate disease presence or progression.
- Enzyme Activity: Assessment of the activity levels of enzymes, which can be indicative of certain diseases or drug metabolism.
- Phosphorylation patterns: Associated with disease states or drug response
3. RNA Biomarkers:
- provide dynamic insights into cellular states and regulatory processes
- mRNA Expression: Analysis of messenger RNA levels to understand gene expression patterns and identify disease-related changes.
- MicroRNA (miRNA): Small RNA molecules that regulate gene expression and can serve as biomarkers for diseases.
4. Epigenetic Biomarkers:
- Epigenetic biomarkers measure disease-associated and drug-associated epigenetic alterations, thus providing decision support for routine clinical treatment and drug discovery.
- DNA Methylation: Changes in the methylation patterns of DNA, influencing gene expression and potentially serving as markers for disease risk or progression.
- Histone Modifications: Alterations in the structure of histone proteins, affecting gene regulation.
5. Metabolomic Biomarkers:
- involve the study of small molecules and metabolites in biological systems.
- Metabolite Profiles: Analysis of small molecules involved in cellular processes, providing insights into metabolic pathways and disease states.
- Drug metabolites that can indicate drug response or toxicity
6. Circulating Tumor DNA (ctDNA):
- Liquid Biopsies: Detection and analysis of tumor-derived genetic material circulating in the bloodstream, providing information about cancer mutations and treatment response.
7. Pharmacogenomic Biomarkers:
- Drug Metabolism Enzymes: Genetic variations affecting the metabolism of drugs, influencing individual responses to medications.
8. Immunologic Biomarkers:
- Immune Cell Activity: Assessment of the activity and composition of immune cells
- relevant for immunotherapy and autoimmune diseases.
9. Next Generation Sequencing (NGS)
- Looks for changes in an individual's DNA or tumor cells which in turn can lead to early diagnoses and treatment plans.
- include various genetic and genomic markers identified through high-throughput sequencing technologies.
When we use Biomarkers to help find the best course of action for a certain disease, it is called “Biomarker Driven Therapies.” We most commonly use it in oncology.
CANCER & TUMORS
WHAT IS IT?
Cancer is a disease in which some of the body’s cells grow uncontrollably and continue to spread to other regions in the body; cancer may start anywhere.
HOW DOES IT WORK/HOW DOES IT SPREAD?
Typically, human cells grow and multiply (through a process called cell division) to form new cells as the body needs them. When cells grow old or become damaged, they die, and new cells take their place. However, disruptions in this orderly cycle can occur, leading to the abnormal growth and multiplication of damaged cells, which may form tumors. Now these cancerous tumors have the potential to invade nearby tissues and spread to distant parts of the body to form new tumors. This process is known as metastasis. Essentially, metastasis involves the spreading out and colonization of cancer cells throughout the body.
Unfortunately, a significant number of cancer-related deaths result from metastatic disease. While treatment may extend life expectancy in some cases, the primary objective in managing metastatic cancer is often to control its development and growth or to relieve the symptoms it causes. It is important to get metastatic cancer treated as it causes severe damage to the way the body functions
TYPES OF GENES THAT CAUSE CANCER
Cancer affects these 3 main types of genes, proto-oncogenes, tumor suppressor genes, and DNA repair genes.
- Proto-oncogenes
- In the human body, the proto-oncogenes have the role of growth and division.
- Sometimes when the proto-oncogenes are altered in a certain way or become hyper-active, they morph into genes that can cause cancer, in other words, oncogenes.
- What this does is that it lets cells grow more and survive when it shouldn't
- Tumor Suppressor Genes
- Tumor suppressor genes also take part in controlling cell division and cell growth.
- If some of the cells are altered this will lead to the cells to divide uncontrollably.
- Tumor suppressor genes also take part in controlling cell division and cell growth.
- DNA Repair Genes
- These genes are vital as they fix damaged DNA.
- If cells have any mutations in these genes, usually the cells start developing other mutations.
- When this happens it could lead to the mutations causing change in the person's chromosomes.
- Ex. some chromosomes could multiply and some parts can be deleted altogether. The combination of all the mutations may make come cells cancerous as a result
- When this happens it could lead to the mutations causing change in the person's chromosomes.
