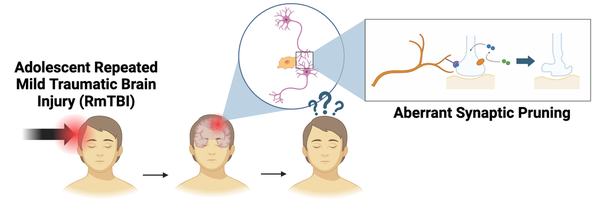
Changes in Complement Protein Expression and Its Role in Microglia-Mediated Synaptic Pruning Following RmTBIs in Adolescent Mice
Wooju Choi
Grade 11
Presentation
Hypothesis
If female adolescent mice either received repeated mild traumatic brain injuries (RmTBI) or sham injuries (no injury), then the mice which received RmTBIs will experience an increase in both C1q and C3 complement protein expression in their motor cortices in relation to the mice which received sham injuries.
Reasoning: Complement expression is expected to increase in order to cause the inflammatory immune response characteristic to TBIs.
Effects: Changes in complement expression may lead to negative changes in microglia-mediated synaptic pruning, which may be responsible for the cognitive deficits observed after a RmTBI.
Research
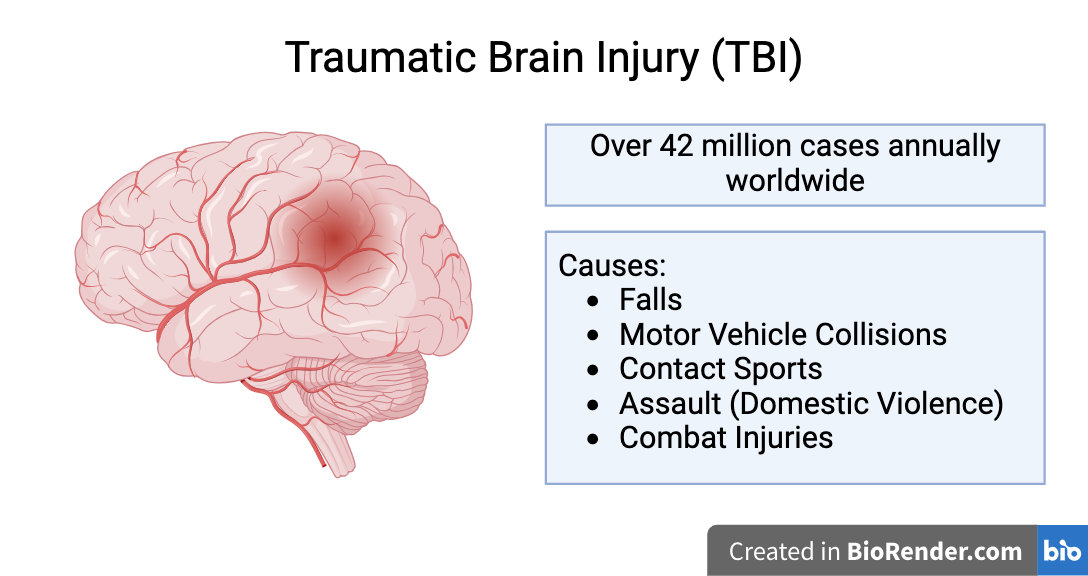
Repeated Mild Traumatic Brain Injury (RmTBI): type of traumatic brain injury (TBI) caused by repeated application of a violent external force to the head
-
Statistics:
-
Over 42 million cases of TBI reported annually worldwide
-
About 80% of TBIs are mTBIs (concussions)
-
High incidence amongst adolescents - 5.5% of adolescents have experienced RmTBIs
-
-
Symptoms: sudden mood changes, confusion, amnesia, attention deficiencies, headaches, dizziness, drowsiness, and insomnia
-
Linked to increased risk of developing neurodegenerative diseases (NDDs) later in life (eg. Alzheimer's disease, Parkinson's disease, chronic traumatic encephalopathy)
-
Pathophysiology:
-
Primary Cascades:
-
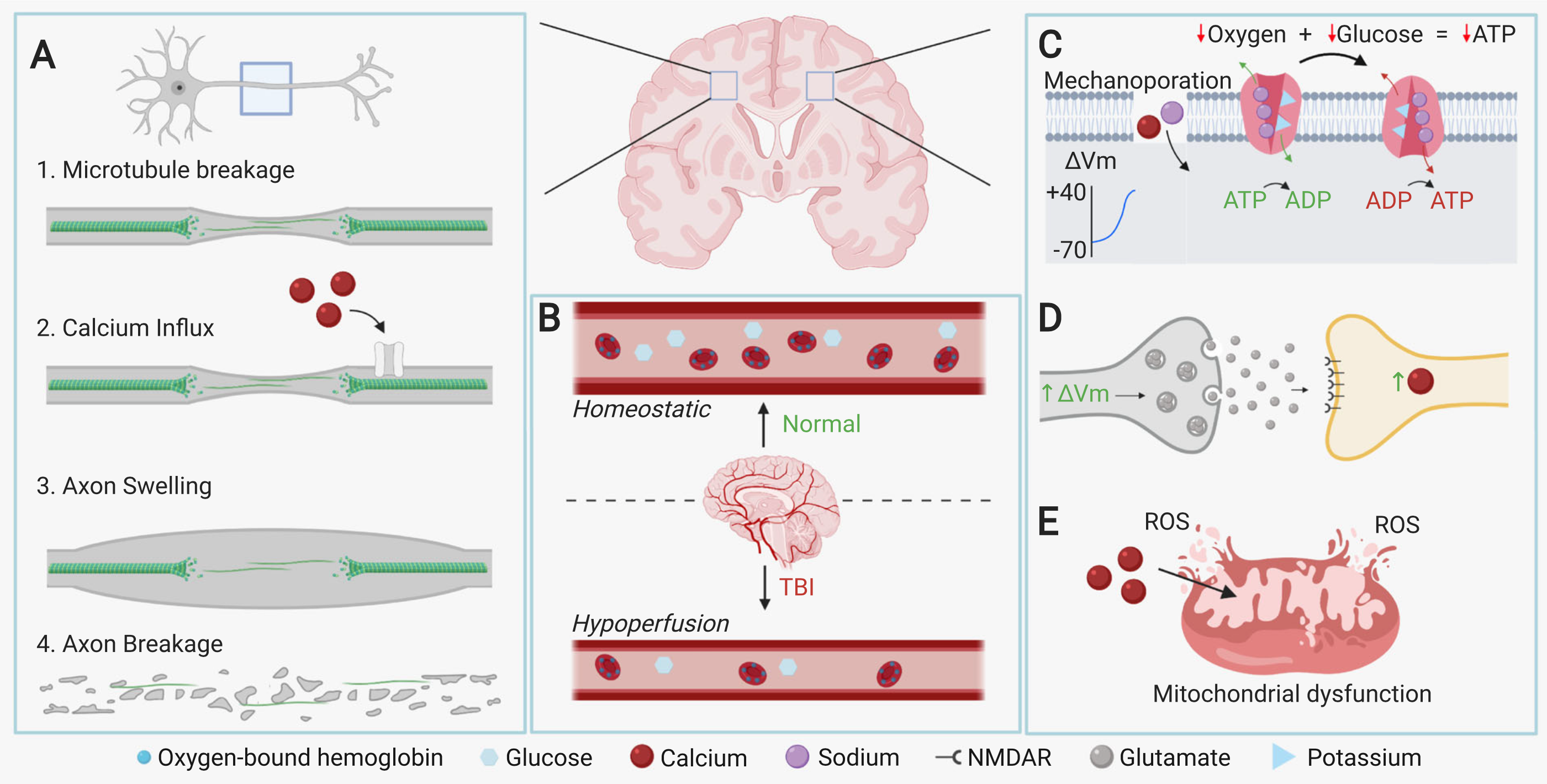
Diffuse Axonal Injury (DAI): different velocities of white matter (neuronal bodies) and grey matter (myelinated axons) upon impact of injury stretches or tears neuron axons, causing neuron death
-
Wallerian Degeneration: axons which are stretched but remain intact experience mechanoporation (creation of holes in membrane), allowing sodium and calcium ions to enter and depolarize the axon, ultimately resulting in apoptosis of the neuron
-
-
Secondary Cascades:
-
Excitotoxicity: depolarization of pre-synaptic neurons cause excessive glutamate neurotransmitter release to the post-synaptic neuron - the post-synaptic neuron subsequently experiences a calcium influx as a result, and the calcium ions either cause mitochondrial dysfunction or the release of cytotoxic molecules, leading to neuron death
-
-
-
Adolescent RmTBIs:
-
Adolescence is a period of critical brain maturation prior to adulthood:
-
Gray matter loss - caused by synaptic pruning (elimination of excess synapses)
-
White matter increase - caused by myelination (formation of myelin sheaths around axon)
-
Neural circuitry refinement
-
-
Adolescents are vulnerable to RmTBIs due to their developing brains:
-
Greater memory deficits
-
Greater risk-taking behaviour
-
Synaptic remodeling (injury response mechanism) impaired by RmTBIs
-
Greater reduction of synaptic density
-
-
Adolescent RmTBIs are overlooked in TBI studies in favour of adults or elderly populations, and are consequently poorly understood.
