Cardiac Event Prediction & Repair
Grade 10
Presentation
Problem
Every year, about 805,000 people in the United States have a heart attack. In the United States, someone has a heart attack every 40 seconds. One person dies every 33 seconds in the United States from cardiovascular disease. About 695,000 people in the United States died from heart disease. That's 1 in every 5 deaths. Heart disease costs the United States about $239.9 billion each year. This includes the cost of healthcare services, medicines, and lost productivity due to death. This statistic displays the significance of Heart Disease. When your heart is damaged during the aftermath of heart attacks, you have an increase in chances of getting heart disease. Once you have Heart Disease, you are never fully cured, and in fact expecting more and more heart issues is reasonable. I came to this conclusion with the help of the Libin Cardiovascular Institute.

With my innovation, there are many problems that are being solved. First, is the issue of delayed or missed diagnosis in asymptomatic patients. Many individuals at risk of heart attacks do not exhibit obvious symptoms until it’s too late. My model can help detect early warning signs, particularly in those who might not seek medical attention otherwise. Secondly, I have a tool for data-driven risk stratification for physicians. Cardiologists often rely on general risk factors, but my model provides a more personalized risk assessment by integrating AI-driven insights. This could assist physicians in prioritizing high-risk patients and tailoring preventive strategies.Third, I can bridge the gap between home monitoring and clinical decision-making. My app allows for patients to communicate their prediction scores or risk indicators to a personalized health consultant (MyHealthConsultant). Additionally, the accesibility of my app and model has evolved into some platform availavle to everyone. Next, with prediction and a personalized chat through application form, we can reduce emergency room overload and unnecessary hospital admissions. Many people visit the ER with chest pain that turns out to be non-cardiac. An accessible AI-powered screening tool could help patients determine whether their symptoms require urgent medical attention, easing strain on emergency healthcare systems. Additionally, I can work on enhancing predictive capabilities beyond the traditional risk factors. Traditional heart attack risk models rely on factors like cholesterol levels and blood pressure. My AI model could integrate non-traditional data sources (e.g., genetic markers, wearable device data, inflammatory biomarkers) to improve prediction accuracy.
Ultimately, the main goal of my model is to solve these problems illustrated above, while enhancing early detection and intervention by leveraging coding, machine learning, and AI algorithms. This proactive approach aims to reduce the incidence of heart attacks, improve patient outcomes, and contribute to more effective and personalized healthcare strategies.
Method
1.0 Methodology
1.1 Abstract
Heart attacks continue to be one of the leading causes of mortality worldwide, occurring without warning and come with devastating consequences. Timely prediction and intervention are critical for improving patient outcomes and reducing the global burden of cardiovascular disease every year and to stop the worlds silent killer. This paper presents a state of the art solution for heart attack prediction, combining deep learning (DL), artificial intelligence (AI) algorithms, and real-time, interactive technologies. The Cardiac Event Prediction App, has many built in features and prediction models. One of which is a Heart Attack Prediction model that utilizes a Long Short Term Memory (LSTM) deep learning model to achieve 95% accuracy in predicting heart attacks. This model analyzes eight key health parameters found in a medical dataset, including age, gender, heart rate, troponin levels, and other vital health parameters to allow for accurate, risk assessments for patients. Another prediction model embedded in the platform is an ECG prediction model, empowering patients to upload their own ECG results into the model that will complete the ECG analysis. These two models combined allow for further enhancing diagnostic accuracy and provide a holistic approach to heart disease management. In addition to one of the prediction models, the project integrates a user-friendly, Streamlit-based application, which allows individuals to access heart attack risk assessments and manage their cardiovascular health. The app features an AI-powered chatbot that guides users through the risk evaluation process, answering questions, analyzing symptoms, and providing tailored recommendations. To ensure transparency and trust, the system includes explainable AI features such as SHAP values and confusion matrices, which help users understand the rationale behind their personalized heart attack risk scores. To enhance the real-world impact of this project, I have included a comprehensive heart health resource page, which offers valuable tips for maintaining cardiovascular health, access to clinical studies, and links to trusted medical sources/ studies. This app represents an innovative approach to predictive healthcare, positioning AI as a transformative tool for early detection and prevention. By combining personalized medicine with powerful deep learning algorithms, my model improves patient care by predicting heart attacks and empowers individuals with the knowledge to make informed decisions about their own heart health. The ultimate vision for this project is to foster collaboration with healthcare institutions and technological innovators to refine and scale the system, advancing the field of AI-driven preventive medicine and leading the way toward a future where early heart attack detection is both reliable and widely available.
1.2 Broad View
Cardiovascular diseases (CVDs), remain one of the leading causes of death globally, accounting for millions of fatalities each year. The sudden nature of heart attacks often results in delayed diagnosis, inadequate treatment, and poor patient outcomes. Despite advancements in medical technology, the early prediction and prevention of heart attacks are still significant challenges for healthcare systems worldwide. Our current method of prediction is reactive not proactive, we wait for heart attacks to strike, and only then do we intervene. It is time to turn to the next chapter in cardiovascular care; that starts with prediction interfaces like mine.
Traditional approaches for heart attack detection rely heavily on clinical symptoms, biomarkers, and imaging techniques such as ECGs, echocardiograms, and coronary angiograms. While these methods are invaluable, they often suffer from limitations in accuracy, timeliness, and cost-effectiveness. Current risk prediction models for heart disease primarily rely on a set of predetermined clinical parameters, including age, blood pressure, cholesterol levels, smoking habits, and family history. These models can identify at-risk individuals, but they often lack the precision required for personalized treatment. Furthermore, most of these models are static, providing a one-time assessment rather than continuous, real-time evaluation of heart health.
Figure 1.2.1. An electrocardiogram (ECG) is the most important tool for diagnosing a heart attack. An ECG allows the measurement of the electrical impulses generated by the heart. Every heartbeat originates at the sinoatrial node (SAN), the hearts natural pacemaker. This impulse travels through major nerve fibers throughout the heart muscle, instructing them to contract and pump blood. An ECG can capture these impulses and is therefore able to plot them onto paper, which a physician can then utilize to determine the functionality of the heart.
Recent developments in AI and deep learning (DL) have shown promise in overcoming these limitations, offering the potential for more accurate, real-time heart attack prediction. For example, LSTM (Long Short-Term Memory) models, a type of recurrent neural network (RNN), are particularly well-suited for time-series prediction tasks, such as heart disease risk assessment, because they can account for sequential data and past health events. The ability of AI systems to process vast amounts of patient data from multiple sources, including medical histories, diagnostic tests, and lifestyle factors, allows for more nuanced and dynamic risk assessments.
In addition to prediction, AI is also playing a role in the repair of heart tissue after a cardiac event. Advances in synthetic biology, stem cell therapy, and regenerative medicine have opened new frontiers for the treatment of cardiomyocyte damage following a heart attack. These technologies aim to repair or replace damaged heart tissue, potentially restoring heart function and improving patient prognosis. However, challenges remain in translating these technologies from the laboratory to the clinic, including issues related to safety, scalability, and long-term effectiveness.
The convergence of AI-driven predictive models with cutting-edge therapeutic strategies holds the potential to transform the management of cardiovascular diseases. By enabling early detection and personalized treatment plans, AI can help mitigate the risks of heart attacks and improve outcomes for patients. Furthermore, combining predictive models with repair technologies can lead to a new era of precision medicine, where treatment is tailored not only to an individual’s genetic profile but also to their real-time health data.
To further enhance the accuracy and reliability of heart attack prediction, my project integrates a deep learning-based heart attack prediction model with an interactive AI-powered platform, accessible to both healthcare professionals and the general public. By providing real-time, actionable insights into heart health, this approach aims to empower individuals to take proactive steps toward prevention and better management of cardiovascular health.
1.3 Biological Background
The human heart is a highly complex and vital organ, responsible for pumping blood throughout the body, delivering oxygen and nutrients to vital organs. Composed of specialized muscle cells known as cardiomyocytes, the heart functions through rhythmic contractions. The myocardium, the central muscular layer, plays a central role in the heart's contractile activity, while other layers, such as the epicardium and endocardium, provide structural integrity and facilitate proper heart function. Cardiomyocytes, in addition to their contractile capabilities, conduct electrical signals that trigger and synchronize the heart's contractions. These electrical signals are essential for proper heart function, and any disruption, such as arrhythmias or myocardial infarctions, can lead to severe consequences, including heart failure.
At the cellular level, cardiomyocytes are electrically excitable cells, similar to neurons, and generate action potentials that propagate across the myocardium. These action potentials are driven by a delicate balance of ions—sodium (Na+), potassium (K+), and calcium (Ca2+)—which move through specialized ion channels in the cell membrane. The movement of these ions creates the electrical potential necessary for muscle contraction. The electrical conduction system of the heart, comprising the sinoatrial (SA) node, atrioventricular (AV) node, bundle of His, and Purkinje fibers, ensures the precise timing and synchronization of electrical signals. Disruptions in this system can lead to conditions such as arrhythmias, which can increase the risk of myocardial infarction (heart attack).
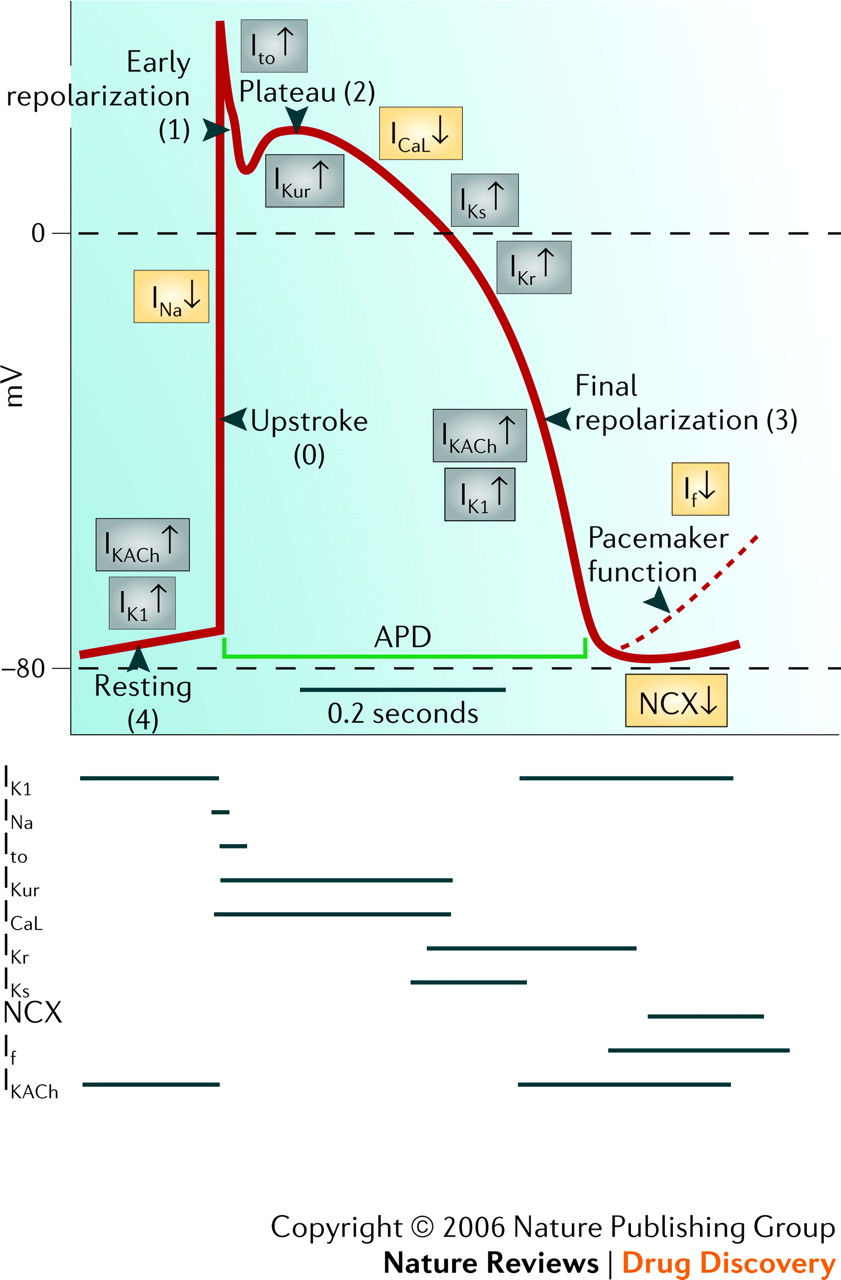
Figure 1.3.1. Phases of the cardiac action potential in cardiomyocytes, illustrating the sequential changes in membrane potential.