Scientists have researched molecular changes that cause cancer and have created a treatment that targets these mutations. Furthermore, they have also found a pattern of certain mutations that are most commonly found in most cancers.
PRECISION ONCOLOGY
Precision oncology is a rapidly evolving approach to cancer treatments that involves customizing therapies based on the individual characteristics of each patient and their tumor allowing for a more targeted and effective approach to cancer care.
- It has the same concept as general precision medicine
A reason as to why precision oncology is more effective than traditional cancer treatments is Disease Heterogeneity.
-
Due to disease heterogeneity, traditional cancer treatments are only effective in a subset of the patient population. Tumors may express different proteins, leading to variable responses to generic treatments as tumors may have underlying genetic causes.
-
Precision oncology addresses this problem by customizing therapy selection for each patient, taking into account their unique genetic and molecular profiles.
TYPES OF PRECISION ONCOLOGY TREATMENTS:
CAR T-CELL THERAPY:
WHAT IS IT?
The concept is identical to administering a "living drug" to patients. The core of CAR T-Cell Therapy is T cells, which play a key role in coordinating the immune response and directly eliminating pathogen-infected cells. Currently, CAR T-cell therapies are tailored to individual patients, aligning closely with the principles of precision medicine. Furthermore, at the moment, these therapies are most commonly used in cases of leukemia and lymphoma.
HOW DOES IT WORK?
The whole function behind CAR T-cell therapies lies in extracting T-cells from the patient and then in a lab, re-engineering them to produce proteins on their surface called chimeric antigen receptors (CARs). These CARs are specifically designed to identify and attach to specific proteins and/or antigens on the surface of cancer cells and kill them.
Step-by-step process of CAR T-cell therapy: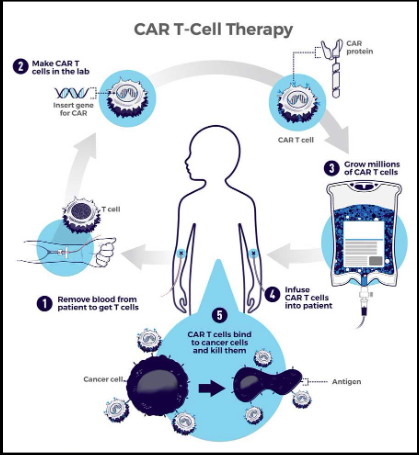
- 1. Patients T-cells are removed from their blood
- 2. The gene for CARs is inserted into the t-cells in a lab
- The gene encodes the engineered CAR proteins that are expressed on the surface of the patient's T cell, creating CAR T cells.
- 3. Millions Of CAR T cells are grown
- 4. Given to the patient through intravenous infusion
- 5. CAR T Cells bind to the antigens on the cancer cells and kill them.
MAKING A CAR T-CELL:
Every CAR spans across the cell membrane, with a portion of the receptor situated outside the cell and another portion within it. Typically, the external segment of the CAR consists of fragments or domains of lab-made antibodies. The choice of domain determines the receptor's ability to recognize and bind to antigens on tumor cells. Additionally, each CAR contains signaling and "co-stimulatory" domains that transmit signals into the cell upon interaction with an antigen on the internal part. Last but not least, it is important to keep in mind that the different domains used may influence the cells' overall function.
If all goes as planned, the CAR T cells will continue to multiply in the patient's body and, with guidance from their engineered receptor (synthetic molecules), recognize and kill any cancer cells that keep the target antigen on their surfaces.
- The CAR T-cell therapies approved by FDA so far target one of two antigens on B cells, CD19 or BCMA.
TUMOR-INFILTRATING LYMPHOCYTES:
WHAT IS IT & HOW DOES IT WORK?
Tumor-infiltrating lymphocytes (TILs) are an experimental cell therapy being developed for treating solid tumors.
Lymphocytes, also known as white blood cells, play a crucial role in the immune system by combating infections and eliminating abnormal cells within the body. Consisting of T cells and B cells, lymphocytes actively patrol the body to detect and eliminate cells that seem abnormal, including cancerous cells. As tumors develop, lymphocytes identify these abnormal cells and infiltrate the tumor, becoming known as tumor-infiltrating lymphocytes (TILs). Once inside the tumor, the TILs began killing the cancer cells. However, they may encounter problems such as being prevented from doing their jobs by brakes in the immune system or signals from the tumor that weaken the immune response. To counteract this, immune checkpoint inhibitors were made to lock some of those brakes and unleash the immune cells to attack cancer.