-
One of the main biological pathways affected by RmTBIs in adolescents is synaptic pruning.
-
Synaptic Pruning: selective destruction of weak or excess synapses by phagocytes
-
The brain overproduces synaptic connections during infancy - synaptic pruning is needed to eliminate weak or excess synapses and strengthen the remaining synapses
-
Synaptic pruning peaks during infancy to adolescence, decreases during adulthood, and returns with NDDs
-
Plays a critical role in adolescent neural maturation - responsible for gray matter loss and reduction in electrical activity during adolescence
-
Key components of synaptic pruning: microglia (phagocyte), complement proteins (opsonins)
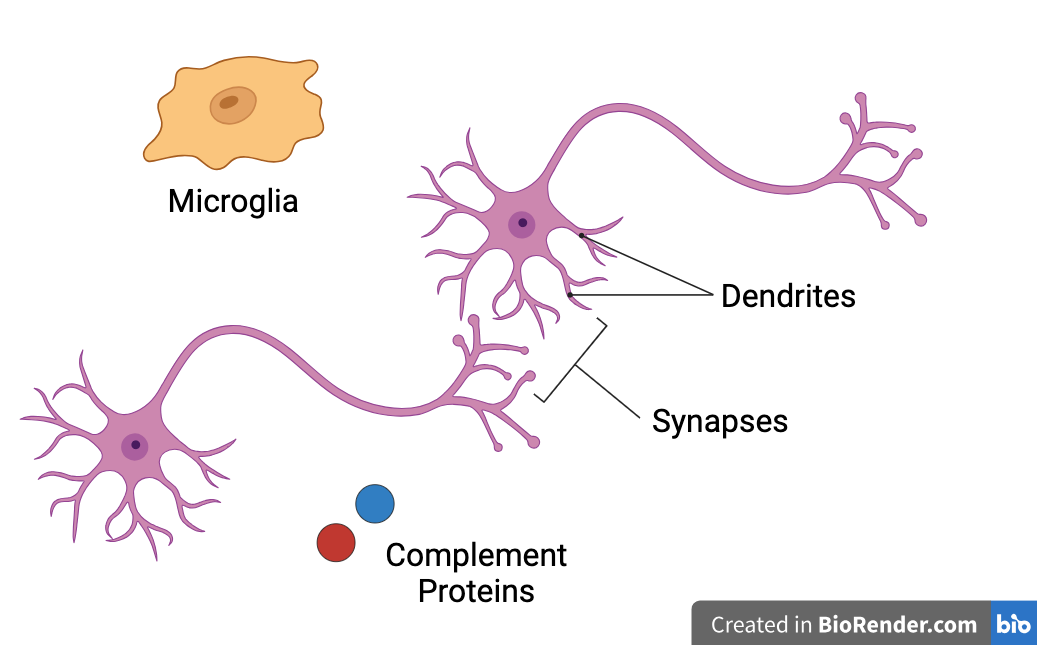
Microglia: glial cells which serve as resident immune cells of the brain
-
Circulatory immune cells cannot enter the brain under homeostatic conditions due to the blood-brain barrier (BBB) - resident microglia are needed to serve as immune cells
-
Microglia response upon infection or injury:
-
Damaged cells release damaged-associated molecular patterns (DAMPs)
-
DAMPs activate microglia from their ramified state to their amoeboid state
-