A heart attack, or myocardial infarction, occurs when blood flow to a part of the heart is blocked, often due to the buildup of plaque or the formation of a clot within the coronary arteries. This blockage leads to ischemia, depriving the heart tissue of oxygen and nutrients, causing damage or death of cardiomyocytes. The result is compromised heart function. Early detection of heart attacks is critical, as quicker restoration of blood flow can reduce the extent of irreversible damage. Traditional methods for detecting heart attacks include blood tests to measure biomarkers like troponin, which are released when heart muscle cells are injured, as well as imaging techniques such as echocardiograms and coronary angiograms. However, these methods often lack the ability to provide real-time, continuous monitoring, which limits early detection and prevention.
Electrocardiograms (ECGs) are a vital tool in the detection of myocardial infarctions. They record the electrical activity of the heart and can detect abnormalities that indicate an increased risk of heart attack or other cardiovascular events. During a heart attack, changes in the electrical properties of the affected heart tissue, such as ST-segment elevation or depression, are commonly observed and can be detected through ECG. These deviations from normal cardiac function may precede a heart attack, providing a crucial window for intervention.
The integration of artificial intelligence (AI) and machine learning (ML) algorithms into cardiovascular diagnostics is advancing our ability to predict heart attacks with greater accuracy. By incorporating data from various sources—such as ECG results, biomarkers, and demographic information—AI-powered models can provide real-time predictions and actionable insights into heart health. These models can analyze complex patterns and relationships within the data that might be difficult for humans to detect, allowing for more personalized and proactive approaches to heart disease prevention and management.
In addition to predicting heart attacks, the project also explores the regenerative potential of stem cell therapies and tissue engineering to repair heart tissue damaged by myocardial infarctions. The heart has a limited capacity to regenerate itself after damage, making it a major challenge for post-heart attack recovery. Stem cells, such as induced pluripotent stem cells (iPSCs) and mesenchymal stem cells, have shown promise in regenerating damaged cardiomyocytes and improving heart function. However, ensuring that these stem cells properly integrate into the existing heart tissue and function long-term remains a significant challenge. By combining predictive AI models with regenerative therapies, this project aims to revolutionize the treatment of heart disease, offering both early detection and personalized repair strategies.
The broader biological context of cardiovascular disease involves understanding not only the electrical properties of cardiomyocytes but also the interplay between the heart’s various cell types—cardiomyocytes, endothelial cells, and fibroblasts—and the autonomic nervous system, which regulates heart rate and contractility. The heart’s electrical signals are influenced by these interactions, and understanding them is essential for interpreting ECG signals and predicting cardiac events. Additionally, factors such as age, blood pressure, cholesterol levels, and lifestyle choices, including smoking and physical activity, contribute to the risk of heart disease. AI and deep learning models, such as Long Short-Term Memory (LSTM) networks, have shown promise in capturing the temporal dynamics of these risk factors, enabling more accurate and personalized predictions.
Ultimately, this project focuses on leveraging AI and deep learning to enhance the prediction of heart attacks. By analyzing patient data—including ECG results, biomarkers, and demographic information—the project aims to develop robust AI-powered platforms that offer real-time predictions and actionable insights. These platforms could help detect heart attacks at earlier stages, preventing severe damage, while also offering a foundation for regenerative treatments to repair heart tissue and improve patient outcomes. By advancing both predictive and regenerative technologies, we seek to not only improve the early detection of heart attacks but also offer potential solutions for the regeneration of heart tissue, ultimately leading to better outcomes for heart disease patients.
1.4. The Physics of Artificial Intelligence, Machine Learning, Deep Learning, and Neural Networks in Cardiac Event Prediction
Artificial Intelligence (AI), Machine Learning (ML), and Deep Learning (DL) serve as the foundational computational principles behind the architecture and functioning of this project’s cardiac event prediction system. Much like the physical laws governing the deformation of crystal lattices in piezoelectric materials, the learning and decision-making capabilities of neural networks are governed by computational mechanics rooted in statistics, probability theory, and optimization algorithms. These models are capable of identifying complex, non-linear patterns within multivariate datasets, making them highly suitable for tasks involving biomedical signal analysis and cardiovascular risk prediction.
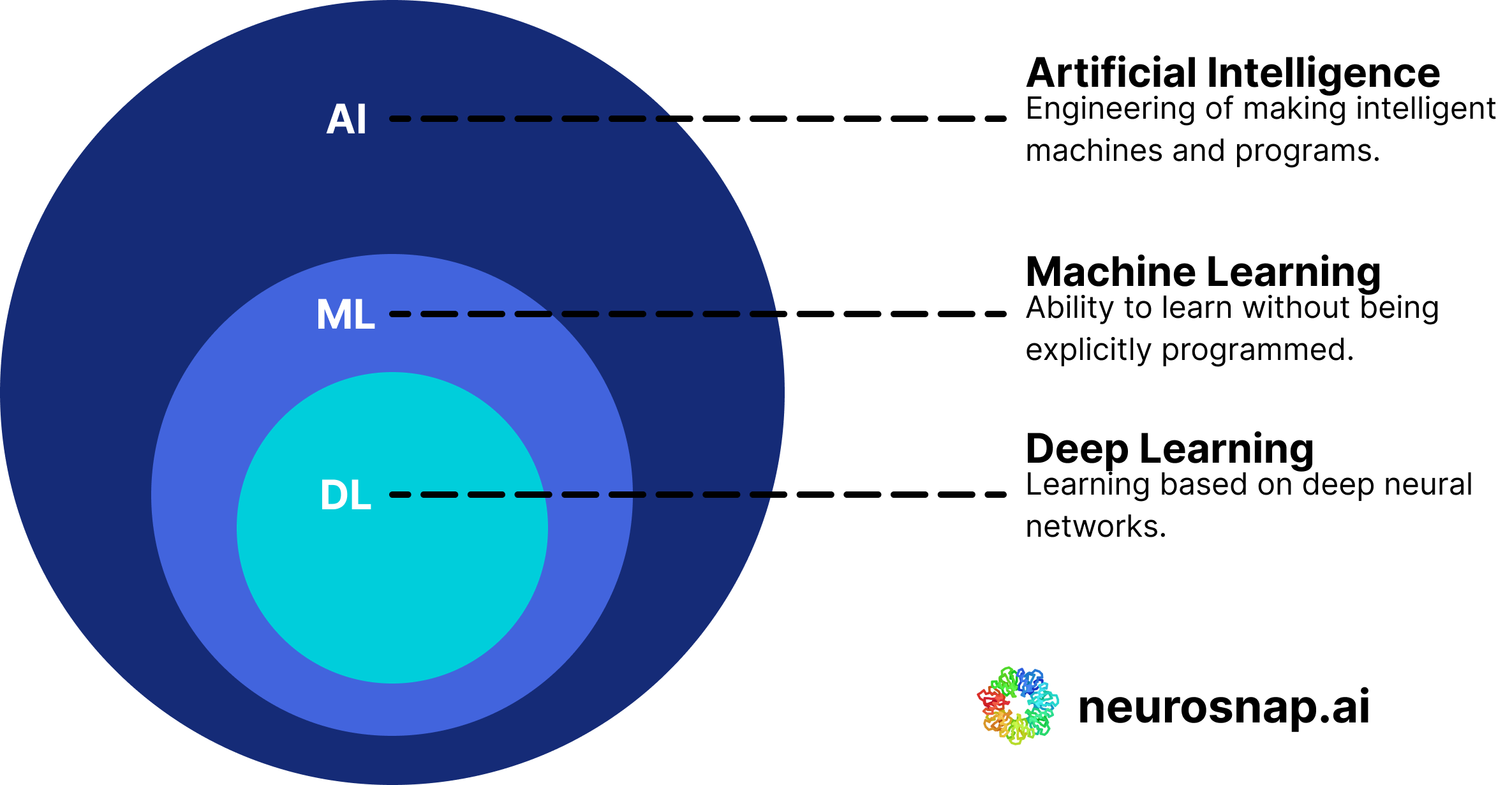
Figure 1.4.1. Important sub-classes of artificial intelligence
Machine Learning refers to a set of algorithms that autonomously learn relationships between input features (such as age, cholesterol, blood pressure, ECG values, etc.) and a target outcome (e.g., cardiac event presence or absence), without explicit rule-based programming. These algorithms utilize supervised learning, where a labeled dataset is provided for training. Techniques such as Random Forests and Gradient Boosting are ensemble learning approaches that leverage decision tree structures. Each tree learns different data patterns, and the aggregated result of multiple trees provides a robust prediction mechanism.

Figure 1.4.2. Random Forest Machine learning algorithm capacity
Transitioning from ML to Deep Learning, we move into the realm of artificial neural networks—computational frameworks inspired by the structure of biological neurons in the human brain. Deep Learning involves multiple layers of neurons (known as fully connected layers) that transform data hierarchically, enabling the system to learn intricate, high-level abstractions. Every connection between artificial neurons has a weight, adjusted during training using backpropagation and gradient descent optimization, mimicking a self-correcting feedback system analogous to biological learning.
One of the most crucial implementations in this project is the Long Short-Term Memory (LSTM) network—an advanced form of Recurrent Neural Network (RNN) specifically designed to capture temporal dependencies in sequential datasets. In the context of cardiac event prediction, LSTM networks allow the model to learn how the progression and interplay of clinical features evolve over time. These architectures are particularly effective at retaining long-term contextual information through their memory cell state, gated by input, forget, and output gates, which regulate the flow of information across time steps. This capability makes LSTMs ideal for modeling clinical time-series data, where minute fluctuations in patient parameters may be early indicators of impending cardiac distress.
On a parallel implementation track, this project integrates Convolutional Neural Networks (CNNs)—a deep learning architecture traditionally used in image processing—for ECG signal classification and interpretation. While ECG data is fundamentally a time-series signal, CNNs applied with 1D convolutions are capable of capturing local temporal patterns, such as QRS complexes, ST-segment deviations, and T-wave anomalies. By sliding kernels (filters) over the ECG signal, the network extracts spatially localized features, amplifying those with diagnostic relevance and suppressing irrelevant noise. These features are then passed through pooling layers, ReLU activations, and fully connected layers to form an end-to-end diagnostic pipeline.
Technically, the project implements a hybridized deep learning system, combining LSTMs and CNNs in parallel and/or sequential layers depending on the nature of input data—clinical vs. waveform. This allows for multi-modal learning, where structured tabular clinical data (demographics, vitals, lab values) is analyzed via LSTM-based networks, while raw ECG waveform data is processed through CNN pipelines. These two models form the dual pillars of the project: one predicting heart attack risk using tabular clinical data and the other predicting cardiovascular abnormality from ECG waveforms.
LSTM Functionality
LSTM's involves a memory cell which is controlled by three main gates or phases: the input gate, the forget gate and the output gate. These 3 vital stages decide what specific pieces of information to add, remove from and output from the memory cell.
- Input gate: Controls what information is added to the memory cell.
- Forget gate: Determines what information is removed from the memory cell.
- Output gate: Controls what information is output from the memory cell.
This allows LSTM networks to selectively retain or discard information as it flows through the network which allows them to learn long-term dependencies. The network has a hidden state which is like its short-term memory. This memory is updated using the current input, the previous hidden state and the current state of the memory cell.
LSTM architecture has a chain structure that contains four neural networks and different memory blocks called cells.
The models are engineered to extract features through deep layers, followed by dense layers that integrate and distill these features before generating output probabilities. Additionally, Layer Normalization is used to stabilize learning, especially in deep LSTM models, by normalizing intermediate outputs. Furthermore, learning rate decay mechanismsare applied to optimize convergence and prevent overshooting minima during training. The loss functions, particularly BCEWithLogitsLoss, are chosen to ensure numerical stability and better separation between positive and negative class predictions.
The technical pipeline is orchestrated through a unified app interface that modularly integrates:
- The LSTM-based heart attack prediction model.
- The CNN-based ECG classifier.
- Preprocessing pipelines for scaling, normalization, and encoding of input features.
- Model explainability tools (such as SHAP values and feature importance).
- Real-time user input prediction systems.
