Building a Hydrogen Engine
Grade 9
Presentation
Problem
The rising global climate has raised awareness for a redefining shift in the energy production industry. Energy production and dependence on fossil fuels, using traditional methods, have significantly caused the accelerated climate change, carrying negative environmental and socio-economic effects. This shows the importance of embracing green energy solutions to mitigate the unfolding crisis.
Fossil fuel-based energy production has been a cornerstone in the global economy for several decades, powering industries, transportation, and residential consumption of electricity. However, the combustion of these fossil fuels, coal, oil, and natural gas, releases copious amounts of greenhouse gases, especially carbon dioxide (CO2), into the atmosphere.
The increase in carbon dioxide content in the atmosphere over the years
This influx of greenhouse gases has led to the intensification of the greenhouse effect, resulting in global warming, altered weather patterns, and the exacerbation of extreme weather events. The interplay between fossil fuel usage and climate change is irrefutable, as evidenced by rising global temperatures, melting polar ice caps, and disruptions to ecological systems.
The U.S. Energy Information Association (IEA) explains climate change
Although the pursuit of green energy solutions faces numerous challenges, it presents a compelling response to climate change. Green energy, renewable and sustainable, offers a vital pathway to mitigating the adverse impacts of energy production on the environment and climate. By adopting green energy sources, we can significantly reduce greenhouse gas emissions, enhance energy security, and foster economic resilience through the creation of green jobs and industries.
Transitioning to green energy will requires the utilization of many renewable sources including, but not limited to, solar, wind, hydroelectric, and geothermal. These sources will extract all the abundant energy within the environment without diminishing limited resources or adding to the increase in greenhouse gases. Furthermore, energy storage technology and upgraded grid infrastructure facilitate integration of renewable energy sources into conventional systems to further improve reliability and scalability.
Method
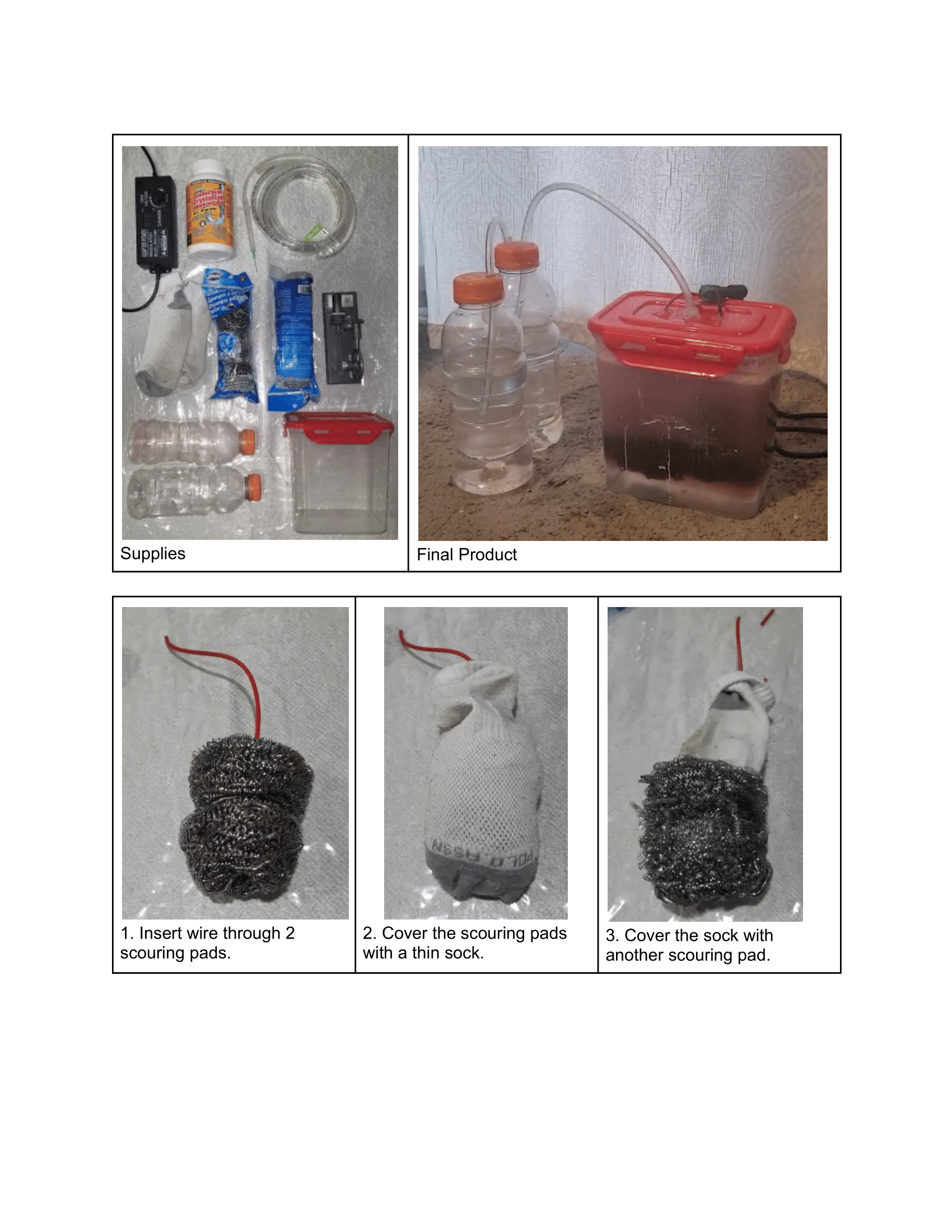
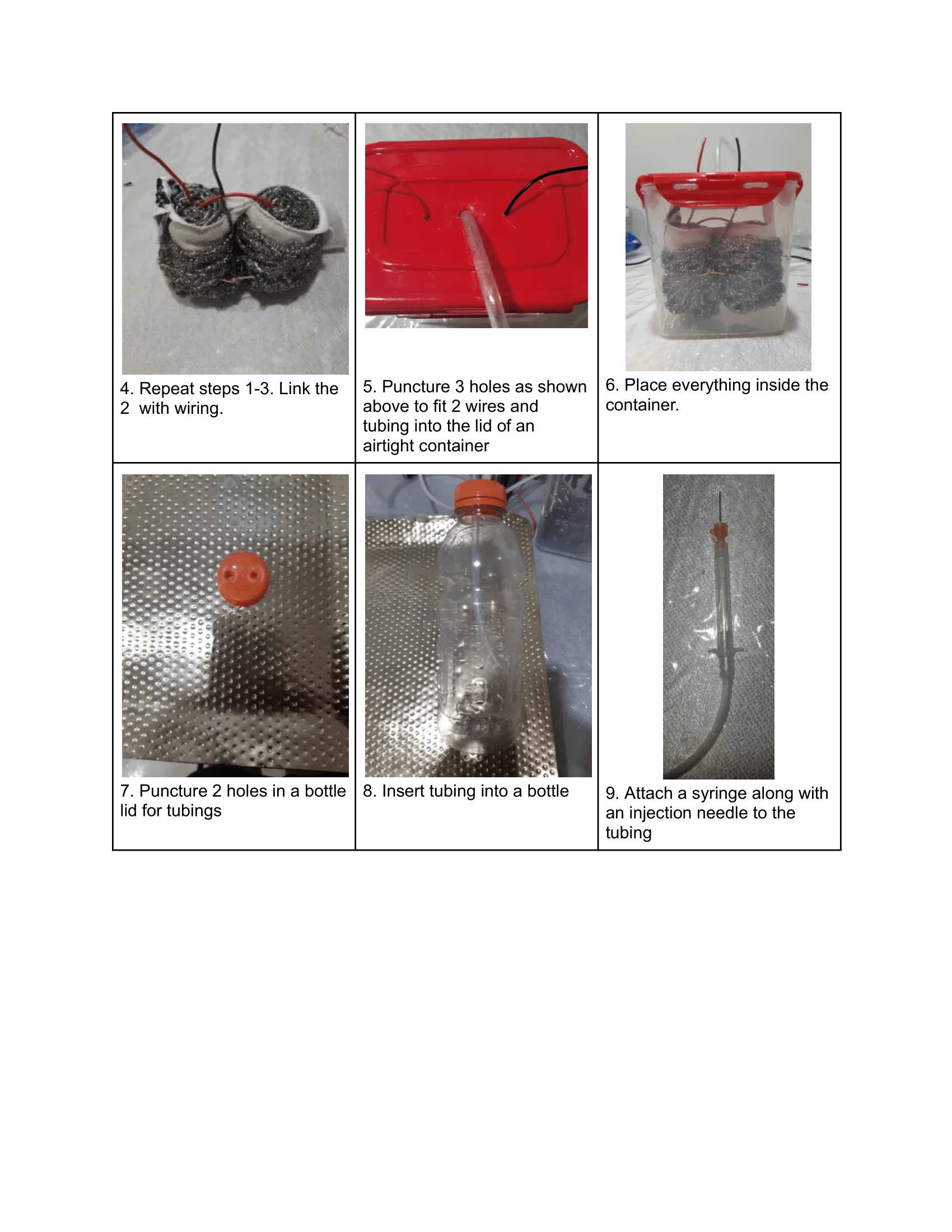
Analysis
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive a chemical reaction. Electrolysis is important as it is used for the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so electrolysis would mean "breakdown via electricity."