Amoeboid microglia phagocytose pathogens or damaged cells and trigger or prevent inflammation (depending on phenotype)
-
-
Microglia also phagocytose opsonized (tagged) synapses during synaptic pruning
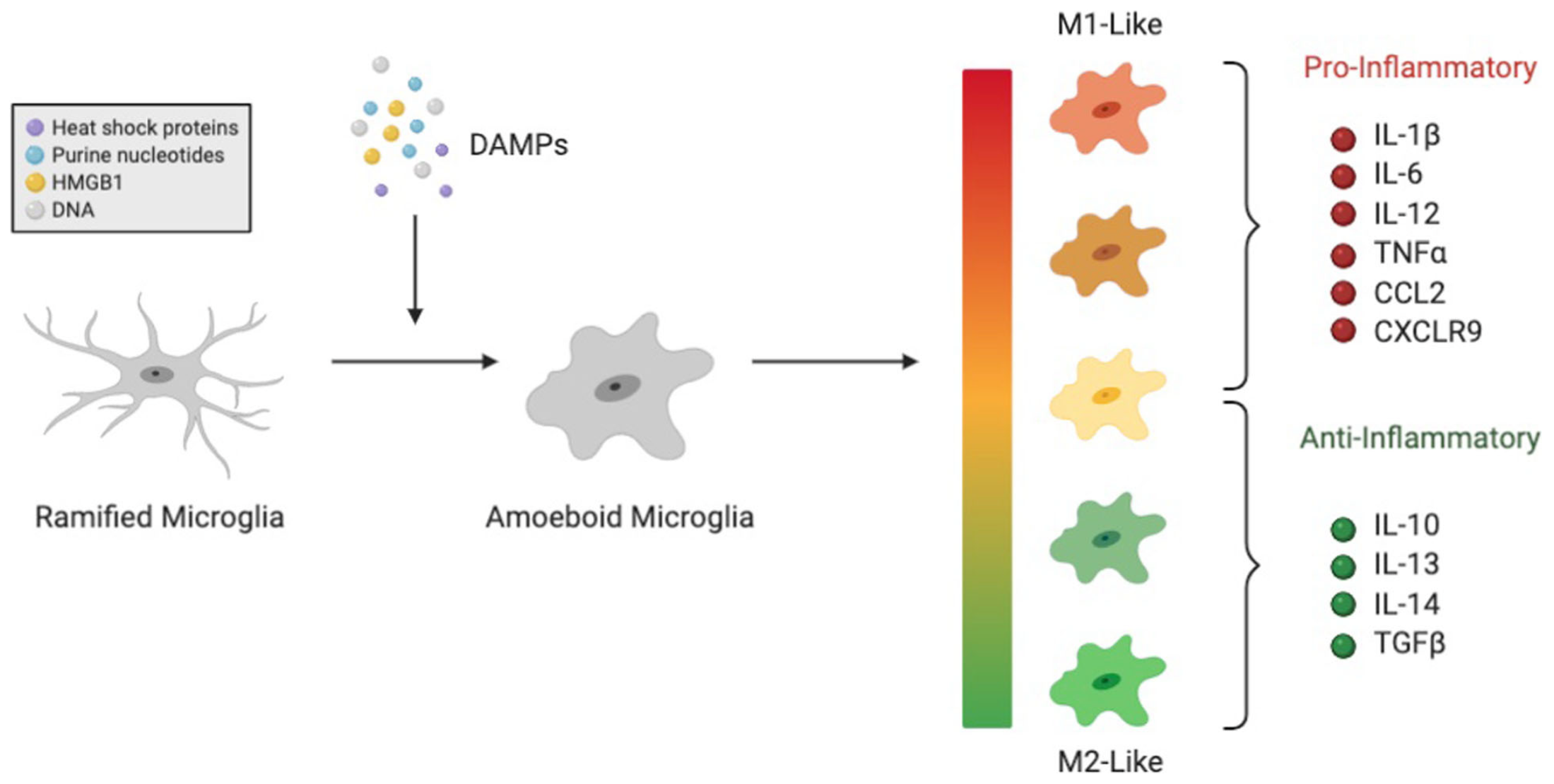
Complement System: set of plasma proteins which opsonize (tag) pathogens for destruction and induce inflammation as part of the body's innate immune system
-
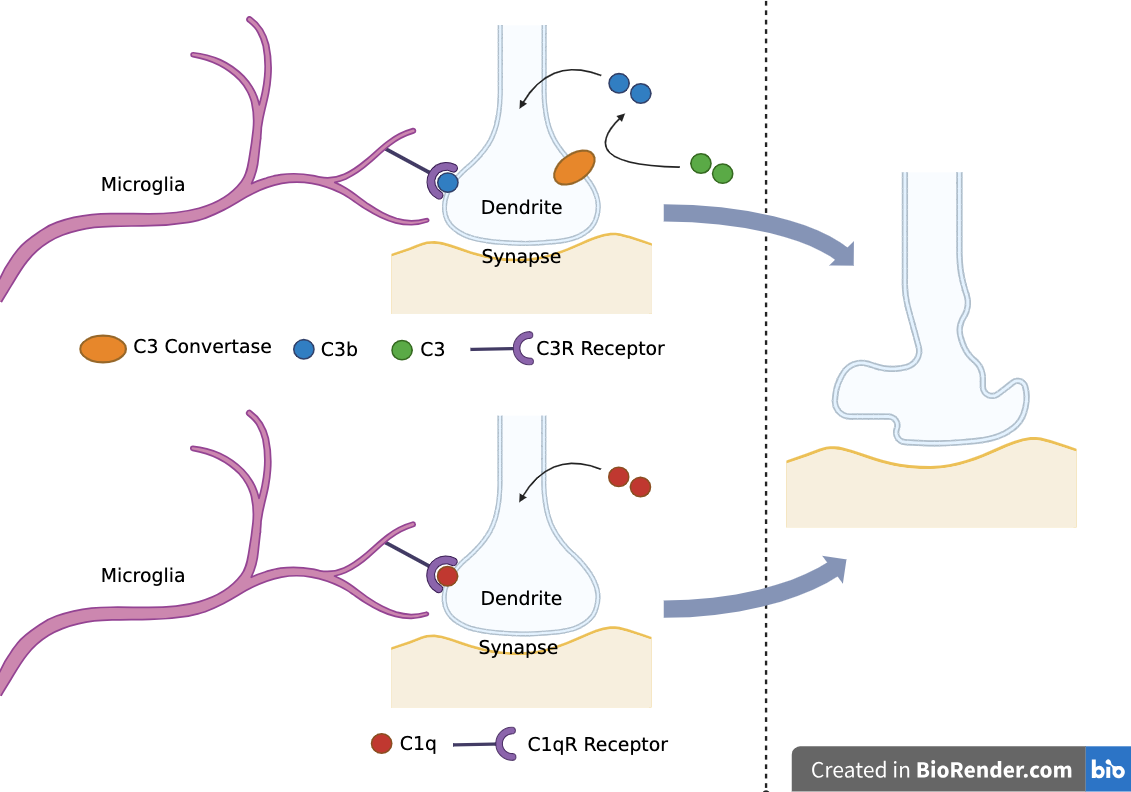
Complement proteins C1q and C3 opsonize synapses for microglia-mediated synaptic pruning
-
Complement system can be activated by classical, lectin, or alternative pathways (only classical pathway thought to be involved in synaptic pruning)
-
C1q:
-
Initiates classical complement activation pathway
-
Opsonizes synapses for pruning - C1qR receptors on microglia recognize C1q proteins
-
-
C3:
-
Cleaved by C3 convertase into C3a and C3b
-
C3a: causes inflammatory response
-
C3b: opsonizes synapses for pruning - C3R receptors on microglia recognize C3b proteins
-
-
-
C1q and C3 are synthesized by neurons in the central nervous system
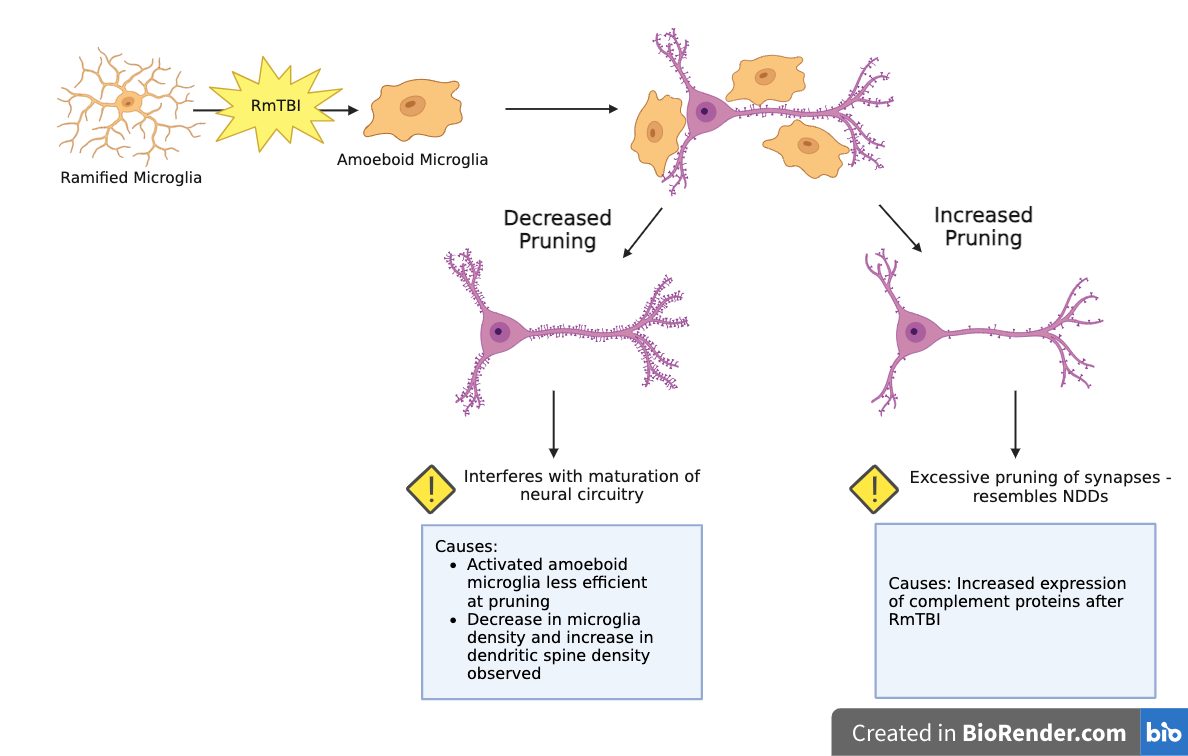
Effect of RmTBIs on Microglia-Mediated Synaptic Pruning in Adolescents:
-