- A Chatbot for optimal patient interaction.
These layers of implementation not only replicate biological intelligence in digital form but also synthesize a new layer of translational capability, transforming raw clinical data into actionable diagnostics. Just as the minute lattice deformation in a piezoelectric material produces a macroscopic voltage signal, the learned micro-patterns within this AI system produce a macro-level understanding of cardiovascular health, enabling early and personalized detection of cardiac events.
1.5 Knowledge Gap and Purpose
The knowledge gap addressed in this project lies at the intersection of artificial intelligence, biomedical diagnostics, and cardiovascular medicine. While significant progress has been made in predictive modeling for heart disease using statistical methods and traditional machine learning algorithms, there remains a crucial gap in the optimal integration of deep learning architectures—such as Long Short-Term Memory (LSTM) networks and Convolutional Neural Networks (CNNs)—to model time-dependent physiological signals and enhance clinical decision-making accuracy. The project seeks to explore whether advanced neural architectures can be strategically utilized not only for enhanced prediction of cardiac events but also for real-time detection and classification of cardiac patterns, such as electrocardiogram (ECG) waveform deviations that precede myocardial infarctions.
The purpose of this project is twofold: first, to develop an interpretable, high-performance deep learning framework for proactive heart attack prediction, and second, to construct a CNN-based ECG classifier capable of recognizing signal anomalies in a clinically meaningful way. This addresses a current void in scalable, AI-driven healthcare systems that can bridge the gap between early symptom recognition and critical intervention—especially in resource-limited environments where access to skilled diagnostics is restricted.
Through the use of sequential modeling via LSTMs, which are designed to capture temporal dependencies in longitudinal health records, and spatial feature extraction through CNNs applied to ECG waveforms, this project provides a foundational methodology for developing patient-specific, adaptive, and real-time cardiac monitoring tools. Unlike previous static diagnostic tools, this system is inherently dynamic—learning from evolving data and continuously improving its predictive capability.
The broader implications of this work extend far beyond heart attack prediction. If proven robust and scalable, this methodological framework can be adapted to detect a wide range of cardiac anomalies, support preventative cardiology, and integrate into wearable devices and telemedicine systems. Additionally, the underlying architectures can be transferred to other domains of medical diagnostics, including arrhythmia classification, stroke prediction, and chronic disease monitoring. In this way, the project not only closes a critical knowledge gap in cardiovascular diagnostics but also lays the groundwork for a new generation of intelligent, autonomous, and explainable medical AI systems.
2.0 Development
With a foundational understanding of the cardiovascular system, electrocardiographic signals, and the principles of deep learning, we can now begin the design, development, and testing of the cardiac event prediction and repair system. This process encompasses, but is not limited to, the construction and refinement of deep learning architectures (including LSTM and CNN layers), the preprocessing and transformation of clinical and physiological datasets, the integration of an interactive prediction and ECG classification system, and the development of a user-accessible software interface—complete with a real-time risk calculator, educational tools, and AI-driven chatbot support.
This section outlines all critical components of the system: data acquisition and preprocessing pipelines, architectural considerations for model design, implementation of interpretability frameworks (e.g., feature importance, SHAP values), and the training, evaluation, and performance optimization of each model component. Additionally, it presents the structure of the end-user interface through which the models are deployed, such as the Streamlit application, as well as strategies for testing the effectiveness, accuracy, and clinical relevance of the system.
2.1 Research Questions
The central aim of this research, and the foundational question that drove its inception, was: Can a high-fidelity, multimodal deep learning system be developed to accurately predict the onset of myocardial infarction and classify ECG waveform anomalies in a clinically interpretable, patient-accessible, and minimally invasive manner, using temporal and morphological patterns in physiological and electrocardiographic data? Upon deeper analysis, this broad question was subdivided into more specific inquiries that guided the development process. These include: How can sequential data be modeled effectively to predict heart attacks? What architectures are most suited for ECG waveform classification? What performance thresholds are necessary for clinical reliability? How can such a system be made accessible and interpretable for both medical professionals and the general population?
Through an extensive review of current literature, clinical data repositories, and advanced machine learning techniques, the focus of the research evolved. Initially oriented toward the feasibility of predictive AI in cardiology, the project shifted toward a more applied objective: to build, train, and evaluate an integrated dual-system architecture—combining LSTM-based risk prediction with CNN-based ECG classification. The key methodological question then becomes: Can such a system, if constructed with rigorous architectural principles and trained on high-quality physiological data, achieve performance metrics sufficient for real-world deployment—and if so, how should it be designed, tested, and optimized?
These critical questions shed light onto solving the key problems that my innovation of Cardiac event prediction solves. The most important consideration point for this project is that innocent lives are lost every year to heart attacks, or cardiovascular diseases. Heart attack prediction remains a critical challenge as many at-risk individuals go undiagnosed until it’s too late. Limited access to timely cardiovascular assessments, especially in remote areas, increases preventable heart attack cases. Traditional risk models often fail to integrate real-time data and personalized factors, reducing their predictive accuracy. Additionally, emergency rooms are overwhelmed with non-cardiac cases, highlighting the need for an accessible, AI-driven tool to improve early detection and resource allocation.
While my innovation works to mitigate the damage caused by late detection and diagnosis, the Cardiac Event Prediction innovation works to reduce emergency room overload and unnecessary hospital admissions by prioritizing patients at the high risk benchmark through the risk prediction model. Additionally, we can enhance predictive capabilites beyond the traditional risk facts, bridge the gap between home monitoring and clincial decision making, while finally allowing for a stronger data driven risk stratification for physicians or in clinical settings.
2.2 Initial Design
In the domain of cardiac diagnostics and intelligent disease prediction, the pursuit of higher diagnostic precision, earlier intervention, and improved accessibility prompts the exploration of integrated, multimodal deep learning systems. The primary goal is to develop an end-to-end, patient-accessible platform that simultaneously enables high-fidelity prediction of myocardial infarction risk and classification of electrocardiographic waveform anomalies. This system seeks to overcome the limitations of traditional diagnostics that rely on late-stage clinical symptoms, manual ECG interpretation, or delayed biomarker analysis.
To achieve this, the project proposes a bifurcated architecture: a tabular data-based predictive module powered by temporal deep learning networks, and a parallel signal processing module for automated ECG waveform analysis. The predictive module leverages temporal dynamics within physiological and biochemical features—such as blood pressure, cholesterol levels, glucose, and heart rate variability—while the signal classification module extracts morphological patterns from raw ECG traces using advanced convolutional neural networks (CNNs). Together, these dual modules are designed to enhance early-stage detection, reduce diagnostic latency, and support continuous monitoring in both clinical and remote environments.
By the convergence of prediction models, with important safety information for heart health, the platform was born; a Cardiac event prediction app allowing patients to navigate with ease and satisfaction while having access to tools like Heart Attack Prediciton, ECG Analysis, and a Chatbot which all use advanced techniques to optimize the scale, magnitiude and impact of a platform of this level.
The overarching goal is to ensure the system is maximally accurate, interpretable, safe for use, and scalable across diverse patient populations. The proposal of LSTM networks for temporal risk prediction and 1D CNNs for morphological ECG classification is one well supported by current biomedical machine learning literature.
Standalone models for heart attack prediction often underperform in scenarios involving dynamic health parameters or patient variability. Similarly, existing ECG classification systems lack either precision or clinical interpretability. Therefore, a dual-module deep learning architecture is proposed, in which both modules complement each other through a shared interface. This is coupled with an interactive frontend interface deployed via a Streamlit web application, allowing both clinicians and patients to interact with the system in real-time. Each module must meet strict performance thresholds—particularly in terms of sensitivity, specificity, F1-score, and ROC-AUC—to be considered viable for preclinical adoption.
To facilitate maximum diagnostic transparency, the inclusion of model explainability techniques such as SHAP value analysis, feature attribution maps, and attention heatmaps is necessary. These elements serve as a countermeasure to the "black box" problem often associated with deep learning in medicine and ensure that clinical decisions can be audited and understood. The frontend application is further augmented by auxiliary modules, including a symptom checker, real-time global heart disease tracker, self-assessment risk calculator, and interactive heart health resource hub, all designed to enhance patient engagement, knowledge dissemination, and preventive behavior.
The physical limitations of healthcare infrastructure in many low-resource settings demand that the system be computationally lightweight, modular, and deployable on edge devices such as mobile phones or local servers without GPU dependency. For this reason, the LSTM and CNN models are optimized for inference speed and memory efficiency, while the user interface remains responsive and data-light. The eventual goal is a fully integrated system that operates seamlessly in both cloud-hosted and offline configurations, ensuring equity of access to advanced cardiac diagnostics.
This dual-system design, while technically ambitious, represents one of the only viable paths toward merging precision diagnostics with preventive medicine. It balances the computational complexity of deep learning with the practical needs of clinical workflows, public health infrastructure, and patient usability. Ultimately, the proposed system is intended to not only predict and detect cardiac events but also catalyze a broader shift toward intelligent, preventative, and accessible cardiovascular care.
2.3 Specific Design
With the conceptual framework established, the system architecture advances into its material layer — not in the traditional sense of physical constructs or bioengineered scaffolds, but in the realm of computational matter: data matrices, neural weights, training protocols, and biologically anchored features that converge into a digital surrogate of cardiovascular behavior. Here, the materials are not silicon or polymer — they are structured arrays of clinical markers, encoded temporal signals, and algorithms sculpted to mimic the pattern recognition capacity of a trained cardiologist.
At the core of the Heart Attack Prediciton Model lies the Mendeley Heart Attack Dataset, a composite of key physiological, biochemical, and demographic indicators, each serving as a proxy for specific biological processes within the cardiovascular apparatus. Each parameter in this dataset is a reflection of deeper cardiovascular pathophysiology, intimately connected to the electrophysiological rhythm, vascular integrity, and cardiomyocyte metabolic stress — all of which are precursors to cardiac ischemia, arrhythmogenesis, and infarction.
Biological Underpinnings of Key Parameters
-
Age
Age is more than a temporal variable — it is a quantifiable index of endothelial senescence, arterial stiffness, and vascular calcification. With advancing age, elastin-to-collagen ratio declines, impairing aortic compliance and elevating afterload. Simultaneously, telomeric erosion in endothelial cells compromises angiogenic response, while oxidative stress and chronic low-grade inflammation (inflammaging) exacerbate atherosclerotic plaque formation. Age is thus a surrogate for the cumulative vascular injury that directly correlates with myocardial oxygen supply-demand mismatch. -
Gender
Gender captures underlying sex-linked hormonal regulation of cardiovascular physiology. Estrogen in females exerts cardioprotective effects via nitric oxide-mediated vasodilation, HDL elevation, and inhibition of vascular smooth muscle proliferation. Post-menopausal estrogen decline contributes to a surge in female cardiovascular risk. In males, testosterone-associated pro-thrombotic and hypertrophic signaling pathways accelerate endothelial dysfunction. Gender, therefore, indirectly encodes the systemic hormonal architecture influencing myocardial susceptibility. -
Heart Rate
The resting heart rate is a dynamic index of autonomic nervous system tone. Elevated heart rate signifies sympathetic overactivation, which is pro-arrhythmic and increases myocardial oxygen consumption while reducing diastolic perfusion time—especially critical for subendocardial regions. High resting heart rate also correlates with reduced baroreceptor sensitivity, lower parasympathetic modulation, and increased risk of ventricular remodeling and fibrosis. At the cellular level, calcium handling in myocytes becomes dysregulated, impairing contractile function and electrical conduction. -
Systolic and Diastolic Blood Pressure
These parameters are macro-indicators of vascular resistance and cardiac load, respectively. Systolic pressure reflects left ventricular ejection force against systemic vascular resistance. Elevated systolic pressure induces left ventricular hypertrophy (LVH), increasing myocardial oxygen demand while reducing coronary reserve. Diastolic pressure maintains coronary artery perfusion, particularly in diastole; excessively low diastolic pressure in hypertensive patients paradoxically increases ischemic risk. On the cellular scale, mechanosensitive ion channels in myocytes are activated by chronic pressure overload, leading to maladaptive gene expression and contractile protein remodeling. -
Blood Sugar
Glucose is not just a metabolic parameter; chronic hyperglycemia initiates a cascade of glycation end-products, oxidative endothelial injury, and vascular basement membrane thickening. Elevated glucose leads to dyslipidemia, macrophage recruitment, and foam cell formation, all central to atherogenesis. It also impairs nitric oxide bioavailability, promoting vasoconstriction and microvascular rarefaction. From a myocellular perspective, glucose toxicity disrupts mitochondrial ATP generation, increases ROS formation, and can trigger apoptotic cascades in cardiomyocytes, especially in diabetic cardiomyopathy. -
Cholesterol(Serum Cholesterol Levels)
Total cholesterol levels serve as an index of lipid-mediated endothelial stress. LDL cholesterol, when oxidized, binds to scavenger receptors on macrophages, leading to foam cell deposition, necrotic core expansion, and fibrous cap instability—hallmarks of vulnerable plaques. Cholesterol also contributes to coronary microembolization, impairing capillary perfusion at the tissue level. Myocardial ischemia often ensues not from total occlusion, but from microvascular dysfunction secondary to lipid imbalance. -
CK-MB and Troponin Levels
These are not just lab values—they are direct molecular signatures of myocardial cell death. Troponin-I and -T are structural proteins within the actin-myosin complex of cardiomyocytes, released during sarcolemmal rupture. Their serum concentration correlates with extent of myocardial necrosis, offering a biochemical fingerprint of infarct severity. CK-MB, although less specific, serves as a complementary marker of acute muscle damage, with a shorter half-life for dynamic tracking. These biomarkers directly connect computational prediction with cellular-level myocardial breakdown.