HOW CAN THESE CELLS BE USED FOR CANCER THERAPY?
As TILs come directly from the tumor itself, they already recognize/identify many targets on the cancer cells, which in turn gives us a real advantage because it prevents the tumor from evading our efforts by hiding one target at a time.
To utilize TILs as a therapy, it's essential to assist them in overcoming obstacles within the tumor environment to effectively remove the cancer. This can be achieved through two approaches:
1. expanding the TILs
2. engineering them with specific attributes.
Expanding the TILs involves increasing the number of immune cells in the patient's body that are already trained at recognizing and targeting the patient's particular tumor. Meanwhile, engineering TILs enhances their efficacy in combating cancer cells. For instance, TILs can be genetically modified to resist signals emitted by the tumor, which typically deactivate T cells, thus boosting their anti-cancer capabilities.
COST:
The cost of precision medicine varies depending on the individual and their case. For example, depending on a certain region's healthcare infrastructure and/or insurance coverage, the cost will differentiate.
The targeted therapies that patients may temporarily receive following genomic sequencing results can come with staggering costs, often exceeding $10,000 per month.
As mentioned, precision medicine is not a one-time thing and often requires continuous monitoring making the price skyrocket. As of this, patients with lower-end salaries and/or insurance may not be able to use precision medicine and will have to deal with a common treatment. Keep in mind that this may not be suitable for their health. Additionally, precision medicine requires a large period of time as mentioned, resulting in the total cost coming out to be $ 250,000 or more.
The cost for precision medicine generally rises as more genetic profiling is required and the case of your illness. Often Precision oncology cases are the most expensive as they include all the general costs of cancer treatment along with the expenses of precision medicine.
PROS VS. CONS
PROS:
1. EMPOWER THE PREVENTION MEDICINE
- shift the emphasis in medicine from reaction to prevention
- predict susceptibility to disease
- improve disease detection
- preempt disease progression
- customize disease-prevention strategies
2. INCREASED EFFICIENCY
- Precision medicine aims to enhance the effectiveness of medical treatment by identifying the most suitable treatment for each patient. This approach allows healthcare providers to steer clear of unnecessary or ineffective treatments, thereby offering significant benefits to patients.
- prescribe more effective drugs
3. IMPROVED PATIENT OUTCOMES
- As patients have treatments designed to their individual needs, there is a better chance that they will react under better circumstances rather than using a general treatment that may not be suitable for that specific individual.
- avoid prescribing drugs with predictable side effects
4. ENHANCED PATIENT EXPERIENCE
- It is widely acknowledged that precision medicine enhances the patient experience by delivering more targeted and efficient treatment options. This tailored approach not only improves quality of life but also minimizes the occurrence of side effects.
5. REDUCED HEALTHCARE COSTS:
- reduce the time, cost, and failure rate of pharmaceutical clinical trials
- Improving patient outcomes and increasing the efficiency of medical treatment is guaranteed with precision medicine, furthermore, it will help you reduce healthcare costs in the long run.
- eliminate trial-and-error inefficiencies that inflate healthcare costs and undermine patient care
CONS:
1. LIMITED AVAILABILITY/ACCESSIBILITY
- Precision medicine is still in its early stages of development and implementation and cannot be something usable in a remote situation.
2. ETHICAL AND PRIVACY CONCERNS
- This is a major con in the world of precision medicine, as precision medicine relies heavily on genetic data and personal information to function, raising concerns about privacy and potential misuse
- protecting this data from unauthorized access and ensuring ethical practices in its usage become critical considerations in the implementation of precision medicine.
3. COMPLEX REGULATORY LANDSCAPE:
- presents unique regulatory challenges, due to the concept of personalized treatments, which may involve the growth of new drugs or diagnostic tools, that require flexible regulations to support innovation while ensuring patient safety.
- developing and implementing such regulations can be a lengthy and complex process, slowing down the common adoption of precision medicine.