RmTBIs can cause increased or decreased microglia-mediated synpatic pruning:
-
Increased Pruning:
-
Potential Causes: Increased expression of complement proteins after RmTBI
-
Effects: Excessive pruning resembles synapse loss observed in NDDs
-
-
Decreased Pruning:
-
Potential Causes:
-
Activated amoeboid microglia less efficient at pruning than dormant ramified microglia
-
Past studies observed decrease in microglia density and increase in dendritic spine density in the motor cortex (MC)
-
-
Effects: Interferes with maturation of neural circuitry in adolescents
-
-
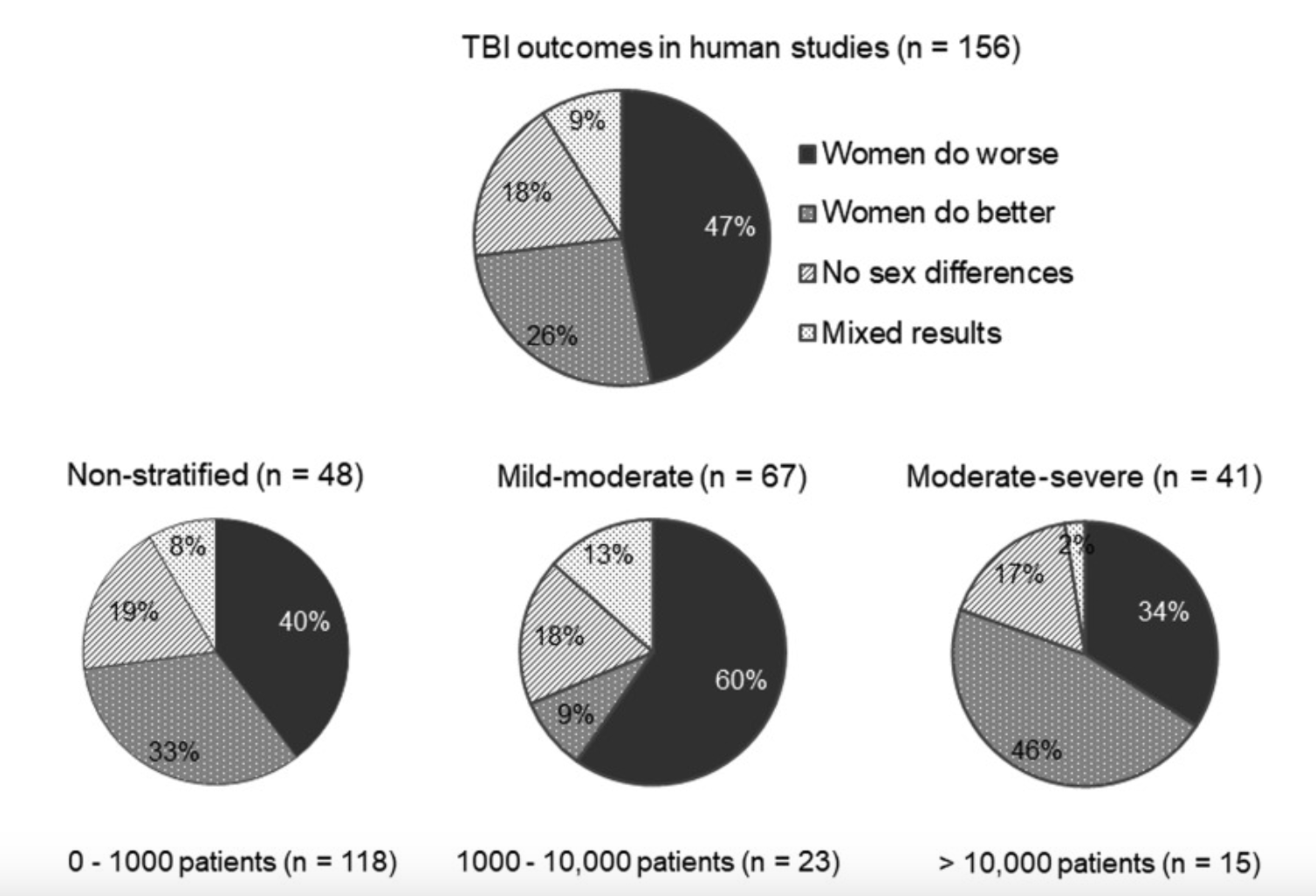
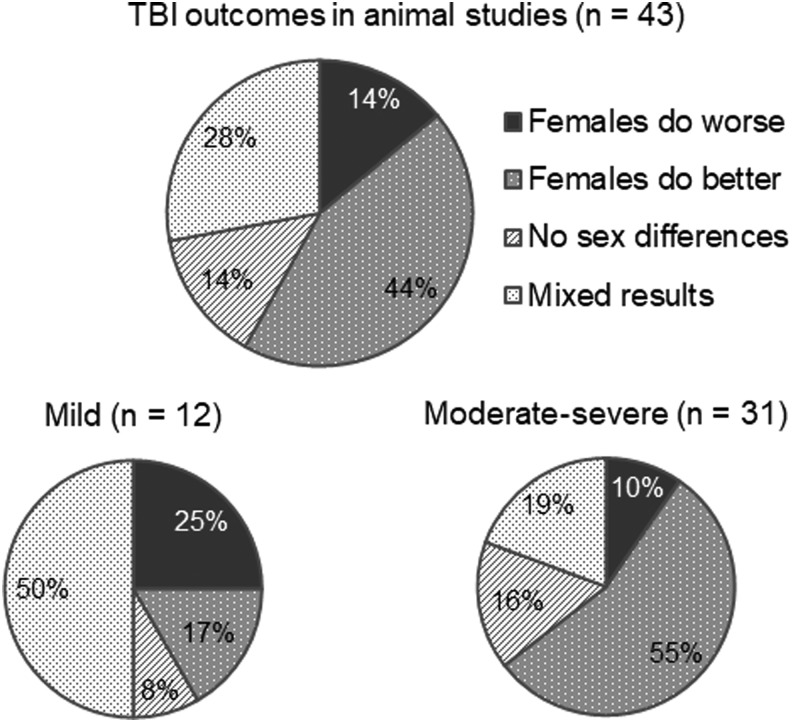
Sex Differences in RmTBI Response:
-
There has been observed to be a sexually dimorphic response to TBIs between males and females
-
Males: greater microglia loss, more severe motor deficits
-
Females: increased loss of consciousness
-
-
However, findings have varied between studies:
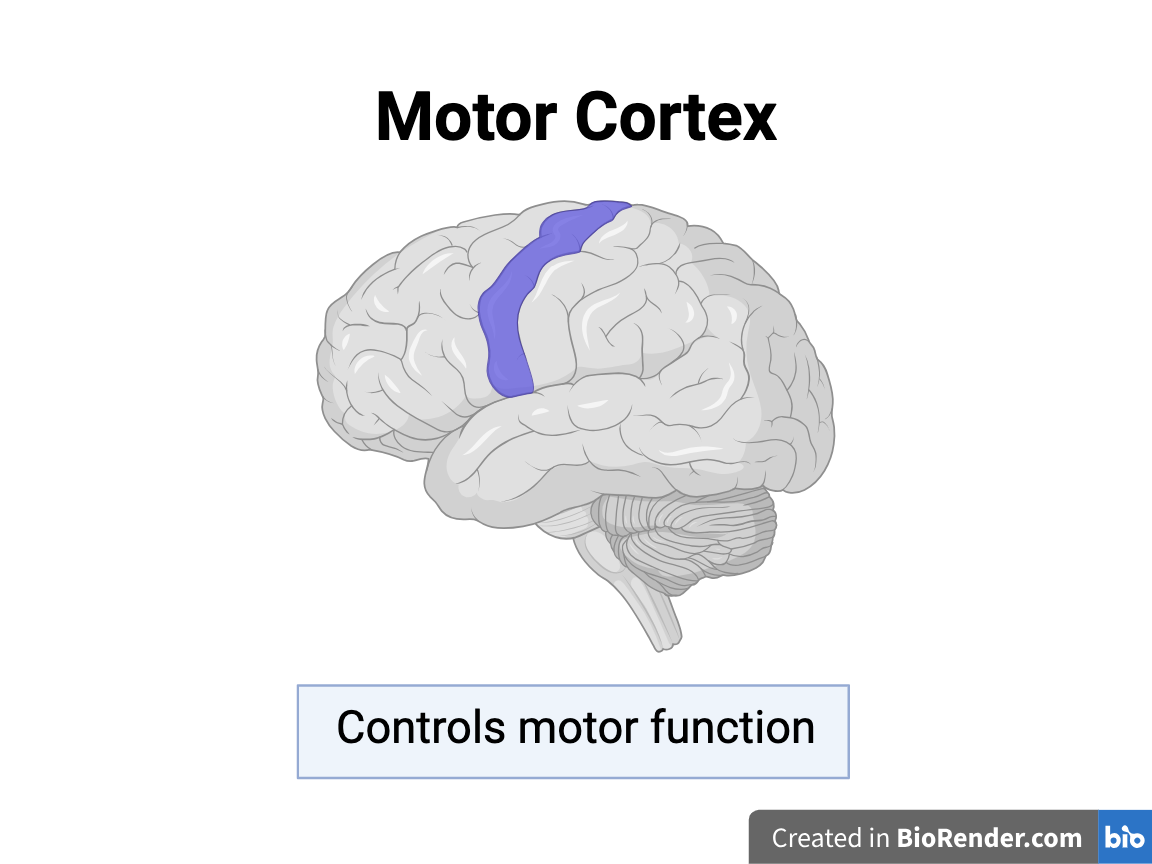
The Motor Cortex:
-
Past studies have observed a decrease in microglia density and an increase in dendritic spine density in the motor cortices of adolescent mice after RmTBIs, which may be caused by changes in synaptic pruning.
