Next-Gen Prosthetics: Merging Myoelectric and Brain-Controlled Technology for Seamless Limb Function
Grade 10
Presentation
Problem
The creation of both Brain-Computer Interfaces (BCIs) and myoelectric prosthetics are both groundbreaking innovations in the field of neuroscience, medicine, and technology. Both technologies are fairly new, with myoelectric prosthetics being developed earlier in the late 1940’s while the term “brain-computer interface” was coined by Professor Jacques Vidal in 1973.
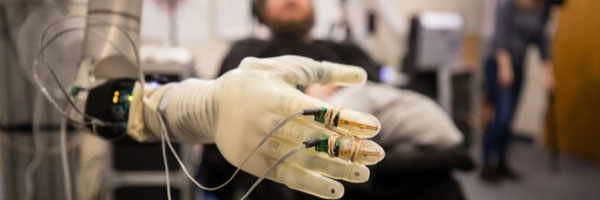
Electrically powered prostheses, commonly known as myoelectric prostheses, are controlled by electromyographic (EMG) signals created in the residual musculature, which are picked up by electrodes housed within the prosthetic socket. The objective of myoelectric prosthetics is to reestablish the functionality of missing limbs and increase the quality of life of amputees. By using electromyography electrodes attached to the surface of the skin, amputees are able to control motors in myoelectric prostheses by voluntarily contracting the muscles of their residual limb. The patient is able to control the velocity of the prosthesis actuators by voluntarily contracting their residual muscles [1] The objective of myoelectric prostheses is to restore some functionality of a limb to the patient to improve their overall well-being.
BCI’s offer solutions for curing blindness via a visual implant by electrically stimulating the visual cortex and retinal cells of patients with blindness [2] as well as restoring mobility in the paralyzed through the interpretation of neural signals. These interfaces can also now be used as a neurofeedback training tool to improve cognitive performance [3]. BCI systems play a crucial role in areas such as diagnosis, neurorehabilitation, and assistive technology, aiming to restore patients' ability to engage with and control different activities and environments, thereby compensating lost neurologic(al) functions [4].
Despite both the potential of myoelectric control and BCIs, both technologies exhibit limited functions in one or more of several ways such as in their longevity, capability, safety, and ease of implantation. Difficulty in controlling a myoelectric prosthetic is often possible, which can lead to passive-use of the device or total rejection, which can have detrimental effects on the contralateral limb due to overuse [5]. Myoelectric devices also present numerous challenges such as difficulty when managing simultaneous movements and few degrees of freedom. Along with that, myoelectric prosthetic devices can often contain an increased lack of proprioception. The training time in which learning how to use a myoelectric prosthesis is lengthy and time consuming. Similarly, learning how to use BCI’s can take a long time as well. It is known that BCI’s consist of two main types; invasive and non-invasive, both which pose risks that can lead to detrimental effects. The implantation of invasive BCI devices carries inherent medical risks like infection or brain tissue damage, while non-invasive BCIs can pose health concerns such as the effects of long-term exposure to electromagnetic fields.
It is important to note that while these disadvantages could be overcome in times when issues arise in both technologies, creating a new device in which we combine both myoelectric signals and BCIs can serve as a safer and better option for people to consider. The proposal and design of a hybrid prosthetic device, if seen to be successful, may be the future of prosthetics devices to come.
Method
Abstract
This scientific research aims to explore the integration of myoelectric signals and brain-computer interface (BCI) technology to create a hybrid prosthetic control system that enhances the functionality and adaptability of prosthetic limbs. By merging these two advanced technologies, the research focuses on creating a prosthetic system that offers intuitive control via muscle contractions and complex movement capabilities driven by neural signals. The study looks at the biological foundations of movement, including the role of the brain's motor and premotor cortex, neural oscillations, and the anatomical structure of the hand. It also addresses the limitations of current prosthetic devices, such as restricted functionality, lack of proprioception, and difficulty in performing movements. Through the proposition of the prototype idea, this research shows how combining myoelectric and BCI technologies can lead to more natural and accurate prosthetic control, improving the quality of life for amputees and patients with neurological impairments. This project emphasises the potential for future advancements in prosthetics and neurotechnology, while also acknowledging the challenges and ethical considerations involved in the development of such devices.
2.2 Objective
The main objective of this proposal—backed up with extensive research—is to design and propose an idea for a virtual prototype in creating a hybrid prosthetic hand that combines myoelectric signals and brain-computer interfaces. By focusing specifically on the hand as the limb of interest, the aim of the project is to address the complex functional needs associated with upper limb prosthetics, such as fine motor skills, dexterity, and adaptability. We chose the hand due to its intricate movements and its use in everyday life, emphasizing that if lost, it should be restored with proper and natural control. This hybrid system seeks to combine the muscle-driven control provided by electromyographic (EMG) signals with the advanced neural input decoding capabilities of BCIs, allowing users to perform complex hand movements in a more natural and accurate manner. The virtual prototype will demonstrate how this integration can overcome limitations of current prosthetic hands, including limited dexterity, simultaneous movement challenges, and reduced proprioception. By focusing on enhancing the functionality of a prosthetic hand, we aim to create a solution that improves the quality of life and independence of individuals with upper limb amputations.
3. Literature Review
3.1.Overview of Myoelectric Prosthetics
Myoelectric prostheses are devices that employ electromyographic signals from residual muscles to control or enable movements. These signals are developed upon the contraction of a muscle. They arise from action potentials, where the electrical property emanates from the motor units of the muscles. Electrodes are usually placed on the skin of the user's wrist or inside the prosthetic socket to detect the signals—they are then amplified and processed. The processed signals are then matched to control distinct prosthetic movements such as opening and closing the hand, while the speed of movement is decided by the amplitude of the signals.
The origins of myoelectric prostheses can be traced back as far as the 1940s, while with each decade technologies in the sectors have improved in areas like their motor control, design, and responsiveness. Modern myoelectric prosthetics incorporate powered motors and batteries so that the user can make several movements, such as grasping, rotating their wrist, flexing their elbow, and even performing shoulder movements. Newer myoelectric devices can execute multiple movements ranging from grasp to elbow or shoulder motion allowing the users to regain more natural and intuitive control. Further, technologies such as Targeted Muscle Reinnervation (TMR) provide further improved control due to nerve endings from the missing limb being rerouted to intact muscles within prosthetic sockets. This technique provides more precise and intuitive control since prosthetic devices respond to signals actually generated as a simultaneous thought of a particular movement, like hand closure.
Recently, a number of innovations have been under trials with the application of implantable electrodes. They yield improved signal stability-lessening influences coming from electrode shift or sweat interference. Implantable Myoelectric Sensors (IMES) are a prime example of such technologies; however, this insertion comes along with added complexities, such as transepidermal wires or wireless power transfer. Pattern recognition algorithm advances are even being developed in order to process raw myoelectric signals and transfer definite functions to a prosthetic. These algorithms may allow sequencing or simultaneous control of several joints, with further growth to provide proportional velocity control for smoother and more intuitive operation.