4. EXPENSIVE & TIME CONSUMING:
- implementing precision medicine involves various processes such as genetic testing, data analysis, and treatment customization, and these procedures come with an expensive cost that may be extremely time-consuming.
Data
EFFECTIVENESS/SUCCESS RATES:
Side note:
I am basing my effectiveness and data on a singular study to avoid my results being mixed.
- It is based on the study:
- A Retrospective Analysis of Precision Medicine Outcomes in Patients With Advanced Cancer Reveals Improved Progression-Free Survival Without Increased Health Care Costs
- Authors: Derrick S. Haslem, S. Burke Van Norman, Gail Fulde, Andrew J. Knighton, Tom Belnap, Allison M. Butler, Sharanya Rhagunath, David Newman, Heather Gilbert, Brian P. Tudor, Karen Lin, Gary R. Stone, David L. Loughmiller, Pravin J. Mishra, Rajendu Srivastava, James M. Ford, and Lincoln D.
- A Retrospective Analysis of Precision Medicine Outcomes in Patients With Advanced Cancer Reveals Improved Progression-Free Survival Without Increased Health Care Costs
BACKGROUND INFORMATION:
They conducted a meticulous matched cohort investigation involving 72 patients diagnosed with metastatic cancer across various subtypes within the framework of a comprehensive, integrated healthcare delivery system. The study analyzed the treatment outcomes of 36 patients who underwent genomic testing and subsequently received targeted therapy (embracing precision cancer medicine) during the period spanning from July 1, 2013, to January 31, 2015. This cohort was meticulously compared with 36 historical control patients who were administered standard chemotherapy protocols.
- The genomic analysis, included NGS-based oligoselective exon sequencing of 96 cancer-related genes:
- ABL1, AKT1, ALK, APC, ATM, AURKA, AURKB, AXL, BCL2, BRAF, BRCA1, BRCA2, CCND1, CDH1, CDK2, CDK4, CDK5, CDK6, CDK8, CDK9, CDK12, CDKN2A, CEBPA, CSF1R, CTNNB1, CYP2D6, DDR2, DNMT3A, DPYD, EGFR, EPCAM, ERBB2, ERBB3, ERBB4, ERCC1, ERCC2, ERCC3, ERCC5, ERCC6, EZH2, ESR1, FGFR1, FGFR2, FGFR3, FGFR4, FLT3, GNA11, GNAQ, GNAS, HNF1A, HRAS, IDH1, IDH2, JAK2, JAK3, KDR, KIT, KRAS, MAP2K1, MAP2K2, MAPK1, MET, MLH1, MPL, MRE11, MSH2, MTOR, MSH6, MYC, MUTYH, NOTCH1, NPM1, NRAS, PARP1, PARP2, PDGFRA, PIK3CA, PMS2, PTCH1, PTCH2, PTEN, PTPN11, RB1, RET, RUNX1, SMAD4, SMARCB1, SMO, SRC, STK11, TET2, TP53, UGT1A1, VEGFA, VHL, and WT1.
THE RESULTS:
- The average progression-free survival was 22.9 weeks for the precision medicine group and 12.0 weeks for the control group
- per patient charges per week were $4,665 in the precision treatment group and $5,000 in the control group
CONCLUSION:
“These findings suggest that precision cancer medicine may improve survival for patients with refractory cancer without increasing health care costs. Although the results of this study warrant further validation, this precision medicine approach may be a viable option for patients with advanced cancer.”
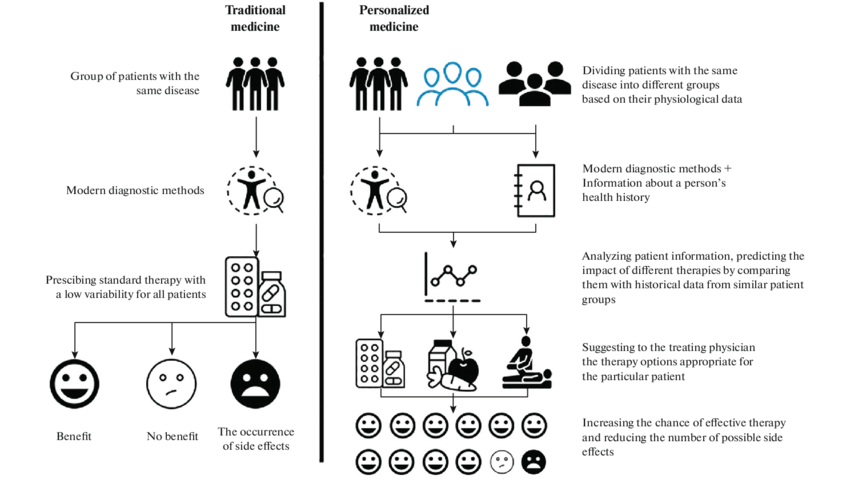
Additionally, I have also attached a chart of precision medicine vs. traditional medicine to showcase my data on how precision medicine works and its difference from generic medical treatments.