In this experiment, I investigated whether RmTBIs can cause changes to the expression of complement proteins C1q and C3 in the motor cortices of female adolescent mice. Changes to complement expression may be responsible for changes in microglia-mediated synaptic pruning, which may subsequently cause the cognitive deficits observed following RmTBIs.
-
To my knowledge, few previous studies have observed changes in complement expression after brain injuries, and no previous studies have observed changes in complement expression after adolescent brain injuries
*Male adolescent mice are undergoing experimentation as of 15 March, 2024. Results from male mice models will be incorporated into the project for the presentation on 12 April, 2024.
Variables
Independent Variable: Presence of a RmTBI in female adolescent mice compared to female adolescent mice subjected to sham injury
Dependent Variable: Number and density of C3 and C1q complement proteins in motor cortex of female adolescent mice after receving a RmTBI or sham inbjury
Controlled Variables:
-
Speed of projectile fired at heads of mice in RmTBI model (5m/s ± 0.2m/s)
-
Head position of mice in RmTBI model (the Lateral Impact Model/LIM)
-
Frequency of injury in RmTBI model (5 injuries/24 hours)
-
Strain of mice (C57BL/6 mice)
-
Gender of mice (female)
-
Age of mice (adolescent/P48)
-
Antibody dilutions in antibody solution
-
Imaging parameters for fluorescence microscopy
Confounding Variables:
-
Anesthesia of mice prior to injury
-
Stress level of mice
-
Natural variation between mice
Procedure
Goal: Calculate density of C1q and C3 complement protein expression in motor cortices of female adolescent mice subjected to RmTBI or sham injury
Animals:
-
Nine female adolescent (P48) C57BL/6 mice
-
RmTBI treatment group: 5 mice
-
Sham injury treatment group: 4 mice
-
Injury Delivery:
RmTBI - Lateral Impact Model (LIM):
-
Mouse anestheized and placed inside Gothenburg Impactor Device
-