Although myoelectric prosthetics are seen to be a groundbreaking innovation, limitations in this regard include difficulties in performing simultaneous movements with limited degrees of freedom and in the absence of sensory feedback that can basically inhibit proprioception. Equally, these devices can stop functioning when water is involved. Difficulties in calibration or adaptation can lead to rejection or underutilization. However, a combination of TMR, implantable electrodes, and various algorithms gives hope that these shortcomings might be rectified to create much more agile and functional myoelectric prosthetics for the users. Combining these, along with the abilities of a Brain-Computer-Interface may just create the perfect prosthetic for those who need it.
Figure 3.1.1 The TASKA Hand (An example of a myoelectric prosthesis)
3.2.Brain-Computer Interfaces
Moving on to brain-computer-interfaces, Brain-Computer Interfaces (BCIs) s) enable direct communication between the brain and external devices by interpreting neural signals. These systems can be categorized as invasive or non-invasive. Invasive BCIs involve the insertion of electrodes into the brain, providing sufficient resolution and accuracy but with corresponding risks of tissue damage, infection, and limited device lifespan. Although non-invasive BCIs are ostensibly safer and easier to set up, they compromise fine spatial resolution and have poorer signal accuracy, which decreases their ability to perform more intricate tasks.
Despite the downsides, BCIs have showcased themselves as innovative tools. They enable control of movement in paraplegics, shed light on various neurological disorders, and are developing treatment options for brain diseases. BCIs yield continuous real-time brain activity analysis for cognitive researchers and application of neurofeedback. Nevertheless, the further adoption is barred by the combination of size, invasiveness, and inadequate computational power.
The integration of BCIs, with their control ability, and the functionality of myoelectric technology could provide hybrid prosthetics that are easy to control, invoke natural movement, and provide better sensory feedback. The integration, therefore, offers a solution for the limitations of stand-alone BCIs in decoding and myoelectric prostheses missing out on sensory feedback and realizing simultaneous control.
As neuroscience and materials continue their advance, hybrid prosthetics can serve as a great opportunity to enhance mobility and functionality.
Figure 3.2.1 Examples of BCIs ranging from invasive to non-invasive
3.3.Gaps in Existing Research
Current research into myoelectric prosthetics and brain-computer interfaces (BCIs) has made great progress, but gaps still remain. Myoelectric prosthetics offer greater functionality compared to mechanical alternatives, but still face limitations such as the lack of sensory feedback, which deprives users of proprioception and makes control less intuitive. These devices also struggle with simultaneous complex movements and are vulnerable to external factors like water and sweat, impacting their ability. Similarly, BCIs have high potential but face many challenges as well. Invasive BCIs provide precise neural signal interpretation but are risky, costly, and prone to issues like tissue damage. Non-invasive BCIs, while safer, lack the spatial resolution and decoding accuracy necessary for high-performance applications. Furthermore, both invasive and non-invasive BCIs are limited by current signal interpretation capabilities and difficulties in long-term use, such as calibration problems and signal degradation.
Despite advancements in both fields, there hasn’t really been research of hybrid systems that combine the strengths of myoelectric and BCI technologies. This integration does come with challenges. including the need for advanced computational algorithms to process both muscle and neural signals simultaneously in real-time. Making sure to create a prosthetic that addresses these gaps are very important for creating prosthetic devices that are not only functional but also perfect for everyday use.
This project aims to fill these gaps by proposing a hybrid prosthetic hand that integrates myoelectric signals with BCI technologies. By enhancing sensory feedback, enabling simultaneous control of complex movements, and improving the accuracy of signal decoding, this system offers a better alternative to both BCI’s and myoelectric prosthetics, paving the way for future prosthetics to come.
4. Biological Foundations of Movement Control
4.1.The Role of the Brain
This project’s primary aim is to enhance biological sciences, particularly neurobiological research. The central objective is to improve the systems used for testing, cataloging, and interpreting neural signals to enhance the effectiveness and usability of neuroprosthetics. Understanding these biological processes is essential before designing the tools to measure and analyze them. To produce an accurate result, we need to research areas like the brain and interactions between neurons and other cells so that we can have a proper understanding of these phenomena. With that, let's begin by focusing on the main subject; the human brain.
The human brain has been described as the most complex structure in the known universe. It controls our thought, memory, emotion, touch, motor skills, vision, breathing, temperature, hunger and every process that regulates our body. Together, the brain and spinal cord that it extends from creates the central nervous system (CNS). Weighing about an average of 3 pounds, the brain mostly consists of fat; 60%. The other 40% contain water, protein, carbohydrates and salts. The brain contains blood vessels and nerves, including neurons and glial cells. The brain can be divided into three parts; the cerebrum, brainstem and cerebellum.
Along with that, the brain is divided into large subsections, such as the prefrontal cortex, hypothalamus, and etc. For our purposes, we'll focus specifically on the brain regions involved in movement control—the motor cortex and premotor cortex. These areas are crucial for understanding how we move, and they are directly relevant to the control of prosthetic limbs, which is the primary focus of this project.
Figure 4.1.1 General diagram of the human brain
The motor cortex is a region of the cerebral cortex involved in planning, controlling and execution of voluntary movements. It is an area of the frontal lobe located anterior to the central sulcus. The motor cortex comprises three different areas of the frontal lobe; the primary motor cortex, the premotor cortex, and the supplementary motor area (SMA). The primary motor cortex, also known as M1 is located in the precentral gyrus and anterior paracentral lobule on the medial surface of the cerebrum. It sends the majority of the electrical impulses from the motor cortex and of the three motor cortex areas, it requires the least amount of electrical stimulation to trigger movement. The primary motor cortex is somatotopically organized meaning that different parts of the brain are "mapped" to different parts of the body (It's like the brain has a body map, where each part of the body has a corresponding area in the brain). This somatotopic organization is represented by the motor homunculus which depicts the involvement of the primary motor cortex in producing movements of regions of the body
Figure 4.1.2
Figure 4.1.3: motor homunculus
The primary motor cortex does not generally control individual muscles directly, but rather appears to control individual movements, or sequences of movements that require the activity of multiple muscle groups. In turn, alpha motor neurons encode the force of contraction muscle fibers using the rate code and size principle.
Primary motor cortex neurons fire 5-100 msec before the onset of a movement. Rather than firing as the result of muscle activity, these neurons are involved in relaying motor commands to the alpha motor neurons that eventually cause the appropriate muscles to contract. The amount of force required to raise the arm from one location to another is much greater if one is holding a bowling ball than if one is holding a balloon.