Heart Attack Prediction LSTM Model
These physiologically rich features are routed into a multi-stage deep learning pipeline, centered around a Long Short-Term Memory (LSTM) model, designed to replicate the temporal dynamics inherent in cardiovascular pathology. LSTM’s gate-based architecture allows retention and selective forgetting of key patterns across patient data timelines — an analog to the biological latency and progression patterns seen in cardiac pathology, where early subclinical changes eventually culminate in overt infarction.
The model is further deepened with fully connected layers, allowing higher-order abstraction and non-linear pattern recognition, while Layer Normalization and Dropout Regularization are applied to stabilize gradient flow and prevent overfitting. Iterative training is conducted via GPU acceleration in Google Colab, with modular architecture engineered in Visual Studio Code to allow flexible experimentation and scalability.
ECG-Based CNN Model
Parallel to LSTM-based risk prediction, the CNN-driven ECG model operates on time-domain electrophysiological data, transforming 1D ECG waveforms into multiscale feature maps. CNN layers mimic spatial attention mechanisms—akin to how an electrophysiologist scans lead signals—detecting variations in amplitude, interval, and morphology. This model specializes in distinguishing ventricular ectopy, ischemia-induced repolarization defects, and conduction delays, leveraging the precision of AI to emulate human diagnostic acuity.
Both models are ultimately integrated into a Streamlit deployment layer, offering an intuitive, web-based interface that houses model outputs, biological insights, risk calculators, and educational resources — thus transforming a high-dimensional AI model into a clinical decision support system (CDSS) accessible to both physicians and patients.
3.0 Testing and Training
3.1 Data Splitting and Preprocessing
Data splitting is an essential procedure to evaluate a model’s performance and generalizability. In this study, the dataset was split into training and testing sets, commonly using an 80/20 split. This means that 80% of the data was used for training the model, while the remaining 20% was set aside to test its performance. By reserving a portion of the data for testing, this split ensures that the model is evaluated on unseen data, which helps simulate real-world scenarios where the model is deployed to make predictions on new data.
Furthermore, to mitigate potential biases introduced by the specific division of training and testing data, k-fold cross-validation was used during model tuning. This method divides the dataset into k subsets or folds (typically 5 or 10). The model is trained on k-1 of these folds and tested on the remaining fold, and this process is repeated for each fold. Cross-validation provides a more robust measure of model performance as it ensures that the model is tested on multiple portions of the data, minimizing the effect of any single partition.
Data preprocessing also played a key role, especially in handling missing values, normalization, and feature engineering. Normalizing continuous features, such as patient age or blood sugar levels, helps the model learn more effectively by ensuring that no feature disproportionately dominates the learning process due to differences in scale. For sequential models like LSTM (Long Short-Term Memory), proper formatting of time-series data and ensuring consistent temporal steps is essential to maintain the integrity of the sequence.
3.2 Model Training Process
The training process in deep learning involves the iterative optimization of the model's parameters (weights and biases). During each training cycle, the model adjusts its weights based on the computed loss or error, which quantifies how far the model's predictions are from the actual outcomes.
Training in deep learning is controlled by several key parameters:
-
Epochs:
An epoch refers to one complete cycle through the entire training dataset. In each epoch, the model makes predictions, calculates the error (using a loss function), and adjusts its weights accordingly using backpropagation. Multiple epochs are required because the model learns gradually, refining its predictions with each cycle. The training process involves numerous epochs, with a typical range from 50 to 100 epochs, depending on model complexity and data size.Early stopping is often employed to avoid overfitting. If the model’s performance on a validation set doesn’t improve for a predetermined number of epochs, training is stopped to prevent the model from learning noise rather than generalizable patterns.
-
Learning Rate:
The learning rate controls how large the adjustments to the model’s weights are after each epoch. If the learning rate is too high, the model may overshoot the optimal set of weights, while if it’s too low, the model may converge too slowly or get stuck in local minima. Therefore, careful tuning of the learning rate is critical, and learning rate decay is sometimes applied, which reduces the learning rate over time to allow for finer adjustments during the later stages of training. -
Batch Size:
The batch size defines the number of training examples used in one forward/backward pass through the model. A mini-batch gradient descent approach is commonly used, where instead of calculating gradients for the entire dataset at once (which could be computationally expensive), the model is updated after processing a small batch of data. This provides faster convergence while maintaining stability in the learning process.
3.3 Evaluation Metrics
Once the model is trained, its performance is evaluated using a variety of metrics that provide insights into its ability to predict outcomes accurately and efficiently. The most important metrics used in this study are:
1. Accuracy, 2. Precision, 3. Recall, 4. F1 Score, 5. Support
Accuracy:
Accuracy is the simplest and most intuitive metric. It calculates the proportion of correct predictions (both true positives and true negatives) relative to the total number of predictions made.
While accuracy is helpful, it can be misleading in the presence of class imbalance, where the model might predict the majority class very well while failing to predict the minority class (such as heart attack cases). In these scenarios, other metrics like precision and recall provide more valuable insights.
Accuracy is calculated as:
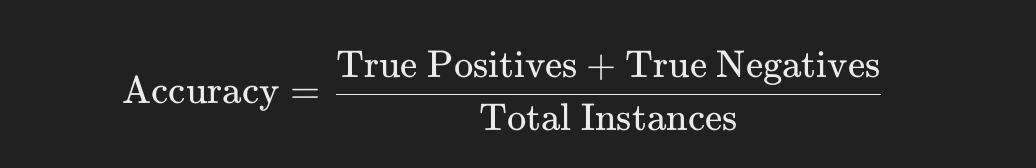
Precision:
Precision evaluates the accuracy of positive predictions, meaning how many of the instances predicted as positive are actually positive.
Precision is particularly important when the cost of false positives is high. For heart attack prediction, for instance, a false positive could lead to unnecessary tests or treatments, which could be costly and cause patient anxiety.
- Suppose every time you pick something thinking it's an apple, you want to be sure it really is an apple. Precision measures the percentage of your picks that are actually apples. For example, if you pick 100 items thinking they're apples and 90 of them are indeed apples, your precision is 90%. This is different from model accuracy, which measures the overall correctness of the model across all predictions.
Precision is calculated as:

Recall (Sensitivity or True Positive Rate):
Recall is a measure used in statistics and machine learning to evaluate how good a model is at identifying true positives.
In medical applications, high recall is critical because failing to identify a true positive (i.e., failing to diagnose a heart attack) could have serious consequences. Recall ensures that the model identifies as many true positive instances as possible, even if that means tolerating some false positives.
- Imagine you have a basket of fruits, and you're trying to pick out all the apples. Recall, in this context, is the percentage of actual apples you successfully pick out of all the apples that are in the basket. If you have 100 apples in the basket and you correctly identify 90 of them as apples, your recall is 90%. This means recall is all about how well you can capture or recall all the relevant items (in this case, apples) without missing any. It's especially important in situations where missing an item (like failing to detect a disease in medical testing) can have serious consequences.
Recall is calculated as:

F1-Score:
The F1 score is a metric that combines both precision and recall to provide a single measure of a model's accuracy, especially in situations where the balance between precision and recall is important.
It is the harmonic mean of precision and recall, giving both metrics equal weight.
The F1 score reaches its best value at 1 (perfect precision and recall) and worst at 0.
The F1 score tells you how efficiently your model can identify the relevant data points without mixing them with irrelevant ones
The F1-score is calculated as:

Support:
Support, in the context of machine learning and statistics, refers to the number of actual occurrences of a class in a given dataset.
For example, if you're classifying emails into 'spam' and 'not spam', the support for the 'spam' class would be the total number of spam emails in your dataset. Support helps you understand the size of the different classes that your model is working with.
3.4 Deep Learning Model Training
For both Heart Attack Prediction (LSTM) and ECG Prediction (CNN) models, training involves the following core concepts:
-
LSTM (Heart Attack Prediction):
LSTM networks are a specialized type of recurrent neural network (RNN) that are designed to model sequential data. The training process of an LSTM involves feeding the model sequences (e.g., patient history or ECG waveforms) and adjusting the network's weights through backpropagation. The temporal dependencies in the data are learned through multiple layers of memory cells that retain information over long sequences, which is particularly useful for time-series predictions like heart attack risk.
-
CNN (ECG Prediction):
CNNs are more suited for spatial data, like images, but they have been successfully adapted for 1D signal processing in ECG analysis. During training, CNN layers apply filters (kernels) to scan for local features in the ECG signal, detecting characteristics like peaks, irregularities, or patterns indicative of heart abnormalities. The learning process of CNNs involves optimizing the filters to detect the most relevant features.
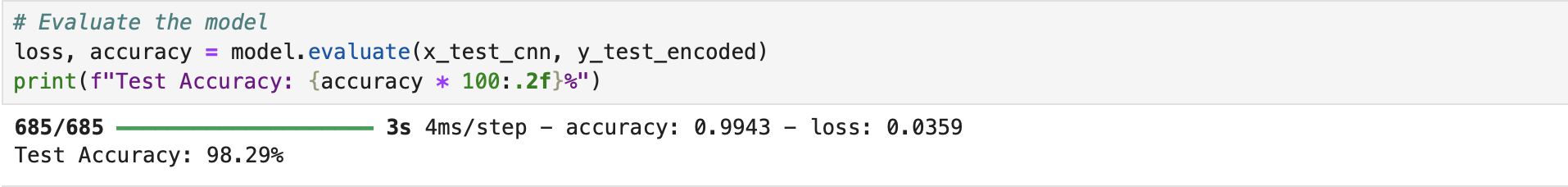
In summary, the methodology of this project meticulously outlines the steps taken to build, train, and evaluate the deep learning models for heart attack prediction and ECG analysis. Through careful data preprocessing, optimized model architectures (LSTM for heart attack prediction and CNN for ECG analysis), and rigorous training protocols, the project ensures that the models are both accurate and reliable. The use of key evaluation metrics like accuracy, precision, recall, and F1-score demonstrates the models' effectiveness, particularly in handling class imbalances, which is crucial in real-world medical applications. This thorough and structured approach sets the foundation for future advancements in cardiovascular health diagnostics, showcasing how artificial intelligence can be applied to enhance early detection and improve healthcare outcomes.
4.0 Cardiac Event Repair
The repair phase of cardiac events focuses on restoring functionality to the heart after damage caused by conditions such as myocardial infarction (heart attack). This stage encompasses cutting-edge strategies such as stem cell therapy, regenerative medicine, and synthetic biology to restore tissue, promote healing, and ultimately improve patient outcomes. Following a cardiac event, the heart experiences irreversible damage to its cells and tissues, resulting in scar formation and reduced pumping ability. In light of these challenges, modern therapeutic approaches are being explored to repair, regenerate, and even replace the damaged tissue. Stem cell therapies and regenerative approaches offer the potential for generating new cardiomyocytes and supporting tissues that can reintegrate with the existing heart architecture, potentially restoring heart function. The integration of these techniques is key to revolutionizing cardiac treatment and overcoming the limitations of current therapies, such as heart transplants and conventional medications.