50g cylindrical projectile fired at head of mouse five times at 24 hour intervals (speed of projectile: 5m/s ± 0.2m/s)
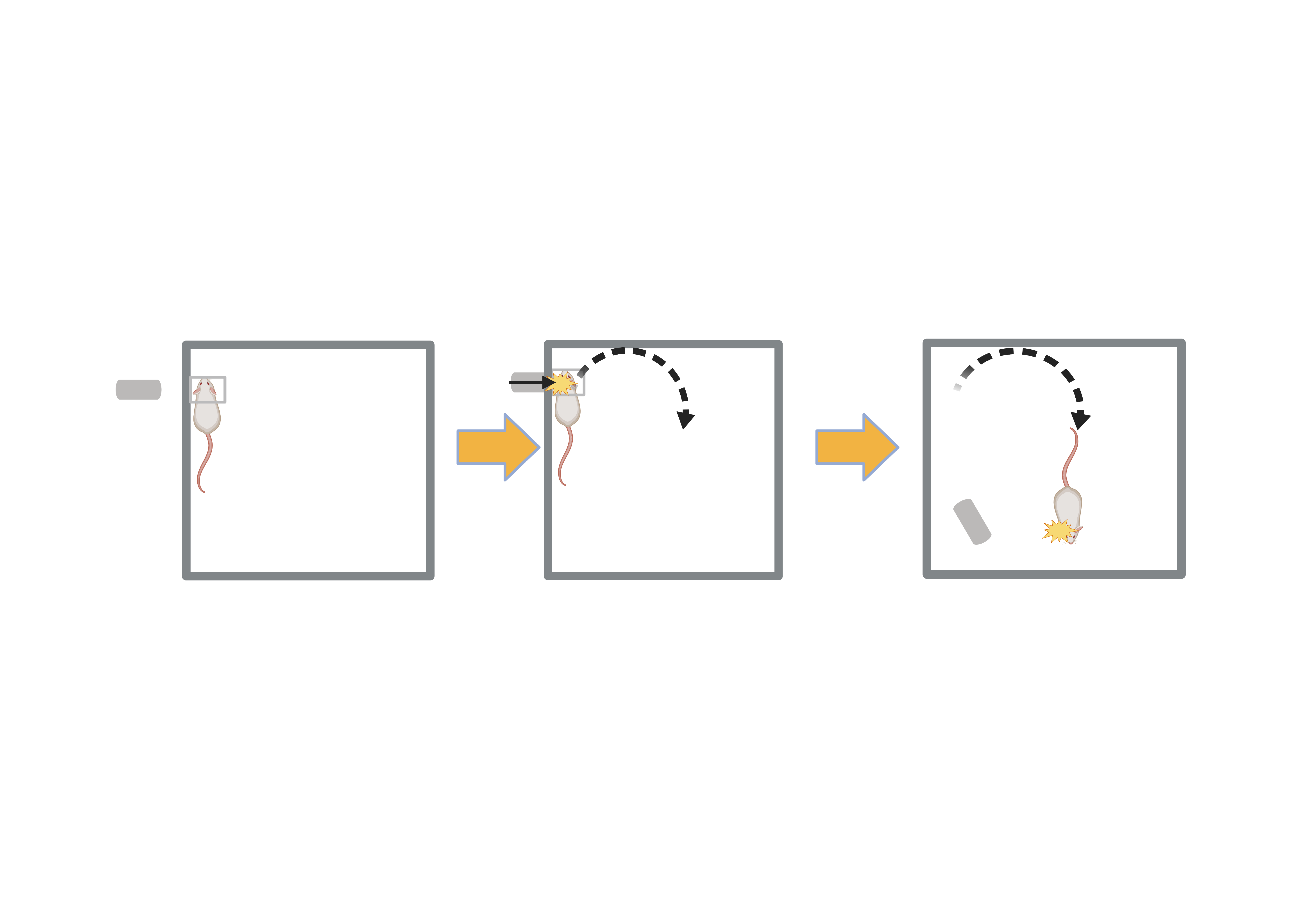

Sham Injury: Mouse anestheized and placed in impactor device but projectile not fired (intended to simulate similar environment as RmTBI mice to minimize effects of stress as a confounding variable)
Tissue Fixation:
-
Mice euthanized five days after injury
-
Transcardial Perfusion: phosphate-buffered saline (PBS) pumped through mice to remove blood
-
4% paraformaldehyde perfused through mice to avoid tissue degeneration in future
-
Brains removed and stored in 30% sucrose solution at 4°C
Cryosectioning:
-
Brain frozen onto circular stand compatible with cryostat using optimal cutting temperature (OCT) compound
-
Stand placed inside Leica HM550 cryostat and handle rotated clockwise to cut brain into 40μm-thick slices
-
Brain slices stored in antifreeze solution at 20°C
-
Three brain slices obtained from each mouse
Immunohistochemistry - Indirect Immunofluorescence:
-
Immunohistochemistry is a staining technique which utilizes specific binding between antigens and antibodies to visualize target antigens
-
Indirect Immunofluorescence:
-
Antigen (in this case, the complement proteins) is tagged using a primary antibody
-
Primary antibody is tagged using a fluorophore-conjugated secondary antibody - the fluorophore allows the antigen to be visualized under a microscope
-
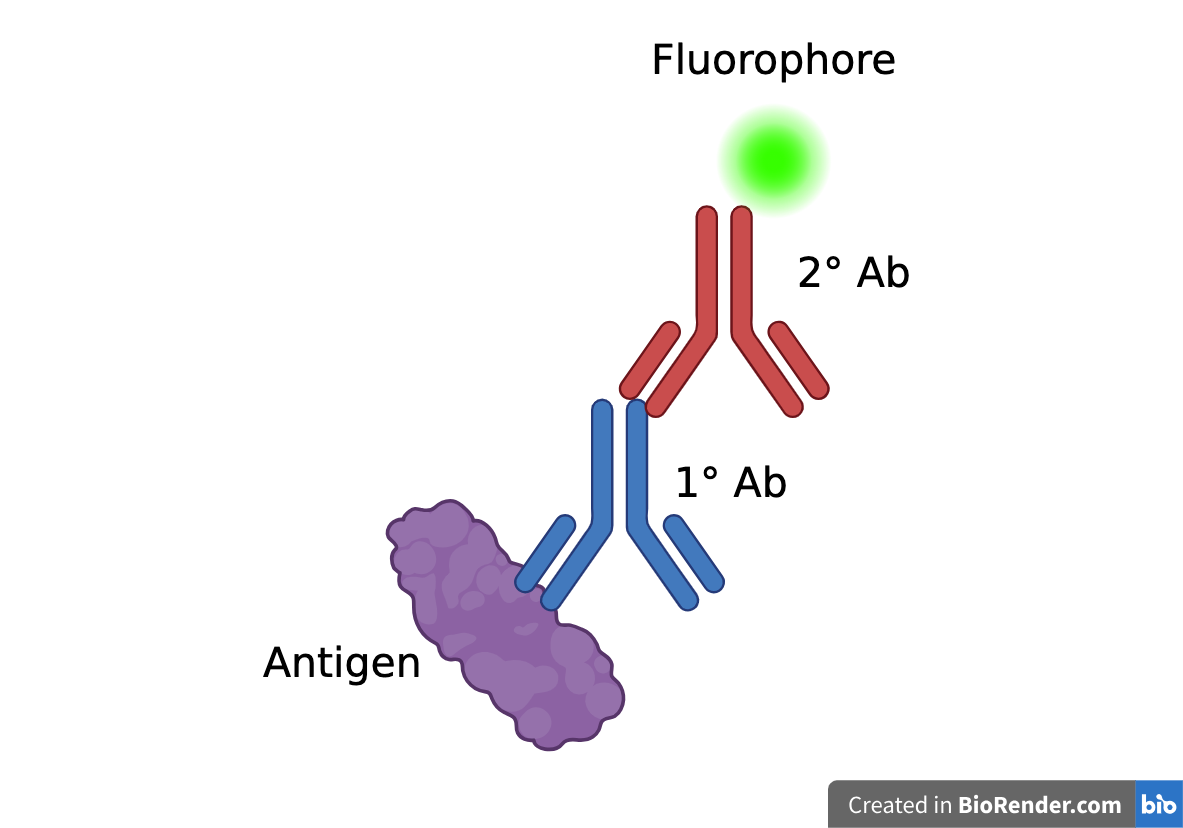
-
Complement Protein (Antigen) → Primary Antibody → Secondary Antibody + Fluorophore
-
C1q → rabbit ɑ-C1q → donkey ɑ-rabbit + AF568
-
C3 → goat ɑ-C3 → donkey ɑ-goat + AF647
-
-
Blocking: brain slices incubated in blocking solution to block non-specific binding sites on non-target antigens which antibodies may erroneously bind to
-
Blocking solution: PBS, 10% donkey serum, 5% bovine serumm albumin (BSA), cold fish skin gelatin, 0.25% triton X-100
-
-