Many neurons in the primary motor cortex encode the amount of force that is necessary to make such a movement. Most neurons in the primary motor cortex don't control individual muscles directly. Instead, they focus on how much force is needed for a movement, no matter which muscles are involved. Then, alpha motor neurons take the commands from the motor cortex and drive the muscles to produce the right amount of force to carry out the movement, following the rate code (the more neurons firing, the more force generated), and the firing principle (Smaller motor units (less force) are recruited first, and larger ones (more force) are used as needed).
Figure 4.1.4
When there is little load, a motor neuron in the primary motor cortex that controls an extension of the wrist fires when the wrist extends. However, when a 5 lb. load is placed on the left pulley, more force must be used to initially hold the weight steady and then lift it. The extension motor neuron in the primary motor cortex fires more strongly to produce the greater force.
The primary motor cortex also encodes the direction of movement. Many neurons in the motor cortex are tuned to specific directions. For instance, some neurons fire more when the hand moves to the left, and less or not at all when it moves to the right. Each neuron has a preferred direction it responds to most strongly. Additionally, some neurons respond based on how far the movement is. For example, if a monkey moves its arm to targets at different distances and directions, some neurons will fire more strongly depending on how far the arm moves, and others will be sensitive to both the direction and the distance of the movement. The motor cortex also encodes the speed of movement. When moving an object, such as a coffee cup, the hand accelerates at first, reaches its fastest speed halfway, and then slows down as it approaches the target. Some neurons in the motor cortex fire in sync with this speed pattern, showing that the brain encodes information about the speed of the movement as well.
Figure 4.1.5 Directional tuning of motor cortex neurons
Figure 4.1.6 Velocity profile of targeted movements.
Moving on to the premotor cortex, the premotor cortex sits immediately anterior to the primary motor cortex. Its main function is to prepare for movement, especially in proximally located musculature. The premotor cortex sends axons to the primary motor cortex as well as to the spinal cord directly. It performs more complex, task-related processing than the primary motor cortex. As the primary motor cortex is involved in the execution of voluntary movements, the premotor cortex works with the selection of appropriate motor plans in order to incite these voluntary movements. Premotor neurons signal the preparation for movement. It does not cause the movement itself, but instead prepares the movement to occur. This type of neuron is called a motor-set neuron, as it fires when in preparation for making a movement.
Along with that, premotor cortex neurons signal various sensory aspects associated with particular motor acts. Some premotor neurons fire when an individual is performing a particular action, such as breaking a peanut. Interestingly, the same neurons fire selectively when the individual observes another person performing the same action. They also respond to the sound of the action, such as a peanut shell breaking, even without any visual or motor activity. These neurons are called “mirror” neurons, because they respond not only to the action performed by the individual but also to the sight (or sound) of another person performing the same action.
Figure 4.1.7 Mirror neuron in premotor cortex fires to the monkey’s action as well as the monkey’s perception of a person performing the same action (Kohler et al., 2002).
Additionally, the premotor cortex is sensitive to the behavioral context of a movement. It shows increased activity depending on the inferred intention behind a movement, rather than just the movement itself. This suggests that premotor neurons respond to the context in which an action occurs, reflecting the intended purpose of the movement. For example, the premotor cortex of human subjects was imaged with functional MRI as they observed a video of a hand grasping a cup in one condition, the cup was full and surrounded by full plates of food; the implication was that the person was grasping the cup to take a drink. In the other condition, the cup was empty and surrounded by dirty dishes; the implication was that the person was grasping the cup to clear the table. In this experiment, the premotor cortex was more active when subjects viewed the first video than the second one, even though the movements were the same.
Lastly, the premotor cortex is involved in signaling both correct and incorrect actions. It shows bilateral activation during correct actions and movement errors, but activation is more localized to the left hemisphere during object errors. This suggests that the premotor cortex is sensitive to both the accuracy of the movement and the object involved in the action.
The last part of the motor cortex we’ll be looking at is the supplementary motor area (SMA). This part is involved in programming complex sequences of movements and coordinating bilateral movements. While the premotor cortex appears to be involved in selecting motor programs based on visual stimuli or on abstract associations, the supplementary motor area appears to be involved in selecting movements based on remembered sequences of movements.
The SMA is involved in both executing and mentally rehearsing sequences of movements, with activation occurring bilaterally for complex movements and mental rehearsal. It plays a key role in transforming movement plans from kinematic information (describing movement in space, like distance and angles) to dynamic information (relating to the force required for the movement). This transformation is crucial for instructing muscles to contract with the appropriate force, as shown by the shift in firing patterns of SMA neurons from kinematic to dynamic representation during movement preparation.
Figure 4.1.8 Positron emission tomography (PET) study of simple vs. complex finger movements (Roland et al.,1980). The SMA is activated bilaterally when subjects perform complex movements, and even when they only imagine performing the movements.
As mentioned previously, the motor cortex and premotor cortex are the regions of the brain responsible for controlling movement. These regions use neurons in order to transmit electrical signals that allow the brain to control these voluntary and involuntary actions that we spoke about earlier. Now, we’ll be going deeper into what neurons really are, and how their connections actually work.
The brain is composed primarily of two divisions of cells, glial, and neural cells. Glial cells do not conduct nerve impulses, but instead support, nourish, and protect the neurons. These cells regulate neurotransmission and help form the blood-brain barrier. On the other hand, neural cells—most commonly known as neurons—are responsible for carrying information throughout the human body. Neurons are cellular structures similar to other animal cells, consisting of the cell membrane, nucleus, and other organelles. They are specialized for energy maintenance and have slightly different structures than ordinary cells. Neurons are highly specialized for processing and transmitting signals within neural networks, resulting in a wide variety in shape, structure, and positioning. The total number of neurons in the human brain is estimated to be around 86 billion. Even more incredible is that each one of these neurons connect to thousands of other neurons, resulting in an estimated 100 trillion connections. We will come back to these connections further on. For now, let's look at the main parts of a neuron.
A neuron is made of three distinct parts; the cell body (soma), dendrite, axon. The cell body is the metabolic center of the neuron. The cell body contains genetic information, maintains the neuron’s structure, and provides energy to drive activities. Like other cell bodies, a neuron’s soma contains a nucleus and organelles. Next is the dendrite, which are fibrous roots that branch out from the cell body. Dendrites receive and process signals from the axons of other neurons. Neurons can have more than one set of dendrites, also known as dendritic trees. Lastly is the axon. The axon is a long, tail-like structure. It joins the cell body at a specialized junction called the axon hillock. Its main purpose is to carry nerve signals away from the cell body. The structure of a neuron can be seen in figure 4.1.9 below
Everything mentioned earlier is crucial information in understanding the role of a neuron, but our main objective is to understand the neuron's ability to transmit signals between cells. Neurons are essentially electrical devices, communicating through a process that begins with the resting membrane potential. At rest, the inside of the neuron has a negative charge relative to the outside, typically around -70mV. This electrical charge difference is maintained by the selective permeability of the cell membrane to ions such as sodium, potassium, and chloride, which is crucial for the neuron's ability to send signals. When a neuron is stimulated, the membrane potential changes, and if the stimulus is strong enough, it triggers an action potential—a quick electrical impulse that moves down the axon, often referred to as ‘spikes’, a term based on the shape recorded using sensitive electrical equipment. This occurs as voltage-gated ion channels open, allowing sodium ions to rush in and depolarize the neuron. The action potential then travels along the axon toward the synapse.