Electrolysis of Water
Electrolysis is the process of using electricity to break down compounds, and water (H₂O) can be split into hydrogen (H₂) and oxygen (O₂) gases through electrolysis. The setup for this process includes two electrodes, an anode and a cathode. A power supply powers the electrolysis process with sodium hydroxide (KOH) added to the water to form an electrolyte.
A diagram showing how to set up an electrolytic cell.
At the anode, hydroxide ions are oxidized to produce oxygen gas, while at the cathode, water is reduced to produce hydrogen gas. The reaction is nonspontaneous and requires energy input to occur. The process relies on these two electrodes for oxidation and reduction.
|
Electrolysis in Water:
|
Electrolytes:
- Ionic compounds contain ions. When ionic compounds are melted or dissolved in water, the ions in the compound are free to move about in the liquid or solution. These ions can be positively charged or negatively charged. When ions move, they can conduct electricity, therefore creating a current. These molten and aqueous solutions are known as electrolytes.
- Sodium Hydroxide: Sodium hydroxide is a strong base and is caustic. When mixed with water, it provides ions in the water that conduct electricity for electrolysis to occur.
- Industrial Uses of Electrolysis: Electrolysis has several important industrial applications, including the extraction and refining of metals, such as aluminum and copper, respectively. It is used in the production of chemicals like caustic soda and chlorine gas, as well as hydrogen. The process also plays a key role in electroplating and electroforming, which involve coating objects with a thin layer of metal and creating exact metal replicas, respectively. Additionally, electrolysis is used in electrotyping to reproduce printing plates and in electro-cleaning to remove rust and contaminants from metal surfaces.
The Invention of Electrolysis (of Water):
In 1785 a Dutch scientist named Martin van Marum created an electrostatic generator that he used to reduce tin, zinc and antimony from their salts using a process later known as electrolysis. Though he unknowingly produced electrolysis, it was not until 1800 that William Nicholson and Anthony Carlisle discovered how electrolysis works.
In the early nineteenth century, William Nicholson and Anthony Carlisle sought to further Volta's experiments. They attached two wires to either side of a voltaic pile and placed the other ends in a tube filled with water. They noticed when the wires were brought together that each wire produced bubbles. One type was hydrogen, the other was oxygen.
Volta’s Experiments:
Alessandro Giuseppe Antonio Anastasio Volta invented the voltaic pile in 1799 and reported the results of his experiments in a two-part letter to the president of the Royal Society, which was published in 1800. With this invention, Volta proved that electricity could be generated chemically and debunked the prevalent theory that electricity was generated solely by living beings. Volta's invention sparked a great amount of scientific excitement and led others to conduct similar experiments, which eventually led to the development of the field of electrochemistry.
Voltaic Pile
The voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit. It was invented by Italian chemist Alessandro Volta, who published his experiments in 1799. Its invention can be traced back to an argument between Volta and Luigi Galvani, Volta's fellow Italian scientist who had conducted experiments on frogs' legs.
The use of the voltaic pile enabled a rapid series of other discoveries, including the electrical decomposition (electrolysis) of water into oxygen and hydrogen by William Nicholson and Anthony Carlisle.
A voltaic pile on display in the Tempio Voltiano (the Volta Temple) near Volta's home in Como, Italy
Hydrogen Fuel
Hydrogen fuel is a clean and sustainable energy source that has the potential to significantly reduce greenhouse gas emissions. When hydrogen is used in a fuel cell, it produces only water as a byproduct, making it an environmentally friendly alternative to fossil fuels. Hydrogen can be produced from various domestic resources, including natural gas, nuclear power, biomass, and renewable energy sources like solar and wind. This versatility makes hydrogen an attractive option for a wide range of applications, including transportation, electricity generation, and home heating.