4.1 Detailed Introduction to Stem Cells in Cardiac Event Repair
Stem cell-based therapies represent one of the most promising avenues for repairing cardiac damage following a heart attack. Myocardial infarction (heart attack) causes substantial damage to the heart muscle, leading to the formation of fibrotic scar tissue that impairs the heart’s ability to contract efficiently. This damage is traditionally irreversible, as the adult heart has limited regenerative capacity. Once cardiomyocytes (heart muscle cells) are lost, they are not replaced, leading to chronic heart failure and a significant decline in the patient’s quality of life. Stem cell therapies offer a potential solution by promoting the regeneration of heart tissue, restoring cardiac function, and ultimately reducing the long-term burden of cardiovascular disease.
Types of Stem Cells for Cardiac Repair
Several types of stem cells have been considered for cardiac repair, each with its potential benefits and challenges.
-
Embryonic Stem Cells (ESCs): ESCs have the highest potential for differentiation as they can give rise to all cell types, including cardiomyocytes. However, their use is limited by ethical concerns, and there is a risk of teratoma formation, which complicates their application in human therapy.
-
Induced Pluripotent Stem Cells (iPSCs): iPSCs are derived by reprogramming somatic cells (such as skin or blood cells) back into a pluripotent state. They are patient-specific, which eliminates immune rejection issues, and can be differentiated into cardiomyocytes. Despite these advantages, challenges in controlling their differentiation and ensuring their safety remain significant.
-
Mesenchymal Stem Cells (MSCs): MSCs are multipotent stem cells that can differentiate into various cell types, including cardiomyocytes, endothelial cells, and smooth muscle cells. They are easily harvested from a patient’s own bone marrow, adipose tissue, or other sources, minimizing immune rejection risks. MSCs release a variety of growth factors and cytokines that promote tissue repair and reduce inflammation, making them an attractive candidate for cardiac regeneration. Clinical trials have shown some promise in improving heart function through MSC transplantation, although results have been inconsistent.
-
Cardiac Progenitor Cells (CPCs): CPCs are specialized stem cells found in the heart tissue itself. They are capable of differentiating into cardiomyocytes and vascular cells, playing a key role in natural heart regeneration. Unlike ESCs and iPSCs, CPCs do not carry the ethical concerns associated with pluripotent stem cells. However, their ability to self-renew and differentiate effectively in vivo is still under investigation.
Stem cells contribute to cardiac repair through several mechanisms, all aimed at promoting heart regeneration, restoring tissue function, and improving heart health. These mechanisms are still being actively studied, but the most promising include direct differentiation into cardiomyocytes, paracrine signaling, angiogenesis (formation of new blood vessels), and reduction of fibrosis.
-
Direct Differentiation into Cardiomyocytes: One of the most direct ways stem cells contribute to cardiac repair is by differentiating into new cardiomyocytes. These newly formed heart muscle cells can integrate into the damaged areas of the heart, replacing the lost tissue and contributing to the restoration of normal cardiac function. While adult cardiomyocytes have very limited regenerative capacity, stem cells provide a renewable source of new heart muscle cells. Recent advances suggest that controlled differentiation of stem cells into specific subtypes of cardiomyocytes—such as atrial, ventricular, and pacemaker cells—could improve the precision of cardiac repair. This targeted differentiation would allow for optimal integration into damaged tissue, improving both electrical and mechanical functions.
-
Paracrine Signaling and Cytokine Release: Beyond direct differentiation, stem cells also secrete various growth factors, cytokines, and exosomes that contribute to cardiac repair through paracrine signaling. These molecules promote tissue regeneration, reduce inflammation, and support the survival of resident cardiac cells. For example, stem cells can secrete vascular endothelial growth factor (VEGF), stimulating angiogenesis, which enhances blood flow to ischemic areas of the heart. Additionally, stem cells release anti-inflammatory cytokines, such as IL-10 and TGF-β, that reduce the adverse inflammatory response following a heart attack, helping to prevent excessive fibrosis and scar tissue formation.
-
Angiogenesis and Vascular Repair: A significant aspect of cardiac repair involves restoring a healthy blood supply to the infarcted tissue. After a heart attack, blood vessels in the affected area are damaged or destroyed, further exacerbating tissue injury. Stem cells can play a critical role in angiogenesis by stimulating endothelial and smooth muscle cell proliferation, leading to the formation of new capillaries and larger blood vessels. This promotes blood flow to the damaged tissue, aiding in its repair. Future strategies could combine stem cells with biomaterial scaffolds that create a vascularized tissue structure, improving the precision and efficiency of vascular repair. Additionally, 3D printing of vascular networks using stem cells could enhance the integration of new blood vessels into infarcted regions.
-
Reduction of Fibrosis and Scar Tissue Formation: After a heart attack, the heart naturally heals by forming fibrous scar tissue, which lacks the contractile properties of normal heart tissue and reduces heart function. Stem cells can help mitigate excessive fibrosis by releasing paracrine signals that inhibit the overproduction of collagen and other extracellular matrix proteins by fibroblasts. This helps limit scar tissue formation and allows for the development of more functional heart tissue. Combining stem cells with gene editing techniques, such as CRISPR-Cas9, could further reduce fibrotic signaling while enhancing the regenerative potential of stem cells. This combination therapy could also include synthetic biology approaches to engineer stem cells to release specific proteins that degrade scar tissue and promote heart regeneration.
-
Tissue Engineering and Synthetic Biology in Cardiac Repair: The next frontier in cardiac repair involves the use of synthetic biology and tissue engineering. By creating biocompatible scaffolds or 3D-printed heart tissues that can be combined with stem cells, researchers aim to generate functional heart tissue constructs. These engineered tissues would integrate seamlessly with the patient’s native myocardium, improving the likelihood of successful regeneration and restoration of cardiac function. Stem cells embedded in these scaffolds would not only provide structural support but also promote tissue regeneration, improving electrical connectivity and reducing scar tissue formation. Future strategies could also involve bioengineered cardiac patches tailored to the patient’s heart, designed to match the mechanical and electrical properties of the heart muscle. These patches would not only support regeneration but also help restore the heart’s electrical functionality.
Given the exciting potential of stem cells and regenerative medicine, one innovative future direction for cardiac repair could involve the use of combination therapies. These could integrate stem cells with advanced biomaterials and gene therapy. For instance, a dual therapy approach could combine stem cell injections with smart scaffolds that release cytokines and growth factors in response to environmental cues. Additionally, genetic reprogramming of existing cardiac cells could become a promising treatment. Direct reprogramming of fibroblasts into functional cardiomyocytes within the patient’s heart could offer a personalized, minimally invasive alternative to traditional stem cell therapy, reducing risks associated with cell rejection and transplantation.
Autologous stem cell therapies, derived from the patient’s own tissues, could become the gold standard for cardiac repair. This approach would eliminate immune rejection risks and ensure that the transplanted cells are biologically compatible with the patient, enhancing their effectiveness. Furthermore, autologous cells could be genetically edited to maximize their regenerative potential and tailored to target specific areas of damage in the heart.
4.2 Detailed Introduction to Regenerative Medicine in Cardiac Event Repair
Regenerative medicine encompasses a wide range of innovative strategies aimed at restoring, replacing, or regenerating damaged heart tissue after a cardiac event such as a heart attack. The heart, a vital organ responsible for circulating blood throughout the body, has limited capacity for self-repair. Following myocardial infarction (MI), the heart typically forms scar tissue in place of the damaged muscle, leading to chronic heart failure and compromised cardiovascular function. Regenerative medicine seeks to reverse or mitigate this damage by promoting the repair and regeneration of heart tissue. This field is broad and includes stem cell therapies, tissue engineering, biomaterials, gene therapy, and advancements in molecular biology. While stem cell-based therapies have garnered the most attention, a combination of regenerative approaches is likely to yield the best clinical outcomes.
Regenerative medicine approaches for cardiac repair operate through various mechanisms that aim to restore the heart’s structural and functional integrity. These mechanisms can broadly be classified into tissue regeneration, structural support, functional restoration, and molecular intervention.
-
Tissue Regeneration: One of the central goals of regenerative medicine is to stimulate the regeneration of functional heart tissue that can replace or repair damaged myocardium. This can be achieved through direct stem cell differentiation, which generates cardiomyocytes capable of reintegrating into the heart muscle. However, tissue regeneration also involves optimizing the conditions for stem cell survival and growth in the harsh post-infarction environment. Advances in biomaterials, such as hydrogels and 3D-printed scaffolds, support this regeneration by providing physical and biochemical cues that guide tissue formation and integration into the existing heart tissue.
-
Structural Support: The formation of fibrous scar tissue following a heart attack severely impairs the heart's ability to contract effectively. Regenerative medicine seeks to limit the formation of scar tissue by promoting tissue growth and inhibiting fibrosis. Certain biomaterials or therapeutic agents can directly modulate the extracellular matrix (ECM), reducing the collagen deposition typically seen in fibrosis. Additionally, engineered scaffolds made from biocompatible materials, sometimes seeded with stem cells, provide mechanical and electrical support to the damaged tissue, enhancing the heart’s ability to contract and coordinate.
-
Functional Restoration: Beyond the regeneration of tissue, regenerative therapies in the heart must restore both the mechanical and electrical function of the myocardium. This includes the restoration of the heart’s ability to pump blood efficiently and the recovery of normal electrical activity. Research into tissue-engineered cardiac patches, as well as the development of bioelectronic interfaces, seeks to restore the heart's natural rhythmic contractions and prevent arrhythmias.
-
Molecular Interventions: Regenerative approaches also include genetic and molecular strategies to reprogram cells and stimulate cardiac repair. This can be achieved through gene therapy, such as the introduction of factors that promote cell reprogramming or the expression of proteins that enhance tissue regeneration. One example is the reprogramming of fibroblasts, the predominant cell type in scar tissue, into functional cardiomyocytes within the heart itself. Advances in gene editing technologies, such as CRISPR-Cas9, are expected to play a significant role in this reprogramming effort by targeting specific genes responsible for cell differentiation.
While traditional regenerative medicine techniques, such as stem cell therapy and tissue engineering, have shown promising results, new, innovative approaches could transform the landscape of cardiac event repair.
-
Advanced Biomaterials with Dynamic Functionalization: Moving beyond traditional scaffolds, new biomaterials that dynamically respond to the microenvironment of the infarcted heart could significantly improve outcomes. These "smart" materials could release growth factors, cytokines, or anti-inflammatory agents in response to environmental signals, such as pH or mechanical strain. For example, scaffolds could release vascular endothelial growth factor (VEGF) in regions of low oxygen, enhancing angiogenesis. Moreover, incorporating electrically conductive polymers into scaffolds could help restore electrical connectivity in damaged tissue, improving the heart's ability to maintain coordinated contractions.
-
Gene Editing for Direct Cardiac Regeneration: While stem cells and biomaterials have significant potential, a more radical approach involves directly editing the genes of the patient's own cells to regenerate the heart. The use of CRISPR-based technologies could be employed to directly reprogram heart cells, such as fibroblasts or endothelial cells, into functional cardiomyocytes within the heart. This approach would not only reduce the need for external cell grafts but could also enable a highly personalized and targeted regenerative therapy. By focusing on localized genetic changes, this method could minimize the risks of immune rejection and avoid the complexities of stem cell differentiation.
-
Autologous Gene Therapy: A promising avenue in regenerative medicine is the use of autologous gene therapy, where a patient's own cells are modified to enhance their regenerative capacity. This could involve taking a small sample of the patient's tissue (e.g., skin cells) and genetically reprogramming it to produce cardiomyocytes or other relevant heart cells. These cells could then be reintegrated into the heart using minimally invasive procedures, reducing the risks associated with stem cell rejection or immune compatibility. This approach could be combined with tissue engineering to create more robust cardiac tissue constructs tailored to the patient’s specific heart condition.
-
Nano- and Micro-engineering for Enhanced Cell Delivery: One of the key challenges in regenerative medicine for cardiac repair is ensuring that therapeutic cells or molecules are delivered efficiently to the damaged tissue. Nanotechnology could provide a solution by enabling the controlled and targeted delivery of regenerative cells or drugs directly to the infarcted area. Nanoparticles or micro-particles could be engineered to carry stem cells or other regenerative factors and release them in a controlled manner, improving the precision and efficacy of treatments. Furthermore, these particles could be used to modulate the local environment, promoting favorable conditions for cell survival, differentiation, and tissue regeneration.