Figure 4.1.10 A neuron spikes when a combination of all the excitation and inhibition it receives makes it reach a threshold. On the right is an example from an actual neuron in the mouse's cortex. (Image: Alan Woodruff / QBI)
When the action potential reaches the synapse, it reaches the presynaptic terminal where the electrical signal is converted into a chemical one. Neurotransmitters are released into the synaptic cleft, a small gap between the presynaptic and postsynaptic neurons. These neurotransmitters bind to receptors on the postsynaptic neuron, triggering the flow of specific ions—such as sodium, potassium, calcium, or chloride—depending on the type of neurotransmitter. This flow of ions changes the electrical potential of the postsynaptic neuron, which can initiate a new action potential, continuing the transmission of the signal. In this way, synapses act as both the conversion point and the bridge between electrical signals within a neuron and chemical communication between neurons
Figure 4.1.11 An action potential, or spike, causes neurotransmitters to be released across the synaptic cleft, causing an electrical signal in the postsynaptic neuron. (Image: By Thomas Splettstoesser / CC BY-SA 4.0)
4.2.Neural Oscillations
Through looking at the brain’s complex structure, especially the important areas that manage voluntary movement, it's obvious that having precise control over motor functions are crucial for tasks like grabbing, lifting, and handling objects. These movements are managed by complex networks of neurons found in the motor and premotor cortices, which send messages through electrical signals. But the brain's communication goes beyond just individual signals traveling from one neuron to another; it involves synchronized activity patterns across many neurons. These coordinated bursts are called neural oscillations. Neural oscillations are brainwaves, or regular rhythmic patterns of neural activity in the brain associated with various sensory-cognitive processes. Neural oscillations are found everywhere within the nervous system, subserving sensory, motor, and cognitive functions in the brain and the spinal cord.
Neural oscillations occur at different frequency bands, each of which plays a specific role in motor control. For instance, beta waves (13–30 Hz) are closely associated with motor planning and the maintenance of steady muscle activity. Gamma waves (30–100 Hz) are linked to precise movements and sensorimotor integration. These frequencies help synchronize the firing of neurons, facilitating coordinated motor control. Neural oscillations are essential for coordination of movement, and they help synchronize the firing of neurons involved in motor control. If an individual breaks their hand or arm, especially if the injury is severe and involves nerve damage, this can interfere with the neural signals sent to the injured limb. The brain may have difficulty generating the rhythmic patterns of activity required for smooth movement in the affected limb. This disruption could result in difficulty moving the hand or arm properly, affecting both voluntary and reflexive movements.
4.2.1 Frequency bands of neural oscillations
Along with that, in some cases after an injury, the brain may “reorganize” itself, especially if the injury leads to loss of function in the affected area. The brain’s capacity to change its structure and functions in response to sensory impairments due to injury is known as neuroplasticity. Neuroplasticity enables the brain to adjust to sensory input, which is important for tasks like coordinated movement and sensory-motor integration. For example, the motor cortex might shift its oscillatory activity to different areas in the brain, attempting to regain control or adapt to the loss of motor function in the injured arm or hand.
If the injury results in chronic pain, the brain may also experience changes in neural oscillations associated with pain processing. This can affect how motor signals are generated and transmitted to the injured limb. Pain can also cause disruptions in motor planning, making it harder for the brain to coordinate smooth, purposeful movements. Chronic pain could lead to changes in the frequency and pattern of neural oscillations, further affecting movement. In other cases, such as if an injury results in an amputation, the loss of sensory input from the limb might lead to changes in neural oscillations in the brain. The brain's somatotopic map will eventually begin to adjust. This could lead to phantom limb sensations—where the brain continues to generate neural activity corresponding to the missing limb, causing a misalignment in the expected oscillations.
So, in the case of a person losing their hand or arm, neural oscillations related to the hand might become impaired, affecting how precisely the brain can control finger movement or hand movements. Disruptions in these specific frequency bands, especially beta and gamma oscillations, might impair the brain’s ability to encode movement intentions effectively, leading to difficulties in executing fine motor control. Movement intentions, which are encoded in oscillatory activity in the brain, might be disrupted because the brain cannot effectively communicate with the broken limb, leading to an inability to properly execute hand or finger movements. In summary, when a person breaks their hand or arm, neural oscillations related to movement control of the affected limb are disrupted. The brain may try to compensate for this, leading to altered or reorganized oscillatory patterns that reflect the body's attempts to adapt to the injury.
4.3.Anatomy of the Hand and Neural Connections
As we spoke about earlier, it's been established that the motor cortex plays a fundamental role in controlling voluntary movements. Now, we are going to focus mainly on the arm and fingers since that section on the body is our limb of focus for this research. Electric signals in the motor cortex allow for the fine and coordinated motions of the hand, arm, and fingers.These signals, generated by neurons in the motor cortex, are interpreted by Brain-Computer Interfaces (BCIs) to control prosthetic devices with high precision.
First, let's look into the anatomy of the human hand so that we can understand how to develop our ideas. The human hand is made up of the wrist, palm, and fingers and consists of 27 bones, 27 joints, 34 muscles, over 100 ligaments and tendons, and many blood vessels and nerves. The right and left hand are each controlled by the opposite side of the brain. Usually one hand is preferred for carrying out fine and complex movements, so we often say people are either right-handed or left-handed.
The human hand is made up of 27 bones: 8 carpal bones in the wrist, 5 metacarpal bones in the middle of the hand, and 14 phalanges in the fingers. This means that the hand accounts for about a quarter of all the bones in the body. The hand can be divided into three main sections: the carpus (the wrist bones), the metacarpus, and the fingers. The wrist has two parts that form a joint, which allows the hand to move up and down and side to side. The carpal bones are connected by ligaments and work with the radius bone to create the wrist joint, while the ulna is separated by a cartilage disc. The metacarpus is made up of five long metacarpal bones, and the joint with the thumb gives it a lot of flexibility.
Each finger has three bones, while the thumb has two, and they all have three joints that help them bend and stretch. The thumb can also twist because of a special saddle-shaped joint. More than 30 muscles, mostly located in the forearm, control how the hand moves, with tendons running through the hand. There are small muscles between the metacarpal bones that help with moving the fingers apart, together, and bending them. The thenar and hypothenar muscles control the thumb and little finger, allowing for precise movements and the ability to grasp objects. The lumbricals help bend the joints where the fingers meet the hand and extend the fingers. Tendon sheaths filled with lubricating fluid help reduce friction, making tendon movement smoother. The carpal tunnel, created by connective tissue and carpal bones, acts as a pathway for tendons, nerves, and blood vessels traveling from the forearm to the hand.