Conclusion
Precision medicine, a cutting-edge approach to healthcare, tailors medical treatment to the individual characteristics of each patient. This includes their genetic makeup, environment, and lifestyle. By using recent advances in DNA sequencing and analysis, as well as other omics technologies, precision medicine aims to understand the unique molecular characteristics of a patient's disease and to customize treatment accordingly. In the context of cancer, precision medicine has shown remarkable success by identifying specific mutations driving the cancer and selecting treatments that target these mutations, leading to improved patient outcomes. Recent breakthroughs in immunotherapy, such as TILs and CAR T cells, demonstrate the potential of precision medicine in revolutionizing cancer treatments. These therapies highlight the effectiveness of tailoring treatments to the unique molecular characteristics of each patient's cancer and tumor, illustrating the promise of precision medicine in improving care. However, a large concern presented with precision medicine are the ethical considerations for several factors. These include privacy, informed consent, and the need for robust ethical frameworks and privacy safeguards to prevent potential misuse. Therefore, before precision medicine can hit the street as a full-time field of medicine, ethical considerations are a critical aspect of implementing precision medicine to safeguard patient rights and ensure equitable and responsible use of genetic and health data. Despite these challenges, with time and resources, precision medicine has the potential to become a truly amazing field of medicine. Ongoing efforts to address ethical and privacy concerns, along with advancements in technology and regulations, can lead to a more equitable and accessible implementation of precision medicine. Last but not least, precision medicine has the potential to reduce treatment costs as you don't have to keep hoping for the best with common treatments and go through trial and error over and over again. This being said precision medicine may be expensive at the start but in the long run may be extremely efficient.
In conclusion, Precision medicine is a revolutionary approach to medicine that considers an individual’s characteristics to determine the best course of treatment. I believe that precision medicine is a groundbreaking approach that holds great promise for optimizing care and improving treatment outcomes, particularly in the context of cancer. While challenges exist, the continued advancement and adoption of precision medicine technologies are expected to lead to greater outcomes and ultimately benefit patients worldwide.
Citations
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8108369/ -
- https://en.wikipedia.org/wiki/RNA -
- https://www.cancer.gov/about-cancer/treatment/research/car-t-cells -
- https://bmcmedgenomics.biomedcentral.com/articles/10.1186/s12920-023-01745-y -
- https://www.sciencedirect.com/topics/computer-science/precision-medicine -
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3841808/ -
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4160907/ -
- https://en.wikipedia.org/wiki/Personalized_medicine -
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6192531/ -
- https://www.fda.gov/medical-devices/in-vitro-diagnostics/precision-medicine#:~:text=Precision%20medicine%2C%20sometimes%20known%20as,genes%2C%20environments%2C%20and%20lifestyles. -
- https://ascopubs.org/doi/full/10.1200/EDBK_174176 -
- https://www.gov.wales/sites/default/files/publications/2019-04/genomics-for-precision-medicine-strategy.pdf -
- https://medium.com/@drugcartsblog/tumors-neoplasm-malignant-tumors-benign-tumors-97b4a294b93b -
- https://cumming.ucalgary.ca/gse/about/programs/precision-health/precision-medicine -
- https://hcp.hms.harvard.edu/news/personalized-medicine-may-come-cost-patients -
- https://www.statista.com/statistics/1420940/average-cost-for-precision-medicine-treatment-globally-by-region/ -
- https://www.testmycholangio.com/molecular-profiling-in-cca#:~:text=Molecular%20profiling%20is%20a%20type,mutations%20unique%20to%20your%20tumor.