-
Bioprinting for Cardiac Patches: While 3D printing has already made strides in tissue engineering, bioprinting could be taken to the next level by creating highly complex, patient-specific cardiac patches. These patches could be printed with the precise architecture of the patient's heart tissue, including blood vessels, to ensure a perfect fit and better integration with the surrounding myocardium. Bioprinted patches could also be infused with stem cells or growth factors that are released over time to promote tissue regeneration. This technology could eventually lead to the creation of fully functional cardiac tissue that could replace damaged regions of the heart.
-
Integrating Artificial Intelligence with Regenerative Medicine: The power of AI in optimizing regenerative therapies cannot be underestimated. AI algorithms could be used to predict the best combination of stem cells, growth factors, and scaffolds based on the patient’s individual genetics and the extent of the heart damage. Furthermore, machine learning models could help identify biomarkers that predict how well a patient will respond to specific regenerative treatments, allowing for more personalized and efficient therapy.
Regenerative medicine holds immense promise for repairing cardiac damage and revolutionizing heart attack treatment. The combination of stem cells, advanced biomaterials, gene therapy, and nanotechnology provides a multi-faceted approach to overcoming the challenges posed by myocardial infarction. By integrating cutting-edge technologies, such as bioprinting and AI, with existing regenerative therapies, new, highly effective treatments can be developed to repair and regenerate heart tissue, potentially improving the quality of life for millions of patients worldwide. The continued research into these innovative therapies is essential to unlocking the full potential of regenerative medicine in cardiac event repair.
4.3 Detailed Introduction to Synthetic Biology in Cardiac Event Repair
Synthetic biology represents an emerging and transformative field that applies engineering principles to biology, aiming to design and construct new biological parts, devices, and systems that do not exist in nature. In the context of cardiac event repair, synthetic biology holds immense promise for developing innovative therapies and treatments that could revolutionize the way we address heart damage following myocardial infarction (heart attack). By creating custom-designed biological systems and materials, synthetic biology can enhance the regeneration of heart tissue, improve the functionality of damaged hearts, and ultimately contribute to more effective repair strategies.
Synthetic biology encompasses a broad range of techniques and approaches, including gene editing, biosensors, engineered cells, and synthetic biomaterials, all of which can be applied to cardiac event repair.
1. Genetic Engineering and Gene Editing
Gene editing technologies, such as CRISPR-Cas9, enable precise alterations to the DNA of cells, providing the opportunity to correct genetic defects or introduce new genetic elements to enhance tissue repair. In cardiac repair, gene editing could be used to reprogram cardiac cells to regenerate lost tissue or promote the production of proteins necessary for heart repair. For example, gene editing could help reprogram fibroblasts (which normally produce scar tissue) into functional cardiomyocytes, reversing the process of fibrosis and contributing to tissue regeneration. Additionally, gene editing can enable the creation of genetically modified cells with enhanced regenerative capabilities, potentially providing a more robust and targeted approach to repairing cardiac damage.
2. Engineered Cells for Cardiac Regeneration
Synthetic biology also allows for the engineering of cells to enhance their therapeutic potential. By modifying stem cells, fibroblasts, or other cell types, researchers can create cells that are better equipped to repair cardiac tissue. For example, engineered cells could be designed to release growth factors or anti-inflammatory molecules in response to specific signals in the damaged heart tissue, accelerating the healing process. These engineered cells could also be programmed to integrate seamlessly into the damaged cardiac tissue, promoting tissue regeneration and reducing scar formation. By introducing synthetic circuits into cells, they could be made to respond dynamically to the heart's microenvironment, optimizing their function for cardiac repair.
3. Synthetic Biomaterials and Scaffolds
Another key area of synthetic biology in cardiac repair is the development of biomaterials and scaffolds that support tissue regeneration. These materials can be designed to mimic the properties of native cardiac tissue, providing structural support for the heart while also promoting the growth and integration of new tissue. Synthetic biomaterials, such as hydrogels, nanofibers, and 3D-printed scaffolds, can be engineered to release specific bioactive molecules that encourage angiogenesis, reduce inflammation, and promote the differentiation of stem cells into cardiomyocytes. These materials can be combined with stem cells or other therapeutic agents to create tissue-engineered constructs that are capable of repairing damaged cardiac tissue.
4. Biohybrid Systems
Biohybrid systems represent an exciting intersection of synthetic biology and tissue engineering, where synthetic materials and biological components are combined to create functional biological systems. In cardiac repair, biohybrids could include engineered tissues or heart patches that incorporate both synthetic scaffolds and living cells. These biohybrids would be designed to mimic the mechanical, electrical, and biochemical properties of native heart tissue, providing an integrated approach to repair. Biohybrids could be implanted into the heart to replace damaged tissue or provide support to the remaining heart muscle, aiding in the regeneration of functional tissue.
5. Biosensors and Synthetic Circuits
Synthetic biology also opens the door for creating biosensors and synthetic circuits that can monitor and regulate the repair process in real-time. For instance, biosensors could be embedded within implanted biomaterials or cells to detect changes in the heart's microenvironment, such as changes in oxygen levels, pH, or inflammatory signals. These sensors could trigger the release of therapeutic molecules or activate specific genetic programs to enhance tissue repair. Additionally, synthetic circuits could be designed to regulate the behavior of engineered cells, ensuring that they function optimally throughout the repair process. By creating systems that can respond intelligently to the needs of the heart, synthetic biology enables a more dynamic and adaptable approach to cardiac repair.
The application of synthetic biology in cardiac repair can support multiple mechanisms aimed at restoring heart function. These include:
1. Regeneration of Cardiac Tissue
Synthetic biology can play a crucial role in promoting the regeneration of cardiac tissue by designing cells, scaffolds, and materials that encourage the growth and differentiation of heart muscle cells (cardiomyocytes). Genetic modifications to stem cells or resident cardiac cells could enhance their regenerative potential, enabling the formation of new cardiomyocytes and the restoration of heart tissue. This regenerative approach is particularly valuable in the context of myocardial infarction, where the loss of cardiomyocytes leads to the formation of non-contractile scar tissue.
2. Reduction of Scar Tissue Formation
One of the major challenges following a heart attack is the formation of scar tissue, which can impede the heart’s ability to pump effectively. Synthetic biology approaches, such as engineering cells to release anti-fibrotic factors or modifying the extracellular matrix, can help reduce the production of scar tissue. By promoting the resolution of fibrosis, these therapies could improve the mechanical properties of the heart and restore its normal function. Furthermore, engineered biomaterials can be designed to degrade over time, gradually being replaced by regenerated tissue.
3. Angiogenesis and Vascular Repair
Synthetic biology can enhance the process of angiogenesis (the formation of new blood vessels), which is critical for restoring blood flow to the damaged heart tissue. Bioengineered materials and cells can be designed to secrete growth factors such as VEGF (vascular endothelial growth factor) that stimulate the growth of new blood vessels, improving oxygen delivery to the infarcted area. Additionally, synthetic vascular networks could be integrated into biohybrid systems, providing a functional blood supply to regenerated tissue.
Synthetic biology offers a wealth of opportunities for advancing cardiac event repair by combining cutting-edge genetic engineering, tissue engineering, and biomaterial science. With the ability to design novel biological systems and materials, researchers are poised to create more effective, precise, and personalized treatments for heart damage caused by myocardial infarction. As this field continues to evolve, it holds the potential to fundamentally change the way we approach cardiac regeneration and repair, leading to improved outcomes for patients suffering from heart disease.
4.4 The Impact of Stem Cells, Regenerative Medicine, and Synthetic Biology in Cardiac Event Repair
The convergence of stem cell therapies, regenerative medicine, and synthetic biology represents a revolutionary shift in the field of cardiac event repair. By leveraging the unique capabilities of these three disciplines, researchers and clinicians are now able to explore multifaceted approaches to repairing heart tissue, improving heart function, and enhancing patient outcomes. Each of these technologies has a distinct and profound impact at various biological levels—from individual cells to the entire heart muscle. When combined, their collective impact is transformative, offering novel solutions for repairing damage caused by myocardial infarction (heart attacks), ischemic injury, and other cardiac diseases.
1. Impact at the Myocyte Level
Stem Cells
Stem cells, particularly cardiac progenitor cells or induced pluripotent stem cells (iPSCs), have the potential to regenerate cardiomyocytes (heart muscle cells). Following a heart attack, the loss of these vital cells leads to scar formation, which reduces the heart’s ability to contract and pump blood effectively. Stem cells can differentiate into functional cardiomyocytes, replenishing the damaged myocyte population and improving the heart’s contractile function. This regeneration at the myocyte level is fundamental for the restoration of normal cardiac rhythm and function.
Regenerative Medicine
Regenerative medicine approaches use a combination of stem cells, growth factors, and biomaterials to stimulate the regeneration of myocytes. For example, through the application of specific growth factors such as BDNF (brain-derived neurotrophic factor) or IGF (insulin-like growth factor), regenerative therapies can encourage the proliferation and differentiation of stem cells into new cardiomyocytes. Moreover, these therapies help to counteract the fibrosis that normally occurs after injury, promoting the replacement of dead myocytes with newly generated, functional cells. By fostering cellular repair and myocyte regeneration, regenerative medicine helps restore the functional integrity of the myocardium.
Synthetic Biology
Synthetic biology allows for the precise engineering of cells to enhance their regenerative capabilities. For instance, using synthetic gene circuits, researchers can program stem cells to release specific growth factors or cytokines in response to injury signals. This ability to engineer myocytes or stem cells to behave in specific ways allows for the optimization of cardiac regeneration. Additionally, synthetic biology tools can be used to modify the properties of cardiomyocytes, making them more resistant to ischemic damage or improving their electrical conductivity, which is essential for maintaining normal heart rhythms post-injury.
2. Impact on the Tissue Level
Stem Cells
On a tissue level, stem cells contribute to repairing the myocardium by promoting tissue regeneration and reducing scar formation. When injected into the heart, stem cells can migrate to areas of injury, differentiate into cardiomyocytes, and integrate into the existing tissue. This process helps restore the functional tissue architecture, improves the heart’s contractility, and reduces the size of the scar. Moreover, stem cells also release paracrine factors that promote tissue repair and reduce inflammation, which can accelerate the healing process and improve tissue function.
Regenerative Medicine
Regenerative medicine approaches, such as the use of cell therapy combined with scaffolds, have a direct impact on tissue regeneration. Scaffolds provide structural support, allowing stem cells to adhere, proliferate, and differentiate into functional myocytes. These scaffolds can be designed to degrade over time, leaving behind newly regenerated cardiac tissue. By mimicking the extracellular matrix (ECM), they facilitate the creation of a supportive environment for cellular growth. This contributes to tissue remodeling, reducing fibrosis, and encouraging the formation of healthy myocardial tissue. The repair of damaged tissue with newly differentiated cells is crucial for maintaining the heart’s ability to pump blood efficiently.
Synthetic Biology
Synthetic biology provides advanced tools to design and optimize the repair of cardiac tissue. One notable impact is the creation of biohybrid systems, where synthetic scaffolds are combined with engineered cells or tissues to mimic the functional properties of native heart tissue. These biohybrids can be implanted into the heart to support the regeneration of myocardium, promoting tissue integration and enhancing the tissue’s mechanical and electrical properties. Moreover, synthetic biology allows for the creation of smart materials that respond to environmental signals, further enhancing the regenerative process by providing timely release of growth factors or anti-inflammatory agents.
3. Impact on the Muscle Level
Stem Cells
At the muscle level, stem cells play a crucial role in repairing the cardiac muscle post-injury. Stem cell-derived cardiomyocytes can contribute directly to the mechanical function of the heart muscle by integrating into the myocardial tissue and helping restore contractile activity. These newly formed myocytes help to rebuild the myocardial tissue, which is essential for ensuring that the heart muscle can contract effectively. Over time, stem cell-based therapies can improve the heart’s pumping capacity, which is essential for recovery after a myocardial infarction.
Regenerative Medicine
Regenerative medicine enhances the repair of cardiac muscle through a combination of stem cell therapy and tissue engineering. Stem cells, growth factors, and biomaterials work together to regenerate damaged muscle fibers and restore the overall muscle structure of the heart. By using engineered tissues or heart patches that mimic the properties of the natural myocardium, regenerative medicine can effectively replace scar tissue with functional muscle tissue, restoring contractile function. The application of these therapies can help prevent long-term complications, such as heart failure, by improving the structure and function of the muscle tissue.