Primary Antibody Incubation: brain slices incubated in primary antibody solution (contains primary antibodies) and then washed in PBS
-
Primary antibody solution: PBS, 5% BSA, cold fish skin gelatin, 0.25% triton X-100
-
Antibody dilutions for C1q and C3:
-
C1q: 1:100
-
C3: 1:250
-
-
-
-
Secondary Antibody Incubation and DAPI Counterstaining: brain slices incubated in secondary antibody solution (contains secondary antibodies and DAPI counterstain) and washed in PBS
-
Secondary antibody solution: PBS, 5% BSA, cold fish skin gelatin, 0.25% triton X-100
-
Antibody dilutions for C1q and C3:
-
C1q: 1:500
-
C3: 1:500
-
-
DAPI counterstain dilution (same for both C1q and C3): 1:1000
-
-
DAPI counterstain provides contrast to principal stain by staining cell nuclei, allowing for easier visualization of principal stain under microscope
-
Mounting: six brain slices mounted onto each slide
Imaging:
-
Slides placed inside Zeiss Celldiscoverer 7 confocal fluorescence microscope
-
Motor cortex delineated using tiling function in each brain slice
-
Confocal z-stacking (collection of 2D images from multiple focal planes to create 3D image) performed at 1.5μm intervals
-
3D images underwent maximum intensity projection to collapse into 2D image and exported as TIFF files
-
Each brain slice produced three images (C1q, C3, and DAPI)

Image Analysis:
-
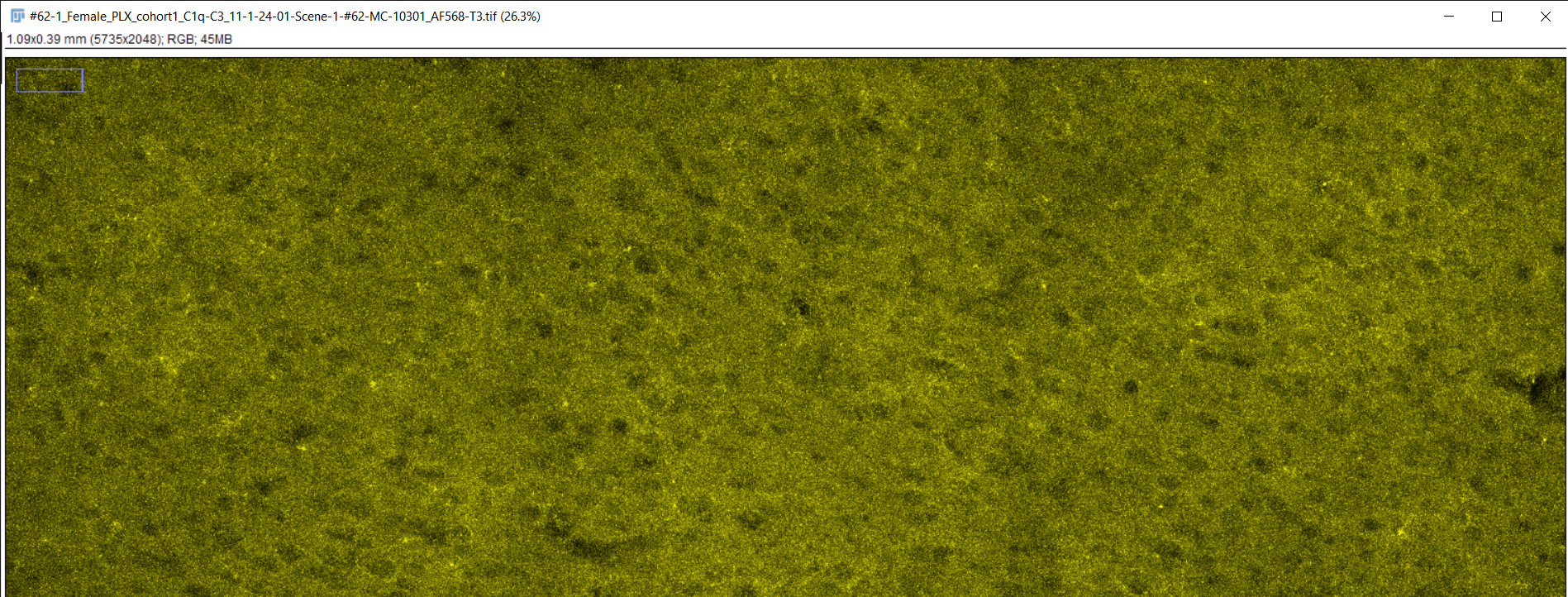
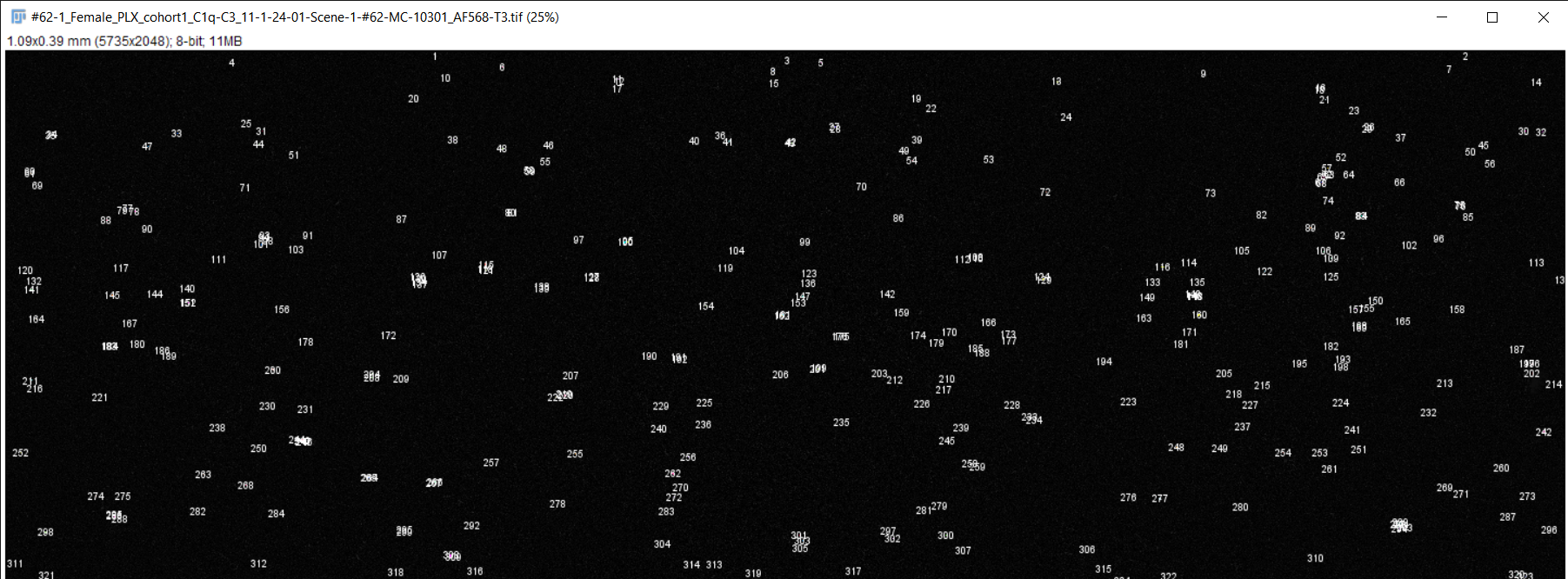
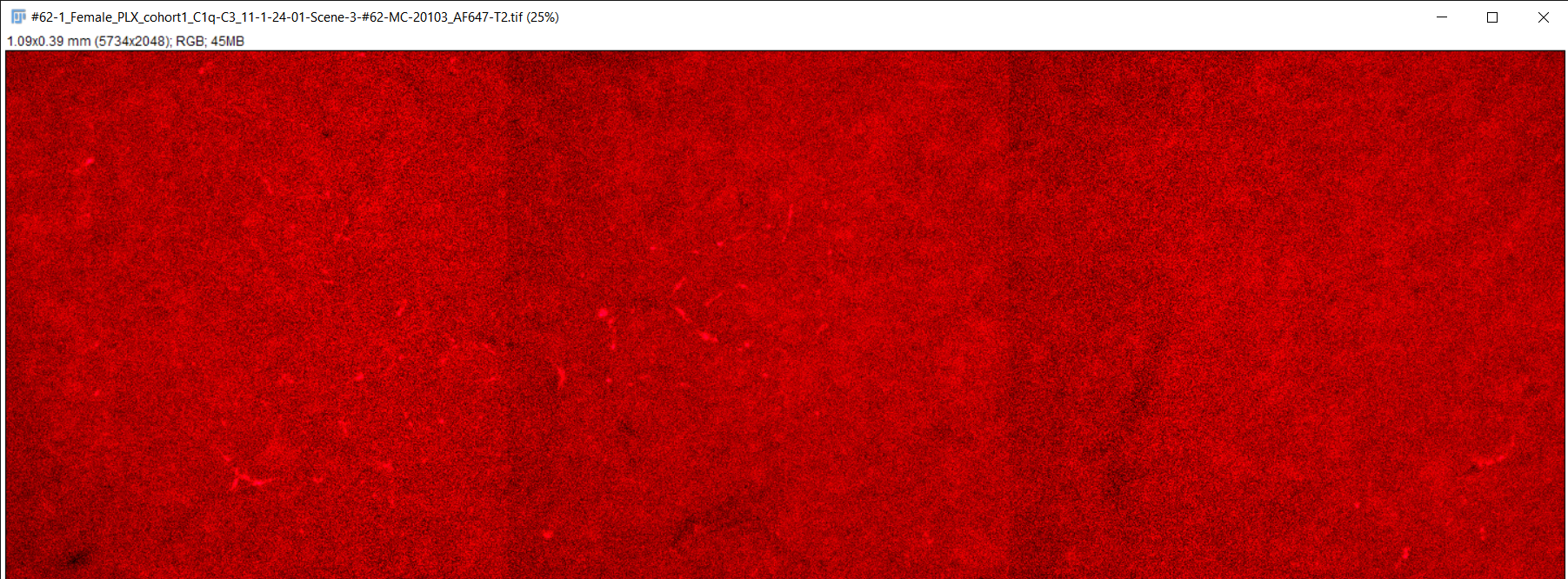
Fiji image analysis software used to obtain complement protein count for each brain slice
-
RGB TIFF converted to 8-bit TIFF
-
Image scale changed to match magnification of microscope
-
Brightness & contrast adjusted, image despeckled, and background subtracting performed to differentiate signal from background staining
-
Image converted to 8-bit/grayscale
-
Thresholding performed to set intensity range of signal
-
Measurements for image analysis set and area of image calculated
-
Number of particles (complement proteins) counted using "analyze particles" function