- https://magazine.ucsf.edu/who-will-benefit-precision-medicine#:~:text=The%20precision%20therapies%20that%20a,more%20than%20%2410%2C000%20a%20month. -
- https://cancer.ca/en/research/understanding-cancer-research/precision-medicine -
- https://www.medicalnewstoday.com/articles/249141#summary -
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8263078/ -
- https://www.genome.gov/genetics-glossary/Precision-Medicine -
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7282491/ -
- https://www.genome.gov/news/news-release/GM8-Looking-across-genomic-medicines-gaps-and-opportunities -
- https://pubmed.ncbi.nlm.nih.gov/33916369/ -
- https://www.mdanderson.org/cancerwise/what-is-tumor-infiltrating-lymphocyte-til-therapy--6-things-to-know.h00-159460056.html#:~:text=Lymphocytes%2C%20made%20up%20of%20T,%2Dinfiltrating%20lymphocytes%2C%20or%20TILs. -
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/tumor-infiltrating-lymphocytes#:~:text=Tumor%2Dinfiltrating%20lymphocytes%20(TILs),density%20of%20the%20TIL%20infiltrate. -
- https://www.nih.gov/about-nih/what-we-do/nih-turning-discovery-into-health/promise-precision-medicine#:~:text=Precision%20medicine%20is%20an%20innovative,grown%20directly%20from%20biomedical%20research. -
- https://www.galaxycare.org/blog/difference-between-tumor-and-cancer/#:~:text=A%20tumor%20is%20an%20abnormal,not%20all%20tumors%20are%20cancerous. -
- https://www.drzilberstein.com/blog/the-difference-between-personalized-medicine-and-traditional-medicine#:~:text=Doctors%20use%20its%20broad%20therapies,diagnosing%20problems%20within%20your%20body. -
- https://www.drzilberstein.com/blog/the-difference-between-personalized-medicine-and-traditional-medicine#:~:text=Doctors%20use%20its%20broad%20therapies,diagnosing%20problems%20within%20your%20body. -
- https://www.webmd.com/cancer/precision-vs-traditional-medicine -
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8282508/#:~:text=%E2%80%9CPrecision%20medicine%E2%80%9D%20is%20a%20term,response%20to%20a%20specific%20medicine. -
- https://www.cdc.gov/genomics/about/precision_med.htm#:~:text=Precision%20medicine%2C%20also%20called%20personalized,will%20work%20best%20for%20you. -
- https://www.researchgate.net/publication/329844619_ABSTRACT_PERSONALISED_MEDICINE_ITS_ADVANTAGES_AND_FUTURE_CHALLENGES_FOR_HEALTHCARE_INDUSTRIES -
- https://europepmc.org/article/MED/12596356 -
- https://www.jax.org/personalized-medicine/precision-medicine-and-you/what-is-precision-medicine
- https://www.babirus.ae/personalized-medicine-benefits/ -
- https://www.babirus.ae/advantages-disadvantages-personalized-medicine/ -
- https://health.ucdavis.edu/precision-medicine/what-is-precision-medicine.html#:~:text=Precision%20medicine%20is%20the%20intersection,change%20and%20interact%20over%20time.-
- https://www.linkedin.com/pulse/basics-precision-medicine-diellza-rabushaj -
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8282508/
- https://www.fda.gov/drugs/biomarker-qualification-program/about-biomarkers-and-qualification#:~:text=Molecular%2C%20histologic%2C%20radiographic%2C%20or,feels%2C%20functions%2C%20or%20survives.-
- https://pubmed.ncbi.nlm.nih.gov/27901055/ -
- https://cordis.europa.eu/programme/id/H2020_SC1-PM-02-2017 -
- https://members.cbio.mines-paristech.fr/~jvert/talks/170306ghent/ghent.pdf -
- https://www.niehs.nih.gov/health/topics/science/biomarkers#:~:text=A%20biomarker%20(short%20for%20biological,warning%20systems%20for%20your%20health.