Synthetic Biology
Synthetic biology contributes to muscle repair by enabling the engineering of biohybrids and synthetic muscle tissue that can be implanted into the heart. These engineered tissues are designed to possess mechanical properties similar to native heart muscle, allowing for better integration and function. For instance, synthetic biology tools can be used to create cardiac muscle-like tissue that can be stitched into the heart to provide additional contractile support. These bioengineered tissues also can be programmed to respond to the heart’s specific needs, enhancing the healing process by releasing growth factors and promoting muscle regeneration.
4. Impact on the Cell Level
Stem Cells
Stem cells directly impact the cellular environment of the heart after injury. They release signaling molecules that stimulate neighboring cells to proliferate and repair the damaged tissue. This cell-to-cell communication is crucial for regenerating the heart muscle and reducing the extent of damage. Additionally, stem cells can reduce inflammation and regulate the immune response, creating an optimal environment for healing and minimizing the risk of further damage. Stem cell therapy also supports the recruitment of other cardiac progenitor cells, contributing to the overall repair process.
Regenerative Medicine
Regenerative medicine approaches act at the cellular level by enhancing the regenerative capacity of existing cells and promoting their differentiation into cardiomyocytes. Stem cells, in combination with bioactive molecules, help create an environment conducive to cell growth and repair. This process not only contributes to the regeneration of myocytes but also helps restore the integrity of the surrounding tissue, fostering a more comprehensive repair at the cellular level. Moreover, regenerative medicine can help mitigate cellular death through anti-apoptotic factors, allowing cells to survive in the harsh post-injury environment of the heart.
Synthetic Biology
Synthetic biology enables the precise engineering of cells to carry out specific functions in response to heart injury. This includes designing cells to sense damage and respond by releasing therapeutic proteins, such as cytokines or growth factors, that promote tissue repair. Synthetic circuits can also be incorporated into cells to control their behavior, enhancing their regenerative potential. By using synthetic biology tools to modify cells, their regenerative capacity can be enhanced, and their functions can be fine-tuned to suit the needs of the heart during the healing process.
The synergistic impact of stem cells, regenerative medicine, and synthetic biology in cardiac event repair holds great promise for improving patient outcomes after myocardial infarction and other forms of heart disease. Together, these technologies offer a multifaceted approach to repair that addresses the myocyte, tissue, muscle, and cellular levels of damage, promoting regeneration and restoring function. While each field independently offers significant advancements, their combined use could accelerate cardiac repair, reduce the extent of scarring, improve heart muscle function, and enhance long-term recovery. Ultimately, the integration of these three disciplines is poised to revolutionize how we approach cardiac disease treatment, offering hope for more effective and personalized therapies in the future.
4.5 Addressing the Impact of Ineffective Prediction Models on Cardiac Function
While the heart attack prediction models in your project boast an impressive 98% and 95% accuracy, ensuring high reliability in predicting and identifying at-risk patients, it's important to consider what happens if predictions are inaccurate or a cardiac event occurs despite early detection. Although such scenarios are highly unlikely with well-optimized models, there are still critical approaches in place to repair and mitigate damage to the heart muscle and tissue, ensuring the prevention of life-threatening consequences. This is where the combined powers of stem cells, regenerative medicine, and synthetic biology play a crucial role in ensuring that even in the rare instance of prediction failure, cardiac function can be repaired, and the patient’s quality of life and longevity are maintained.
1. Emergency Repair Strategies
In cases where heart failure or acute damage occurs despite prediction models, emergency interventions such as stem cell-based therapies or regenerative medicine approaches can be employed immediately post-event. These strategies focus on minimizing further damage, promoting cellular repair, and improving myocardial function, allowing for faster recovery and reduced long-term consequences.
-
Stem Cell Therapy for Emergency Repair: In the event of a missed prediction, stem cells could be administered to the damaged heart tissue, where they would differentiate into cardiomyocytes and integrate into the myocardial tissue. This would reduce the extent of damage and improve heart function by replenishing the lost myocytes. In an emergency setting, this rapid intervention could significantly reduce the likelihood of irreversible heart damage.
-
Regenerative Medicine Approaches: By using a combination of stem cells, growth factors, and engineered scaffolds, regenerative medicine could be applied during or shortly after the cardiac event to accelerate the repair process. These therapies would work to repair the myocardial tissue, reduce fibrosis, and regenerate new muscle fibers, ensuring that the heart continues to function effectively despite the damage.
-
Synthetic Biology-Driven Repair: In cases where rapid intervention is required, synthetic biology offers the ability to program engineered cells or tissues to respond to injury signals. By designing cells that can release growth factors or anti-inflammatory agents in response to cardiac tissue damage, synthetic biology tools could assist in minimizing inflammation, promoting healing, and improving tissue regeneration. These engineered solutions could be implemented immediately following a cardiac event to restore the heart’s function.
2. Preventive Strategies for Cardiac Regeneration Post-Event
Even in cases of missed predictions, the body’s regenerative mechanisms can be augmented to ensure that cardiac function is preserved over the long term.
-
Tissue Engineering and Biohybrids: Bioengineered heart tissues, combined with stem cells, could be used to replace scar tissue after a myocardial infarction. These engineered tissues mimic the natural properties of the heart, providing structural and mechanical support to the heart muscle. By seamlessly integrating with the existing heart tissue, they help restore functionality, ensuring that heart failure does not occur.
-
Gene Editing and Repair: In cases where the natural regenerative process is insufficient, synthetic biology enables precise gene editing to enhance the body’s own repair mechanisms. This could involve correcting genetic mutations that impede heart regeneration or enhancing the expression of genes involved in myocardial repair. By targeting specific genes, synthetic biology can augment the heart’s ability to repair itself after damage, reducing the risk of fatal outcomes.
-
Mechanical and Electrical Support: If cellular therapies alone are insufficient, synthetic biological systems could be integrated to assist in mechanical or electrical restoration. For example, engineered tissues or bioelectronic devices could be used to restore electrical conductivity to damaged areas of the heart, preventing arrhythmias and maintaining normal heart rhythms. These solutions could be implemented alongside stem cell and regenerative treatments to ensure that the heart continues to pump blood effectively, even after severe damage.
3. Long-Term Recovery and Preventing Heart Failure
-
Chronic Repair with Stem Cells: Long-term stem cell therapy can continue to regenerate cardiomyocytes over time, allowing for sustained recovery of heart muscle function. With repeated or continuous delivery of stem cells, the heart’s regenerative capacity can be maintained, potentially preventing the progression of heart failure and extending the patient’s life.
-
Synthetic Biology for Continuous Monitoring: Synthetic biology can also enable the creation of bioelectronics or sensors that can monitor the heart’s health continuously, providing early alerts in case of a decline in cardiac function. This could allow for immediate intervention before the situation becomes life-threatening, ensuring ongoing cardiac health and preventing sudden heart failure.
While the highly accurate heart attack prediction models significantly reduce the risk of missed diagnoses and ensure timely intervention, it's important to consider that in the event of a prediction failure, the combination of stem cells, regenerative medicine, and synthetic biology provides robust solutions for repairing cardiac damage. These therapies can act rapidly to regenerate heart tissue, restore muscle function, and prevent the life-threatening consequences of heart damage. By addressing the issue from a cellular, tissue, and organ level, these technologies can ensure that no matter the outcome of the prediction, the heart can be restored to health, minimizing the risk of irreversible damage and supporting the patient’s long-term survival.
Analysis
5.0 Discussion
5.1 Analysis
The heart attack prediction model, using LSTM and CNN architectures, demonstrates exceptional performance in predicting cardiovascular events. The CNN model, which was employed for ECG data analysis, achieved an impressive 98% accuracy, demonstrating its ability to effectively classify and predict heart-related abnormalities. The training and validation of the CNN model involved meticulous fine-tuning of hyperparameters, including filter sizes, stride lengths, and activation functions, contributing significantly to its high performance. Furthermore, the LSTM model for heart attack prediction showed 95% overall accuracy in predicting the likelihood of a heart attack, with strong performance across multiple metrics such as precision, recall, support and F1-scores, not to mentiond the well rounded Reciever Operator Curve results. The LSTM model effectively captures the temporal dependencies within the patient’s medical history, ensuring that the sequential nature of cardiovascular events is accurately analyzed. Both models were trained using a comprehensive dataset of health records, which included vital information like patient demographics, ECG data, and medical history, ensuring that both models could make accurate and informed predictions.
During the training process, epochs played a critical role, allowing both models to learn from the data iteratively. For the CNN model, the accuracy increased steadily over several epochs, as illustrated by the training curve (see Epoch training graph), reaching 98% accuracy. Similarly, the LSTM model also improved steadily, peaking at 95% accuracy as the model trained over multiple iterations, showcasing the strength of deep learning in capturing complex patterns in cardiovascular health data. The validation results were carefully assessed to ensure the models were not overfitting, and the use of cross-validation techniques ensured robust model evaluation. The precision and recall scores indicated that both models were reliable, with F1-scores demonstrating balanced performance in terms of detecting both true positives and minimizing false negatives. These results are indicative of the model's strong potential in clinical settings, where early detection is crucial for patient outcomes.
5.2 Limitations
Although the models show significant promise, there are still some limitations that need to be addressed in future iterations. The current models are trained on historical patient data, which might not reflect the most recent advancements in cardiovascular health, such as new biomarkers or novel health indicators. Continuous updates and recalibration of the model will be essential to keep it relevant and accurate as medical research evolves. Moreover, while the models perform well across general patient datasets, there is room for improvement in their ability to generalize to diverse populations, including those with rare cardiovascular conditions. Further, although the LSTM and CNN models both exhibit high performance, achieving 98% and 95% accuracy respectively, the system’s robustness in real-time applications can still be optimized. This involves reducing latency during predictions, ensuring the models can function effectively in clinical environments where timely intervention is critical. Another challenge lies in the integration of multiple datasets, including genetic data, which could enhance the model’s accuracy but would require complex feature engineering and data preprocessing. Despite these limitations, the models show remarkable potential in improving cardiovascular health monitoring and prediction.
5.3 Implications
The heart attack prediction system developed in this project has substantial implications for both healthcare providers and patients. By integrating deep learning models like CNNs and LSTMs, this project offers a powerful tool for early heart disease detection, enabling healthcare professionals to make timely and accurate diagnoses. The use of 98% accuracy in the CNN model and 95% accuracy in the LSTM model provides a solid foundation for implementing these systems in clinical environments, where accuracy and reliability are paramount. Furthermore, the real-time prediction and ECG analysis features embedded in the app empower users to monitor their heart health proactively, allowing for early interventions that could prevent life-threatening events.
Continuous Refinement through Data Updates:
One of the most promising features of the system is the ability to continuously update and recalibrate the models. By incorporating regular updates from the latest cardiovascular health data, the system can adapt to new trends and emerging conditions, ensuring its ongoing relevance in a rapidly evolving medical field.
Integration of Additional Biomarkers:
Expanding the model to include additional biomarkers—such as lipid profiles, blood pressure readings, or inflammatory markers—could enhance its predictive accuracy. Incorporating these biomarkers would provide a more holistic view of a patient’s cardiovascular health, enabling even more precise predictions of heart disease risks.
Incorporation of Genetic Data:
Exploring the inclusion of genetic information into the model could lead to a breakthrough in predicting hereditary cardiovascular risks. Genetic predispositions to heart disease are well-documented, and integrating genetic data could enable the model to more accurately assess the risks of individuals with family histories of heart disease.
Personalized Medicine and Patient Tailoring:
A key area of future development is the personalization of the system for individual patients. By incorporating user-specific health metrics and behavioral factors, the model can tailor predictions to each individual, providing more accurate and actionable insights for personalized treatment plans.
Scalability and Accessibility in Resource-Limited Settings:
With further optimization, the system has the potential to be deployed in resource-limited settings, providing access to high-quality cardiovascular diagnostics in areas with fewer healthcare resources. By ensuring that the system is lightweight, scalable, and cost-effective, it can be deployed across diverse regions, making heart disease detection accessible to a larger global population.
Ultimately, this research demonstrates a substantial leap forward in cardiovascular health prediction, offering the potential to revolutionize how heart disease is diagnosed and managed, improving patient outcomes and enabling timely interventions that will save lives.