The production of hydrogen can be achieved through several methods, such as natural gas reforming, electrolysis, and solar-driven processes. Natural gas reforming involves reacting steam with hydrocarbons to produce hydrogen, while electrolysis uses an electric current to split water into hydrogen and oxygen. Solar-driven processes utilize sunlight to drive chemical reactions that produce hydrogen.
Natural Gas Reforming
The majority of hydrogen generated in the US today is created using the well-established steam-methane reforming process, which uses high-temperature steam (700°C to 1,000°C) to create hydrogen from a methane source, like natural gas. In steam-methane reforming, methane and steam combine with a catalyst at pressures ranging from 3 to 25 bar (1 bar = 14.5 psi) to create hydrogen, carbon monoxide, and a small quantity of carbon dioxide. Steam reforming is endothermic, meaning that for the reaction to occur, heat must be applied to the process.
Although today most hydrogen is produced from natural gas, the Hydrogen and Fuel Cell Technologies Office is exploring a variety of ways to produce hydrogen from renewable resources.
Solar Driven Processes
Light is the agent used in solar-powered procedures to produce hydrogen. Several processes, such as photobiological, photoelectrochemical, and solar thermochemical, are powered by the sun. Hydrogen is produced by photobiological processes using the natural photosynthetic activity of bacteria and green algae. Specialized semiconductors are used in photoelectrochemical processes to split water into hydrogen and oxygen. Concentrated solar energy is used to power water splitting reactions in solar thermochemical hydrogen generation, frequently along with additional species like metal oxides.
The photobiological hydrogen production process uses microorganisms and sunlight to turn water, and sometimes organic matter, into hydrogen.
Industrial Uses for Hydrogen Fuel
Hydrogen fuel is utilized in various sectors due to its clean energy properties and efficiency. In the transportation sector, hydrogen fuel cells power buses, trucks, trains, and ships, offering long-range travel and quick refueling. In industries, hydrogen is used as a feedstock in processes like steel production, ammonia synthesis, and petroleum refining. Additionally, hydrogen fuel can serve as a backup power source for critical infrastructure, such as hospitals and data centers, ensuring reliable energy supply during outages.
Hydrogen Vehicles
Hydrogen cars, such as the Toyota Mirai and Hyundai Nexo, are equipped with hydrogen fuel cells that convert hydrogen into electricity to power an electric motor. These vehicles emit only water vapor, making them an environmentally friendly alternative to traditional combustion engines. While hydrogen cars are still relatively rare due to the lack of widespread refueling infrastructure and higher costs compared to battery-electric vehicles, they represent a promising technology for reducing greenhouse gas emissions in the transportation sector.
|
Toyota Mirai
The 2nd generation of Toyota’s hydrogen fuel cell cars. It provides a range of up to 647 KM on a single tank of hydrogen, while emitting only water in passing. |
Hyundai Nexo
The first automotive manufacturer to make a hydrogen fuel cell EV available to the Canadian public. The Nexo is an SUV with up to 612 km of range in just 5 minutes (of refuelling) and emits water and purified air. |
While less notable, there are other hydrogen powered cars, which use a hydrogen combustion engine rather than hydrogen fuel cells. Similar to the Hyundai Nexo and Toyota Mirai, these vehicles emit only water vapor, in addition to nitrogen oxide. These cars are less popular in the consumer market, but they stand as a testament of the unique uses that hydrogen offers as opposed to traditional fossil fuels and electricity. Some notable examples include the Toyota Corolla Cross H2 and the BMW Hydrogen 7
|
Toyota Corolla Cross H2 The Toyota Corolla Cross H2, while similar to the Toyota Mirai, features some notable differences, including the modified engine. It boasts a refueling time of just 1 and a half minutes. |
BMW Hydrogen 7 The BMW hydrogen combustion series is another example of a hydrogen combustion vehicle. This is the company's first attempt at producing a hydrogen car, and while only a concept, it represents BMW’s commitment in searching for a sustainable energy source. |
Stirling Engine
In the Stirling engine, a working fluid (e.g. air) is heated by energy supplied from outside the engine's interior space (cylinder). As the fluid expands, mechanical work is extracted by a piston, which is coupled to a displacer. The displacer moves the working fluid to a different location within the engine, where it is cooled, which creates a partial vacuum at the working cylinder, and more mechanical work is extracted. The displacer moves the cooled fluid back to the hot part of the engine, and the cycle continues.
Robert Stirling was a Scottish clergyman and engineer. He invented the Stirling engine and was inducted into the Scottish Engineering Hall of Fame in 2014.