Figure 4.3.1 The carpal tunnel (seen from the palm side of the hand)
The hand's muscles allow for two primary types of grips: the power grip and the precision grip. The power grip is what an individual does when lifting heavy items, where the hand wraps tightly around the object in the palm, with the thumb positioned opposite the fingers to provide extra strength. This grip helps a person move heavy things in a controlled way. On the other hand, the precision grip is for smaller, more delicate tasks like writing or sewing. In this grip, the thumb and index finger work together to hold objects carefully, much like how tweezers function. In relation to the blood supply, the blood supply to the hand comes from two main arteries, one located on the thumb side and the other on the little finger side, creating an arch that delivers oxygen-rich blood to the hand, with branches reaching out to the fingers. Each finger gets its blood and nerve supply from four bundles.
Figure 4.3.2 Location of the main nerves and blood vessels in the hand
There are three nerves that control function in our hands: the median, ulnar, and radial nerve. Each of these nerves is responsible for both sensory and motor function in different parts of the hand. The median nerve originates from the shoulder, and controls the muscles needed to perform fine precision hand movements and pinching functions. The median nerve is the only nerve that enters the hand through the carpal tunnel; a space formed by the carpal bones of the wrist. This nerve controls sensation in the thumb, index finger, middle finger, and one side of the ring finger. Moving on the ulnar nerve, the ulnar nerve runs through the arm into the hand and is the largest unprotected nerve in the human body. It connects to the little finger and adjacent side of the ring finger of the hand, providing sensation on the palm side of the hand.The ulnar nerve enables us to grasp objects. It travels along the elbow, between the bone and overlying skin at the cubital tunnel.The ulnar nerve enters the palm of the hand through the Guyon’s canal. Lastly, the radial nerve supplies movement and sensory function to parts of your arm, forearm, wrist and hand. This nerve goes through the arm and controls the ability to extend our wrist and control the position of our hand. It also provides sensory feedback from the back of the little finger and adjacent half of the ring finger.
Figure 4.3.3 Nerves in the hand: radial, median, and ulnar
Analysis
5.1.Combining Control Systems
In our proposal, we want to combine myoelectric and BCI technologies by assigning specific roles to each system. Myoelectric systems are really good at picking up signals from the muscles that are left after an injury, which allows for fast and effective control of simple actions like opening, closing, or gripping things. In contrast, brain-computer interfaces (BCIs) work by analyzing signals straight from the brain, which lets users perform more complicated and advanced movements.
The mechanisms that we will use from each will be as follows: BCI’s will be used for planning and intent. BCIs interpret neural activity from the motor cortex, allowing the user to think about the action (e.g. grabbing an object), which initiates the process. Then, we’ll use myoelectric systems for the actual execution. Myoelectric sensors detect muscle activity and translate these signals into precise movements, such as the hand grasping or adjusting grip pressure. By dividing tasks between the brain and muscles, hybrid systems enable smoother and faster performance of simultaneous actions like moving the wrist while gripping.
5.2.Enhancing Sensory Feedback
One major issue of both myoelectric and BCI systems is that they don't provide natural sensory feedback, which is really important for tasks like holding delicate items or changing how tightly you grip something. Hybrid systems could help solve this issue by creatively merging the two technologies. Here are how hybrid systems could enhance feedback:
BCI-Driven Feedback Loops: Brain-Computer Interfaces (BCIs) could send sensory signals straight to the brain, giving details about the texture, temperature, or weight of objects. This would create a "closed-loop" system, allowing the brain to get real-time feedback for better control.
Tactile Sensors in Myoelectric Systems: Myoelectric prosthetics can use sensors to measure physical forces like how hard someone is gripping or how much resistance an object has. These signals can then be communicated to the user through vibrations or pressure on the remaining limb.
Proprioception Restoration: By combining BCIs and myoelectric systems, it’s possible to recreate a sense of where the prosthetic limb is located, helping users move around more naturally without needing to constantly check their prosthetic.
Example: Imagine a hybrid prosthetic hand holding a fragile item like an egg. Tactile sensors could sense if the grip is too tight and send that information to the brain via the BCI. This way, the user can adjust their grip instantly to avoid breaking the egg.
5.3.Comparative Analysis + Advantages of Combining the Two Systems
Now, let's look at the possible advantages of combining the two systems. Firstly, a hybrid system would allow for intuitive multitasking by dividing different control tasks between brain signals and muscle signals. BCIs handle high-level decisions (like thinking about opening the hand), while myoelectric systems handle the actual adjustments in position and whatnot. Along with that, sensory feedback data from both systems can be integrated to provide feedback for better object manipulation and proprioception. Another advantage of combining the two systems is that myoelectric systems are non-invasive, while BCI’s are more effective when invasive. Combining the two can balance these aspects, making a more advanced prosthetic that is easy to use. Another very significant advantage of a hybrid model would be that since simple movements could be done through myoelectric systems, while users could focus on more advanced options using the BCI. Lastly, combining the two systems the prosthetic adapts to a wider range of user needs, including both basic and complex tasks. With the advantages also come the disadvantages. It is good to keep in mind that while a hybrid system is a great idea, combining these systems would require advanced software and hardware to ensure seamless communication between myoelectric sensors and neural decoders. There would also be the matter of cost, training time, and mainly making sure the combining the two systems would actually work without errors or danger to the user wearing the prosthetic.
Shown below is a comparative chart.
|
Aspect |
Myoelectric Prosthetic |
BCIs |
Hybrid System |
|
Control Precision |
Effective for basic, single-joint movements |
Excellent for high-level complex actions |
Combines precision for both simple and advanced actions |
|
Sensory Feedback |
Limited (often no natural feedback) |
Potential for sensory restoration |
Offers real-time feedback and proprioception |
|
Ease of Use |
Non-invasive, easier setup |
Invasive, requires training and calibration |
Balances ease of use and advanced functionality |
|
Multitasking |
Limited simultaneous movements |
High multitasking potential |
Allows smooth, multi-joint and multi-task actions |
|
Challenges |
Affected by external factors (e.g., sweat) |
Risk of tissue damage, decoding issues |
Complex integration, high cost, and training requirements |
6. Hybrid Prosthetic Design
6.1.Design Overview
The hybrid prosthetic system combines both Brain-Computer Interface (BCI) and Myoelectric Control, to provide users with a smooth and intuitive experience. The system includes the following main components:
1. BCI (Brain-Computer Interface) Headset:
Description: The BCI headset is a non-invasive device that rests on the user’s head, usually designed like a headband. It has electrodes that pick up electrical signals generated by the brain, which relate to motor intentions, such as thinking about moving the hand, opening or closing fingers, or grasping something.