- https://www.phgfoundation.org/blog/biomarkers-and-cancer-precision-medicine#:~:text=Biomarkers%20are%2C%20as%20their%20name,to%20predict%20a%20patient's%20outcome -
- https://www.frontiersin.org/articles/10.3389/fgene.2019.00267/full#:~:text=Precision%20medicine%20is%20a%20rapidly,fuller%20promise%20of%20precision%20medicine.-
- https://www.cancer.gov/about-cancer/treatment/types/biomarker-testing-cancer-treatment#:~:text=Yes%2C%20biomarker%20testing%20is%20an,other%20substances%20in%20your%20body.
- https://www.atsjournals.org/doi/10.1164/rccm.201507-1428ED
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5813864/ -
- https://www.pfizer.com/science/innovation/precision-medicine#:~:text=These%20include%20high%20throughput%20omics,real%20time%20using%20mobile%20sensors).-
- https://www.nature.com/articles/nrc.2016.56 -
- https://www.almacgroup.com/diagnostics/diagnostic-resource/an-introductory-guide-to-biomarkers-precision-personalised-and-stratified-medicine/ -
- https://www.cancer.gov/about-cancer/treatment/types/biomarker-testing-cancer-treatment -
- https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2020.00628/full#h3 -
- https://www.pfizer.com/science/innovation/precision-medicine -
- https://www.phgfoundation.org/blog/biomarkers-and-cancer-precision-medicine -
- https://www.ncbi.nlm.nih.gov/books/NBK573223/ -
- https://www.precisionmedicine.columbia.edu/content/oncology -
- https://lcfamerica.org/about-lung-cancer/diagnosis/biomarkers/#:~:text=Biomarker%20(or%20Genomic%20Diagnostic)%20testing,the%20DNA%20of%20tumor%20cells.
- https://accp1.onlinelibrary.wiley.com/doi/abs/10.1002/jcph.765
- https://www.cdc.gov/genomics/disease/pharma.htm#:~:text=Pharmacogenomics%20looks%20at%20how%20your,you%20or%20has%20no%20effect.-
- https://www.futuremedicine.com/doi/10.2217/epi.09.6#:~:text=Epigenetic%20biomarkers%20measure%20disease%2Dassociated,clinical%20treatment%20and%20drug%20discovery.-
- https://pubmed.ncbi.nlm.nih.gov/34952790/#:~:text=RNA%20biomarkers%20provide%20dynamic%20insights,provide%20more%20information%20than%20DNA.-
- https://newsinhealth.nih.gov/2013/12/personalized-medicine#:~:text=%E2%80%9CIf%20doctors%20know%20your%20genes,variants%20before%20prescribing%20certain%20drugs.-
- https://learn.genetics.utah.edu/content/precision/intro#:~:text=At%20the%20center%20of%20precision,likelihood%20of%20getting%20certain%20diseases.-
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2949280/ -
- https://www.drugtargetreview.com/article/110998/precision-medicine-exploring-the-impact-of-dna-testing/ -
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6164147/ -
- https://www.cancer.gov/about-cancer/understanding/what-is-cancer#:~:text=Cancer%20cells%20can%20break%20away,and%20spread%20into%20surrounding%20tissues.\ -
- https://www.arigobio.com/til
- https://academic.oup.com/database/article/doi/10.1093/database/baad006/7059703?login=false
- https://ascopubs.org/doi/full/10.1200/jop.2016.011486 -
- https://www.statista.com/topics/4345/personalized-medicine/#topicOverview -
- https://www.researchgate.net/figure/Comparison-of-traditional-and-personalized-medicine_fig1_357389115
Acknowledgement
Firstly, I would like to express my deepest gratitude to my teachers for their guidance and support throughout this project as their encouragement and insights have been greatly valuable. I would also like to thank my friends and family for their unwavering encouragement and belief in my abilities. Their endless support has been a constant source of motivation. Next, I would like to give a special thank you to my science fair coordinator for their dedication and hard work in organizing this event. Their efforts have provided me with the opportunity to showcase my passion for science and innovation. Lastly, I am incredibly grateful to the Calgary Youth Science Fair (CYSF) for providing me with the platform to explore my interests and expand my knowledge beyond the classroom. This experience has been truly memorable, and I am thankful for the opportunity to participate in such an amazing extracurricular activity.
Thank you to everyone who has played a role in making this science fair possible. Your support has been great in my journey, and I am deeply appreciative of all that you have done.
THANK YOU!