The Stirling Cycle, by Nik Schulz
Conclusion
In conclusion, our project successfully created a significant amount of hydrogen gas using our HHO generator. Our objective to use the hydrogen to create a small and controlled flamer to then utilize the high burning temperatures (of hydrogen) to power a Stirling engine was only partly accomplished. While it was possible to ignite the hydrogen flame, it was challenging to maintain as it would burn back into the flashback arrestors within a very brief time. This limitation prevented the continuous operation of the Stirling engine.

Despite all this, the project provided wonderful insight into the reality and feasibility of hydrogen fuel. It raised significant questions regarding improved flame control and safety protocols for future endeavours. With a bit more time, we are confident that we would have sorted out better how to proceed and operate the Stirling engine for extended periods of uninterrupted power. Overall, the project showed the potential along with the pitfalls of using hydrogen as an energy solution, and we hope there will be further investigation and progress, either by our hands or by others.
Citations
- YouTube. "Electrolysis of Water - Electrochemistry" YouTube, 2019, www.youtube.com/watch?v=zMLNHm4nUCQ.
- "Stoichiometry." Wikipedia, Wikimedia Foundation, 13 Feb. 2025, en.wikipedia.org/wiki/Stoichiometry.
- "Oxyhydrogen." Wikipedia, Wikimedia Foundation, 13 Feb. 2025, en.wikipedia.org/wiki/Oxyhydrogen.
- Labster. "Electrolysis." Labster, theory.labster.com/electrolysis/.
- "Electrolysis." Wikipedia, Wikimedia Foundation, 13 Feb. 2025, en.wikipedia.org/wiki/Electrolysis.
- "Alkaline Water Electrolysis." Nel Hydrogen, Nel ASA, 13 Feb. 2025, nelhydrogen.com/glossary/alkaline-water-electrolysis/#:~:text=Potassium%20hydroxide%20is%20a%20strong,one%20electrode%20to%20the%20other.
- "Voltaic Pile." Wikipedia, Wikimedia Foundation, 13 Feb. 2025, en.wikipedia.org/wiki/Voltaic_pile.
- "Alessandro Volta." Wikipedia, Wikimedia Foundation, 13 Feb. 2025, en.wikipedia.org/wiki/Alessandro_Volta.
- "Greenhouse Gases." U.S. Energy Information Administration, 13 Feb. 2025, www.eia.gov/energyexplained/energy-and-the-environment/greenhouse-gases.php.
- "Keeling Curve." National Geographic Education, 13 Feb. 2025, education.nationalgeographic.org/resource/keeling-curve/.
- "Hydrogen Fuel Basics." U.S. Department of Energy, 13 Feb. 2025, www.energy.gov/eere/fuelcells/hydrogen-fuel-basics.
- "Hydrogen Production: Natural Gas Reforming." U.S. Department of Energy, 13 Feb. 2025, www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming.
- "Hydrogen Production: Photobiological." U.S. Department of Energy, 13 Feb. 2025, www.energy.gov/eere/fuelcells/hydrogen-production-photobiological.
- "Toyota Mirai Overview." Toyota Canada, 13 Feb. 2025, www.toyota.ca/toyota/en/vehicles/mirai/overview.
- "2024 Hyundai Nexo." Hyundai Canada, 13 Feb. 2025, www.hyundaicanada.com/en/vehicles/2024-nexo.
- "Prototype Corolla Cross Hydrogen Concept." Toyota Europe, 13 Feb. 2025, www.toyota-europe.com/news/2022/prototype-corolla-cross-hydrogen-concept.
- "BMW iX5 Hydrogen." BMW USA, 13 Feb. 2025, www.bmwusa.com/ix5-hydrogen.html.
- "Stirling Engine." Wikipedia, Wikimedia Foundation, 13 Feb. 2025, en.wikipedia.org/wiki/Stirling_engine.
- "Two-Can Stirling Engine." Make:, Makezine.com, 13 Feb. 2025, makezine.com/projects/two-can-stirling-engine/.
Acknowledgement
We would like to thank from the bottom of our hearts everyone who motivated and supported us while we were making our hydrogen engine prototype. Thanks to our teacher, Ms. Behairy, for her guidelines and valuable tips. We are also so grateful to our parents for their ongoing support and motivation. Last but not least, we would also like to thank our friends and classmates, whose encouragement and belief in us helped turn this project into a reality.