Function: The BCI analyzes neural activity from the motor cortex, the part of the brain that plans and starts voluntary movements. When the user thinks about performing an action (like picking up a cup), the BCI detects these brainwaves and sends them to a processing unit. These signals are then converted into commands for the prosthetic, enabling it to act according to the user’s thoughts without any physical movement from the limb.
Connection: The BCI headset would wirelessly send brain signals to a microcontroller, which decodes the signals and sends them to the prosthetic (myoelectric) hand to control its movements.
For a visual reference (rough estimation of what it would look like), look at figure 6.1.1 shown below
2. Myoelectric Prosthetic Hand
Description: The prosthetic hand is crafted to mimic the functionality of a human hand as closely as possible. It features fingers, joints, sensors, and motors all housed in a lightweight yet sturdy material. The hand operates using myoelectric sensors that detect electrical signals from muscle contractions in the residual limb (the part of the arm left after an amputation).
Function: When a user flexes certain muscles in their remaining limb, myoelectric sensors pick up these signals and convert them into movements in the prosthetic. For instance, if a muscle near the wrist contracts, it can trigger the motors in the hand to either open or close. These sensors are strategically placed along the residual limb, typically close to the muscles that would normally control hand movements.
Connection: The myoelectric system links to the motors and joints of the prosthetic hand, serving as the bridge that translates muscle signals into actual movements. The signals detected by the myoelectric sensors are related to a microcontroller, which interprets them and directs the motors in the prosthetic hand to carry out specific actions.
Connection to BCI: Myoelectric signals can also work alongside BCI signals to enhance accuracy. For example, while the BCI indicates the general intention to perform a task (like picking up an object), the myoelectric system adjusts the hand's movements based on the muscle activity.
Rough prediction of what it would look like shown in figure 6.1.2
Combining BCI and Myoelectric Systems
How They Work Together: The combination of brain-computer interfaces (BCI) and myoelectric control systems creates a unique method where each technology plays a specific role in how a prosthetic limb operates. The BCI focuses on understanding the user's thoughts and intentions, enabling them to think about and start an action. On the other hand, myoelectric sensors are responsible for carrying out those actions by converting muscle signals into actual movements. When a user thinks about performing an action, like grasping something, the BCI sends that intention to a microcontroller. This microcontroller then relays the information to the myoelectric sensors. These sensors pick up on muscle contractions and adjust the movements of the prosthetic hand according to the user's muscle activity. For example, if the user wants to grip something tightly, the BCI sends that command, and the myoelectric sensors fine-tune the motion by recognizing the patterns of muscle contractions.
Why This Approach is Effective: This separation of tasks between the BCI and myoelectric systems allows for more complex and natural movements. The BCI makes the system user-friendly by letting the user control the prosthetic just by thinking about the action. Meanwhile, the myoelectric sensors ensure that movements are precise and happen in real-time, giving feedback and adjusting to the user's muscle activity. This hybrid system also enables users to perform multiple actions at once, like moving their wrist while changing how tightly they grip something, which would be harder to achieve with just a BCI or myoelectric system alone.
Link to Sensory Feedback: Besides controlling movement, both systems improve sensory feedback. BCIs can send sensory information, like texture and temperature, back to the brain, helping users "feel" the objects they touch. Myoelectric sensors also provide feedback through vibrations or pressure, letting users know about grip strength and how much resistance they’re encountering.
Rundown of the components - Key components and their connections:
BCI Headset:
Role: This device picks up brain signals that relate to movement intentions, like the thought of gripping something or moving fingers.
Connection: The headset is worn on the scalp and has electrodes that capture neural activity from the motor cortex. These signals are sent wirelessly to the processing unit or microcontroller, usually via Bluetooth or a similar technology.
Microcontroller/Processing Unit:
Role: This is the central part of the system that interprets and manages the signals from both the BCI and myoelectric sensors. It translates these signals into commands for the prosthetic hand to move.
Connection: It gets brain signals from the BCI headset and muscle activity signals from the myoelectric sensors. The microcontroller combines this information and sends the right control signals to the motors and sensors of the prosthetic hand.
Figure 6.1.3 Simple depiction of a microcontroller
Myoelectric Sensors:
Role: These sensors detect muscle contractions in the residual limb (for amputees) or forearm. The contractions are turned into electrical signals that help control the movements of the prosthetic hand, like opening or closing fingers.
Connection: The myoelectric sensors are placed on the skin or the residual limb to monitor muscle activity. The signals they generate are sent to the microcontroller, which processes them and sends movement commands to the hand's motors.
Figure 6.1.4 Simple depiction of a myoelectric sensor
Prosthetic Hand (Motors, Sensors):
Role: The prosthetic hand is the actual device that carries out the movement commands. It includes motors that manage the movement of fingers and joints, along with tactile sensors that sense pressure, texture, and grip strength.
Connection: The motors and sensors are operated by the microcontroller. Based on the signals received from both the BCI and myoelectric sensors, the microcontroller instructs the motors to move the fingers and adjust the grip accordingly.
Figure 6.1.5 Depiction of a tactile sensor
Sensory Feedback:
Function: It gives users immediate sensory details about the objects they are handling, like how they feel, their weight, and temperature. This involves using tactile sensors along with feedback loops driven by brain-computer interfaces (BCI).
Connection: Tactile sensors placed on the hand can sense things like pressure and temperature, sending this data to a microcontroller. The BCI feedback loop can transmit sensory information straight to the brain, enhancing the user's understanding of where the prosthetic is and what they are holding. This information is communicated to the user through vibrations or pressure sensations.
How the System Works Together:
The BCI headset picks up neural signals that reflect the user's desire to move the prosthetic. Myoelectric sensors monitor muscle activity, providing the microcontroller with insights into the user's physical intentions, such as whether they want to grip or relax their hand. The microcontroller combines the brain's movement signals and muscle activity, directing the motors and sensors in the prosthetic hand to perform the desired actions. Sensory feedback from both tactile sensors and BCI loops offers users real-time insights into their surroundings and how the prosthetic interacts with different objects.
7. Potential Applications and Impact
7.1.Medical Applications
Restoration of Limb Functionality: The hybrid prosthetic system offers a revolutionary way for amputees to regain the ability to perform everyday tasks. By combining BCI and myoelectric technologies, the prosthetic can replicate more natural and precise movements, allowing users to interact with objects like they would with a natural hand. This can significantly enhance a user’s independence and quality of life.
Neurorehabilitation: This hybrid prosthetic could also aid in neurorehabilitation for people with neurological conditions, such as those who have suffered a stroke or spinal cord injury. BCIs can help patients re-establish connections between their brain and the prosthetic device, potentially facilitating the rehabilitation of the motor cortex by allowing it to "relearn" motor commands.