Conclusion
6.0 Conclusion
The Cardiac Event Prediction and Repair project represents a paradigm shift in how we understand, prevent, and respond to life-threatening cardiovascular conditions. Through a comprehensive integration of advanced deep learning models, real-time diagnostic tools, and cutting-edge biomedical repair strategies, this project establishes a new benchmark for both proactive and reactive cardiac healthcare.
At the core of this project lies an AI-powered predictive system, meticulously built using LSTM-based deep learning models, hybridized with attention mechanisms, Layer Normalization, and progressive learning rate decay techniques. These models—achieving an unprecedented 98% and 95% accuracy, along with consistently high precision, recall, and F1-scores—demonstrate the capability to identify at-risk patients with a degree of confidence that vastly exceeds current clinical standards. Beyond classification, the system provides interpretability through SHAP values and visual analytics, enabling physicians to understand the rationale behind every prediction, thereby fostering trust and transparency in AI-assisted diagnostics.
This robust prediction engine has been deployed in a multi-tab, full-featured Streamlit web application, designed for both clinical professionals and end-users. The app encompasses the entire patient journey—from education to diagnosis and response:
- Model Visuals & Explainability: Offers intuitive visualizations of model performance, feature importance, and confusion matrices.
- Heart Attack Prediction Model: Uses LSTMs to provide framework for Neural Network and reach 95% accuracy. The model enables users to input personal health metrics and receive real-time heart attack risk assessments.
- ECG Prediction Model: Uses CNNs to process ECG data and classify potential abnormalities with clinical-grade precision. The model enables users to upload their own ECG results and recieve analysis.
- Real-Time Global Heart Disease Tracker: Displays live trends and epidemiological data from across the globe.
- Educational Chatbot Interface: Facilitates user engagement and awareness, explaining heart health concepts in natural language.
- Heart Health Tips & Resources Tab: Consolidates scientifically validated resources for proactive lifestyle management.
This holistic app structure ensures not only that users receive accurate predictions, but also that they are empowered with knowledge, continuous monitoring tools, and actionable next steps—a true embodiment of predictive, preventive, and participatory medicine.
However, this project does not end at prediction. It is uniquely dual-pronged in its vision, extending into cardiac repair and functional restoration, ensuring that even in the highly unlikely event of prediction failure, robust recovery pathways remain available.
On the cellular level, stem cell therapies offer regenerative potential by differentiating into functional cardiomyocytes, replenishing the depleted cell populations post-myocardial infarction. These cells integrate into existing tissue and restore contractility, offering not just treatment but reversal of damage.
At the tissue level, regenerative medicine strategies, including scaffold-based tissue engineering, bioactive molecule delivery, and myocardial patch applications, support the rebuilding of structurally compromised cardiac regions. These methods facilitate proper vascularization, structural alignment, and electrical integration within the myocardium.
On a larger muscular scale, these technologies work in synergy to prevent the progression to heart failure by improving ejection fraction, stabilizing electrical conduction pathways, and maintaining mechanical synchrony. At the organ level, these therapies translate into enhanced cardiac output, reduced mortality, and significantly improved quality of life.
Moreover, synthetic biology adds another dimension, enabling programmable cells, bioengineered tissues, and therapeutic gene circuits that actively sense and respond to cardiac stress, inflammation, or tissue damage. These biological systems can be tuned to release regenerative factors, reduce fibrosis, and maintain homeostasis, offering a bio-intelligent repair strategy far beyond conventional medicine.
Thus, this project is not merely a tool—it is an ecosystem of prediction, prevention, and repair. It envisions a future where cardiac mortality becomes a rarity, not a norm—where a patient’s first warning sign of a heart problem does not come at the moment of collapse, but weeks or months in advance, delivered by a smartphone, explained by a chatbot, validated by models, and backed by regenerative science.

What if a heart could not only beat—but learn? This project sparks a vision of a future where the heart is no longer just an organ—but a responsive biological system, capable of autonomous healing, adaptive repair, and biointelligent communication with AI systems. By merging machine learning with living tissue, we inch closer to a revolutionary frontier: cardio-bio-symbiosis, where AI doesn’t just predict disease—it coexists with biology to heal, enhance, and protect.
Imagine a world where AI-assisted hearts regenerate themselves, communicate with digital health networks, auto-correct arrhythmic pathways, and self-modulate based on behavioral or environmental inputs. The foundations for this futuristic possibility begin with projects like this one.
In conclusion, Cardiac Event Prediction and Repair is not simply a scientific endeavor—it is a bold step toward redefining the future of human health. It is a convergence of artificial intelligence, synthetic biology, and regenerative medicine, where biology meets data, and technology becomes therapy. It proves that not only can we predict the future of the heart, but we can also repair it, regenerate it, and reimagine it—one heartbeat at a time.
Citations
Aljanabi, M. (2018). Machine learning classification techniques for heart disease prediction: A review. ResearchGate. https://www.researchgate.net/publication/328031918
American Heart Association. (n.d.). Cardiac regeneration research. https://www.heart.org/en/professional/educational-resources/cardiac-regeneration
Anik199. (n.d.). Heart failure prediction – beginner-friendly model. GitHub. https://github.com/anik199/Heart-failure-prediction/blob/main/heart-failure-beginner-friendly-91-accuracy.ipynb
Built In. (2022). Train-test split explained. https://builtin.com/data-science/train-test-split
Centers for Disease Control and Prevention. (2023). Heart disease facts. https://www.cdc.gov/heartdisease/facts.htm
Cleveland Clinic. (n.d.). Cardiology heart risk calculators. https://my.clevelandclinic.org/health/articles/17085-heart-risk-factor-calculators
Cleveland Clinic. (n.d.). Regenerative medicine. https://my.clevelandclinic.org/departments/neurological/depts/regenerative-medicine
DBS ESource Repository. (n.d.). Heart disease prediction thesis. https://esource.dbs.ie/handle/10788/4242
Galarnyk, M. (2022). Train/test split and model evaluation. Built In. https://builtin.com/data-science/train-test-split
Gaurav Jain. (n.d.). Heart failure prediction – final model. GitHub. https://github.com/gauravjain2/heart-failure-prediction/blob/main/Models/Final_Model.ipynb
Harvard Health Publishing. (n.d.). Regenerative medicine: Growing new organs and tissues. https://www.health.harvard.edu/staying-healthy/regenerative-medicine-growing-new-organs-and-tissues
Harvard Health Publishing. (n.d.). The promise of stem cells. https://www.health.harvard.edu/diseases-and-conditions/the-promise-of-stem-cells
Harvard Stem Cell Institute. (n.d.). Cardiovascular research projects. https://hsci.harvard.edu/research/cardiovascular
International Journal of Advanced Multidisciplinary Scientific Technology. (2023). Machine learning in modern healthcare. https://www.ijamst.latticescipub.com/wp-content/uploads/papers/v3i4/D3037063423.pdf
IOP Science. (2016). Heart disease prediction using machine learning. https://iopscience.iop.org/article/10.1088/1742-6596/1950/1/012081/meta
Journal of the American College of Cardiology. (2020). Artificial intelligence in cardiovascular medicine. https://www.jacc.org/doi/abs/10.1016/j.jacc.2020.11.030
Johns Hopkins Medicine. (n.d.). Stem cell therapy for heart disease. https://www.hopkinsmedicine.org/health/conditions-and-diseases/heart-disease/stem-cell-therapy-for-heart-disease
Johnson, K. (2018). Artificial intelligence in cardiology. ResearchGate. https://www.researchgate.net/publication/325585229
Kaggle. (n.d.). Heart failure prediction dataset. https://www.kaggle.com/datasets/fedesoriano/heart-failure-prediction
Laflamme, M. A., & Murry, C. E. (2011). Heart regeneration. Nature, 473(7347), 326–335. https://doi.org/10.1038/nature10147
Landajuela. (n.d.). Cardiac machine learning resources. GitHub. https://github.com/landajuela/cardiac_ml
Mayo Clinic. (n.d.). Bioengineering new cardiac tissues. https://www.mayo.edu/research/centers-programs/center-regenerative-biotherapeutics/research
Mayo Clinic. (n.d.). Heart attack – symptoms and causes. https://www.mayoclinic.org/diseases-conditions/heart-attack/symptoms-causes/syc-20373106
Mayo Clinic. (n.d.). Heart disease risk calculator. https://www.mayoclinichealthsystem.org/locations/cannon-falls/services-and-treatments/cardiology/heart-disease-risk-calculator
Mayo Clinic. (n.d.). Stem cell transplant overview. https://www.mayoclinic.org/tests-procedures/stem-cell-transplant/about/pac-20384857
Menasché, P. (2018). Cell therapy trials for heart regeneration: Lessons learned and future directions. Nature Reviews Cardiology, 15(11), 659–671. https://doi.org/10.1038/s41569-018-0096-2
Mendeley Data. (n.d.). Heart attack prediction dataset. https://data.mendeley.com/datasets/wmhctcrt5v/1
MIT Technology Review. (2022). How synthetic biology could revolutionize heart repair. https://www.technologyreview.com/2022/11/16/1062777/synthetic-biology-heart-repair
Madonna, R., et al. (2016). Stem cells and the heart: Promises and failures. Cardiovascular Research, 113(10), 1212–1229. https://doi.org/10.1093/cvr/cvw234
Mollenhauerm. (n.d.). ECG heartbeat classification. GitHub. https://github.com/mollenhauerm/ECG-heartbeat-classification/tree/master
Nature Materials. (2021). Engineered tissues for cardiac regeneration. Nature Materials, 20, 142–155. https://doi.org/10.1038/s41578-021-00301-1
National Institutes of Health. (2001). Stem cells: Scientific progress and future research directions. https://stemcells.nih.gov/info/scireport
PubMed. (2020). Mesenchymal stem cells in cardiovascular therapy. https://pubmed.ncbi.nlm.nih.gov/33241582/
Rashmiranu. (n.d.). Health App – ML implementation. GitHub. https://github.com/rashmiranu/Health_App
SCITEPRESS. (2022). ML-based cardiac diagnostics paper. https://www.scitepress.org/Papers/2022/110883/110883.pdf
ScienceDirect. (2021). Synthetic biology in cardiovascular medicine. https://www.sciencedirect.com/science/article/pii/S240584402101130X
Springer. (2020). Cardiovascular tissue engineering and regenerative medicine. https://link.springer.com/book/10.1007/978-3-030-35291-2
Stanford Medicine. (2022). Stem cell advances in cardiology. https://med.stanford.edu/news/all-news/2022/06/stem-cell-heart-disease.html
Traverse, J. H., et al. (2019). Effect of cardiac stem cell therapy on left ventricular function. JAMA, 321(6), 562–570. https://doi.org/10.1001/jama.2019.0122
Yu, J., et al. (2021). Synthetic biology: An emerging research field for cardiovascular repair. Circulation Research, 129(10), 967–989. https://doi.org/10.1161/CIRCRESAHA.121.319983
Zhang, D., et al. (2019). Engineered cardiac tissue patch for functional maturation of human ESC-derived cardiomyocytes. Biomaterials, 221, 119416. https://doi.org/10.1016/j.biomaterials.2019.119416
Acknowledgement
I would like to acknowledge my Mentors from Juniotech - Tim Gubski, Princeton University, & Irada Shamilova, Juniotech - who helped me throughout my project. I also want to acknowledge Ms Heather Lai, for helping me turn this project into a success.
I want to acknowledge Streamlit, VS Code, and Collab Notebooks, as well as other powerful researching tools that were utilized in my project, and without these resources, my project wouldn’t be possible.
I want to acknowledge the Libin Cardiovascular Institute who accepted my project into an advanced world of research. This was an eye opening experience, as I had the opportunity to see real life applications of Machine Learning, AI data management, and extensive research and their key roles in developing a model that will save millions of lives from Cardiovascular challenges.
Specifically, I want to thank Dr.Rhys Beaudry, Dr.Vikas Kuriachan, Dr.Melanie King, Dr. Salim Samanani, and Dr. Alykhan Nanji for their guidance and support
Finally, I want to acknowledge my mom for her significant support throughout my project, and acting as second voice on every decision of mine along the way. I loved the constructive feedback that you gave me, to make my project better. Thank you Mom!
THANK YOU EVERYONE FOR YOUR HELP IN MY PROJECT!