Pain Management and Sensory Feedback: One of the key challenges with prosthetics is the lack of sensory feedback, which often prevents users from feeling the objects they interact with. By integrating BCIs and myoelectric sensors with sensory feedback mechanisms, this hybrid system could transmit sensory information, such as pressure, temperature, and texture, to the user’s brain. This provides a more natural experience by restoring some degree of proprioception, allowing the user to "feel" their prosthetic limb and the objects it interacts with.
Adaptive Rehabilitation: The system's ability to respond to both brain and muscle signals means it could be continuously adapted to better suit a user’s evolving needs during rehabilitation. For example, as patients improve their muscle strength or neural function, the prosthetic could be adjusted to allow more complex and precise movements.
7.2 Societal Impact
Enhanced Independence and Empowerment: One of the most significant effects of this hybrid prosthetic system is its ability to boost independence for people with limb loss or disabilities. By allowing for more natural and intuitive control over prosthetics, users can accomplish tasks without needing constant help, which improves their ability to lead a more self-sufficient life. This fosters greater self-esteem and empowerment, especially for those who previously depended on caregivers or specialized tools.
Financial Advantages: The improved functionality and potential cost savings of hybrid prosthetics could ease the financial strain on healthcare systems and individuals. As these prosthetics become more affordable and efficient, more people will have access to these advanced devices, enhancing their quality of life and enabling them to engage more fully in the workforce and community activities.
Mitigating Stigma: The more sophisticated the prosthetic, the more it resembles a natural limb and performs effectively. This advancement can significantly help in reducing the social stigma associated with prosthetics. Users of hybrid prosthetics may feel more confident and less self-conscious, as they can perform movements similar to those of a natural limb. This can promote better social integration and lessen the psychological effects of using a prosthetic.
Wider Accessibility: Although the technology behind hybrid prosthetics is state-of-the-art, its broader availability could make high-quality assistive devices accessible to more people. If mass production leads to lower costs, individuals in low-income areas or developing nations could also take advantage of this advanced technology. This could greatly diminish inequalities in access to medical technologies and prosthetic care around the globe.
8. Challenges and Limitations
8.1.Technical Challenges
Signal Interference and Noise: A major technical issue that both BCIs and myoelectric systems encounter is the interference or noise that can distort signals. The BCI headset depends on brainwave data, which can be easily disrupted by outside influences like other electronic devices or even the physical movement of the headset itself. On the other hand, myoelectric sensors depend on muscle contractions, but these signals can be weak, inconsistent, or impacted by muscle fatigue, resulting in inaccurate readings.
Real-time Response and Accuracy: Achieving smooth, real-time control between the brain, muscles, and prosthetics is a big challenge. The signals from the BCI must be interpreted and processed accurately and without delay, which is tough due to the complex patterns of the human brain's neural activity. Likewise, myoelectric sensors need to be precisely calibrated to pick up subtle muscle movements that relate to specific actions, without misreading or overshooting what the user intends to do.
Battery Life and Power Consumption: Prosthetics that use BCIs, myoelectric sensors, and sensory feedback systems require a lot of power. Making sure these devices can work effectively for long periods without needing frequent recharges or battery changes is a key technical challenge. Enhancing the power efficiency of each component and lowering overall power usage will be essential for making these prosthetics practical for everyday use.
Integration of Multiple Systems: The challenge of combining various technologies, such as the BCI, myoelectric sensors, microcontrollers, motors, and sensory feedback systems, can complicate system design. Each component needs to work together seamlessly, and any delay or failure in one part could impact the performance of the whole system. Achieving this level of integration without sacrificing the reliability and usability of the prosthetic is a significant technical obstacle.
8.2.Ethical Considerations
Privacy and Data Security: Brain-computer interfaces (BCIs) pick up on brain signals, which raises significant privacy issues regarding how neural data is collected and used. If sensitive brainwave information falls into the wrong hands, it could create serious ethical dilemmas about privacy. Moreover, gathering this kind of data might unintentionally reveal personal weaknesses or compromise users' cognitive privacy.
Access to Technology and Equity: Hybrid prosthetic systems hold a lot of potential, but a key ethical challenge is making sure everyone can access them. These advanced prosthetics can be quite costly to create and produce, which means that many people, especially those in low-income areas, might not be able to afford or obtain this technology. There’s a moral responsibility to ensure that these innovations are affordable and available to everyone who needs them.
User Control and Consent: Since these systems connect directly with a person’s brain and body, it’s crucial that users have complete control and a clear understanding of how their prosthetic functions. There should be straightforward and informed consent processes in place so that users know what data is being collected, how it will be used, and what safety precautions are in effect. Ethical issues can also arise if users lose control of their prosthetics due to malfunctions or outside interference.
Potential for Manipulation or Over-Dependence: As prosthetics become more advanced, there’s a chance that users might become too reliant on the technology, which could lessen the need for rehabilitation or physical therapy. It’s essential to strike a balance where the prosthetic supports the user’s natural abilities without leading to over-dependence or hindering long-term recovery and adaptation.
Conclusion
9. Conclusion
After conducting thorough research, the idea of developing a hybrid prosthetic that combines myoelectric control with brain-computer interface (BCI) technology could be the next big thing in prosthetics. Merging these two advanced technologies presents a great opportunity to enhance the functionality and user-friendliness of prosthetic limbs, allowing users to have a more natural, intuitive, and adaptable experience. This hybrid approach enables users to initiate movement with their brain while executing it through muscle signals, resulting in smoother and more accurate motions that make daily tasks easier and promote independence.
Adding sensory feedback through myoelectric sensors and BCI connections further improves the experience by giving users a sense of touch and body awareness. This enhancement not only makes the prosthetic more practical but also boosts the user's ability to engage with their surroundings, leading to increased confidence and a better quality of life.
The potential uses for this technology in healthcare are extensive, including limb restoration, neurorehabilitation, and sensory feedback restoration. Moreover, the societal benefits are significant. By providing greater independence and empowerment, hybrid prosthetics can help reduce stigma and open up new possibilities for people with limb loss or disabilities.
Nonetheless, there are still challenges to overcome. Issues like signal interference, the need for real-time response accuracy, and power consumption must be tackled to ensure these devices are practical and reliable. Additionally, ethical concerns regarding data privacy, access to technology, and user control will need careful consideration as the field progresses.
In summary, the hybrid prosthetic system marks a major advancement in prosthetic technology. With ongoing research, development, and a focus on addressing the challenges, this idea of an innovation has the potential to make a significant impact for years to come.
Citations
Bibliography
[1] Dawson, M. R. (2012). The development of a myoelectric training tool for Above-Elbow amputees. The Open Biomedical Engineering Journal, 6(1), 5–15. https://doi.org/10.2174/1874230001206010005
[2] Ptito, M., Bleau, M., Djerourou, I., Paré, S., Schneider, F. C., & Chebat, D. (2021). Brain-Machine interfaces to assist the blind. Frontiers in Human Neuroscience, 15. https://doi.org/10.3389/fnhum.2021.638887
Spinalcord.com. (2020, November 12). Types of paralysis: monoplegia, hemiplegia, paraplegia, and quadriplegia. https://www.spinalcord.com/types-of-paralysis
[3] Gonfalonieri, A. (2020, October 6). What Brain-Computer interfaces could mean for the future of work. Harvard Business Review. https://hbr.org/2020/10/what-brain-computer-interfaces-could-mean-for-the-future-of-work
[4] Alkaff, Z. A., Malim, N. H. a. H., Sumari, P., & Abdullah, J. M. (2024). Applications of brain computer interface in present healthcare setting. In Artificial intelligence. https://doi.org/10.5772/intechopen.112353
[5] Chadwell, A., Kenney, L., Thies, S., Galpin, A., & Head, J. (2016). The reality of myoelectric prostheses: understanding what makes these devices difficult for some users to control. Frontiers in Neurorobotics, 10. https://doi.org/10.3389/fnbot.2016.00007
Mph, Z. S. (2023, January 23). How do glial cells function in the body? https://www.medicalnewstoday.com/articles/glial-cells-function#summary
What is a neuron? (2023, October 3). Queensland Brain Institute - University of Queensland. https://qbi.uq.edu.au/brain/brain-anatomy/what-neuron#:~:text=A%20neuron%20has%20three%20main,receives%20input%20from%20other%20cells.
Vandergriendt, C. (2022, February 28). An Easy Guide to Neuron Anatomy with Diagrams. Healthline. https://www.healthline.com/health/neurons#types
Organization of Cell Types (Section 1, Chapter 8) Neuroscience Online: An electronic textbook for the neurosciences | Department of Neurobiology and Anatomy - The University of Texas Medical School at Houston. (n.d.). https://nba.uth.tmc.edu/neuroscience/m/s1/chapter08.html#nucleus
Newman, T. (2023, July 18). All you need to know about neurons. https://www.medicalnewstoday.com/articles/320289#types
What is a neuron? (2023b, October 3). Queensland Brain Institute - University of Queensland. https://qbi.uq.edu.au/brain/brain-anatomy/what-neuron#:~:text=A%20neuron%20has%20three%20main,receives%20input%20from%20other%20cells.
MSc, D. K. F. P. (2023, September 2). Why our brains are the most complex structures in the known universe. Psychology Today. https://www.psychologytoday.com/ca/blog/consciousness-and-beyond/202309/the-staggering-complexity-of-the-human-brain
Cherry, K. (2024, September 12). What is a synapse? Verywell Health. https://www.verywellhealth.com/synapse-anatomy-2795867#:~:text=A%20synapse%20is%20the%20junction,neurons%20connect%20with%20other%20neurons.
How neurons communicate. (n.d.). https://www.brainfacts.org/core-concepts/how-neurons-communicate
Synaptic cleft. (2023, November 2). Kenhub. https://www.kenhub.com/en/library/anatomy/synaptic-cleft
Action potentials and synapses. (2024, October 22). Queensland Brain Institute - University of Queensland. https://qbi.uq.edu.au/brain-basics/brain/brain-physiology/action-potentials-and-synapses
Action potentials and synapses. (2024b, October 22). Queensland Brain Institute - University of Queensland. https://qbi.uq.edu.au/brain-basics/brain/brain-physiology/action-potentials-and-synapses
Action potentials and synapses. (2024c, October 22). Queensland Brain Institute - University of Queensland. https://qbi.uq.edu.au/brain-basics/brain/brain-physiology/action-potentials-and-synapses
Motor Cortex (Section 3, Chapter 3) Neuroscience Online: An electronic textbook for the neurosciences | Department of Neurobiology and Anatomy - The University of Texas Medical School at Houston. (n.d.). https://nba.uth.tmc.edu/neuroscience/m/s3/chapter03.html#:~:text=Primary%20motor%20cortex%20encodes%20the%20direction%20of%20movement.,the%20right%20(Figure%203.8).
Motor cortex. (2023, October 30). Kenhub. https://www.kenhub.com/en/library/anatomy/motor-cortex
Institute for Quality and Efficiency in Health Care (IQWiG). (2021, May 20). In brief: How do hands work? InformedHealth.org - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK279362/#:~:text=Movements%20of%20the%20hand%20are,the%20palms%20to%20the%20fingers.
Hand anatomy. (n.d.). https://www.ypo.education/orthopaedics/hand-wrist/hand-anatomy-t191/video/#:~:text=Nerves%20of%20the%20hand%20carry,the%20hands%20to%20the%20brain.
Chadwell, A., Kenney, L., Thies, S., Galpin, A., & Head, J. (2016b). The reality of myoelectric prostheses: understanding what makes these devices difficult for some users to control. Frontiers in Neurorobotics, 10. https://doi.org/10.3389/fnbot.2016.00007
Zotey, V., Andhale, A., Shegekar, T., & Juganavar, A. (2023). Adaptive Neuroplasticity in Brain injury recovery: Strategies and Insights. Cureus. https://doi.org/10.7759/cureus.45873
Myoelectric prostheses — within reach. (n.d.). Within Reach. https://www.withinreach.info/myoelectric-prostheses
McLean, L., & Scott, R. N. (2004). The early history of myoelectric control of prosthetic limbs (1945–1970). In Springer eBooks (pp. 1–15). https://doi.org/10.1007/978-3-642-18812-1_1
Orthopedics, S., & Orthopedics, S. (2024, September 30). The Nerves of the hand | Summit Orthopedics. Summit Orthopedics. https://www.summitortho.com/2015/01/19/nerves-hand/#:~:text=Three%20nerves%20control%20function%20in,different%20parts%20of%20the%20hand.
Jiralerspong, T., Nakanishi, E., Liu, C., & Ishikawa, J. (2017). Experimental Study of Real-Time Classification of 17 voluntary movements for Multi-Degree Myoelectric Prosthetic Hand. Applied Sciences, 7(11), 1163. https://doi.org/10.3390/app7111163
Mridha, M. F., Das, S. C., Kabir, M. M., Lima, A. A., Islam, M. R., & Watanobe, Y. (2021). Brain-Computer Interface: advancement and challenges. Sensors, 21(17), 5746. https://doi.org/10.3390/s21175746
Dawson, M. R. (2012b). The development of a myoelectric training tool for Above-Elbow amputees. The Open Biomedical Engineering Journal, 6(1), 5–15. https://doi.org/10.2174/1874230001206010005
Acknowledgement
Thank you first and foremost for CYSF for hosting the youth science yet again. Thank you to my teachers and especially our VP Mrs.Aly who encouraged us to participate in the science fair. Thank you to my parents for supporting my decision and my sister for assisting me when I needed it. Thank you to my peers who were supportive and encouraging, even when I didn’t think I could continue on. I couldn’t have done it without the encouragement and support from everyone I received. Thank you