Data Refinement:
Calculating particle density:
-
Image volume calculated: volume = area*depth of image
-
Particles density of image calculated: density = particles count/volume
Filtering outliers within each mouse:
-
Interquartile range (IQR) of particle densities for each mouse calculated
-
First and third quartiles of particle densities for each mouse calculated
-
Upper and lower bounds of particle densities for each mouse calculated
-
Upper bound = Q3 + IQR*1.5
-
Lower bound = Q1 - IQR*1.5
-
-
Determined whether any of the brain slices had particles densities which were outliers
-
Particle density is outlier if: particle density < lower bound OR particle density > upper bound
-
-
Average particle densities of each mouse calculated (excluding any outliers)
Filtering outliers within either treatment group:
-
Process for filtering outliers within each mouse repeated, but using average particle densities of each mouse instead of particle densities of each brain slice
-
Average particle densities of either treatment group calculated (excluding any outliers)
Statistical Analysis:
-
GraphPad Prism statistical anaylsis software used
-
Input average particle densities from each mouse, dividing them into the assigned treatment groups
-
Parameters for statistical analysis set:
-
Two-sample (RmTBI and sham injury)
-
One independent variable (treatment type)
-
Paired t-test (samples originate from same population but were subject to different treatments)
-
Welch's correction (do not assume both populations have same standard deviation)
-
95% confidence interval
-
-
Perform noramlity and lognormality test
-
Perform Student's t-test
-
Determines if there is statistically significant difference between average particle densities of RmTBI and sham injury groups
-
-
Graph data with standard error of the mean (error bars)
Observations
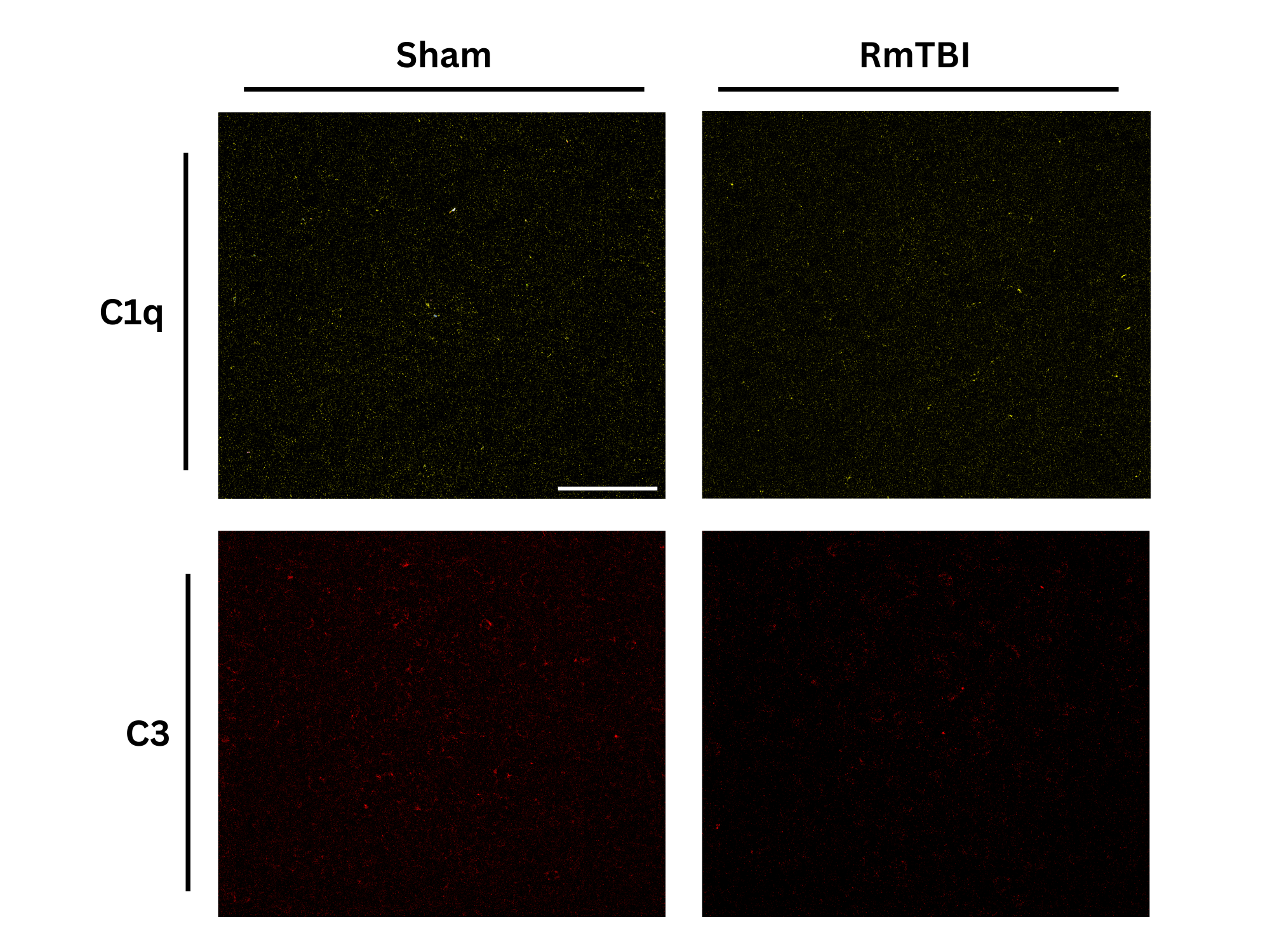
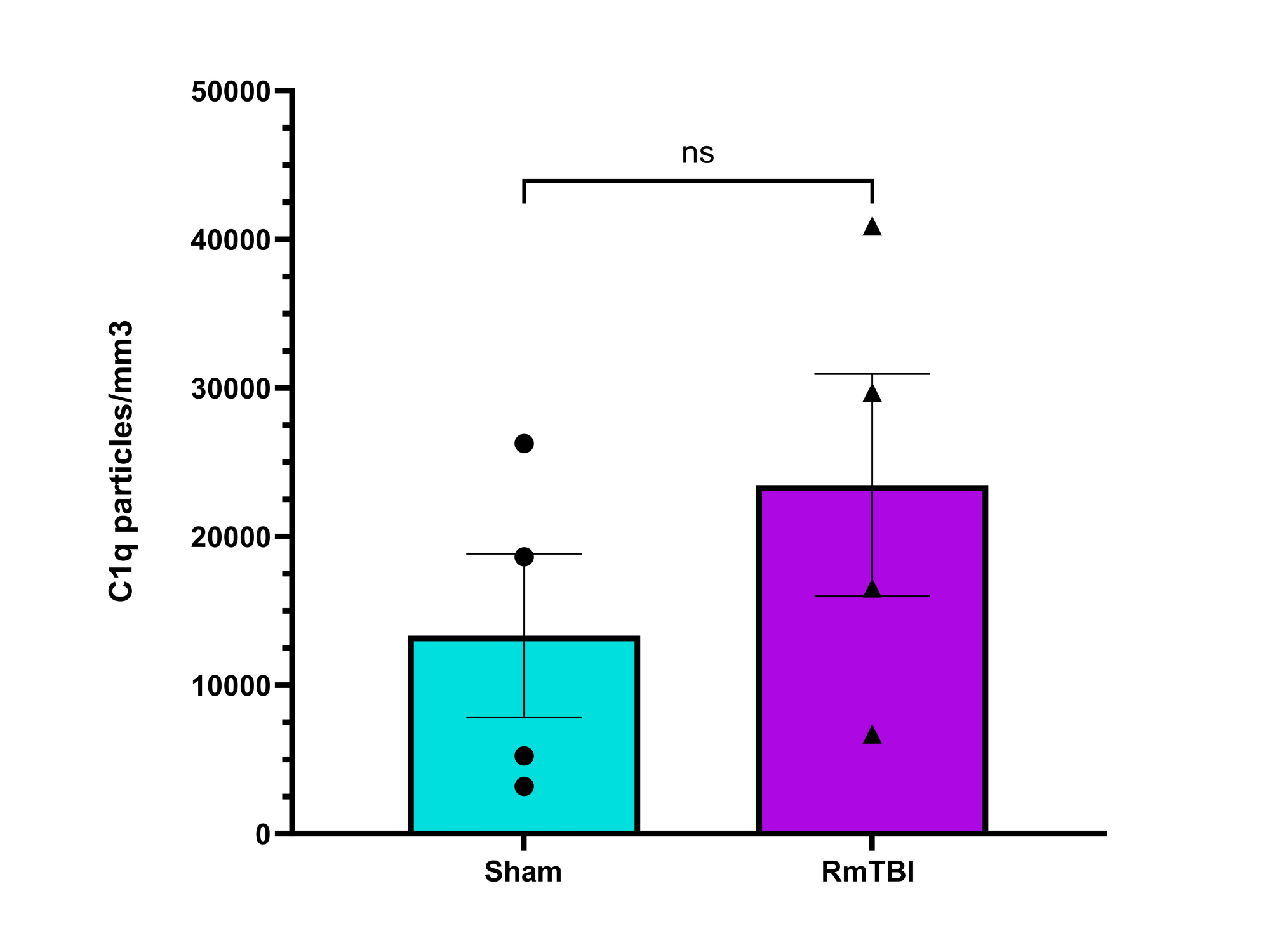
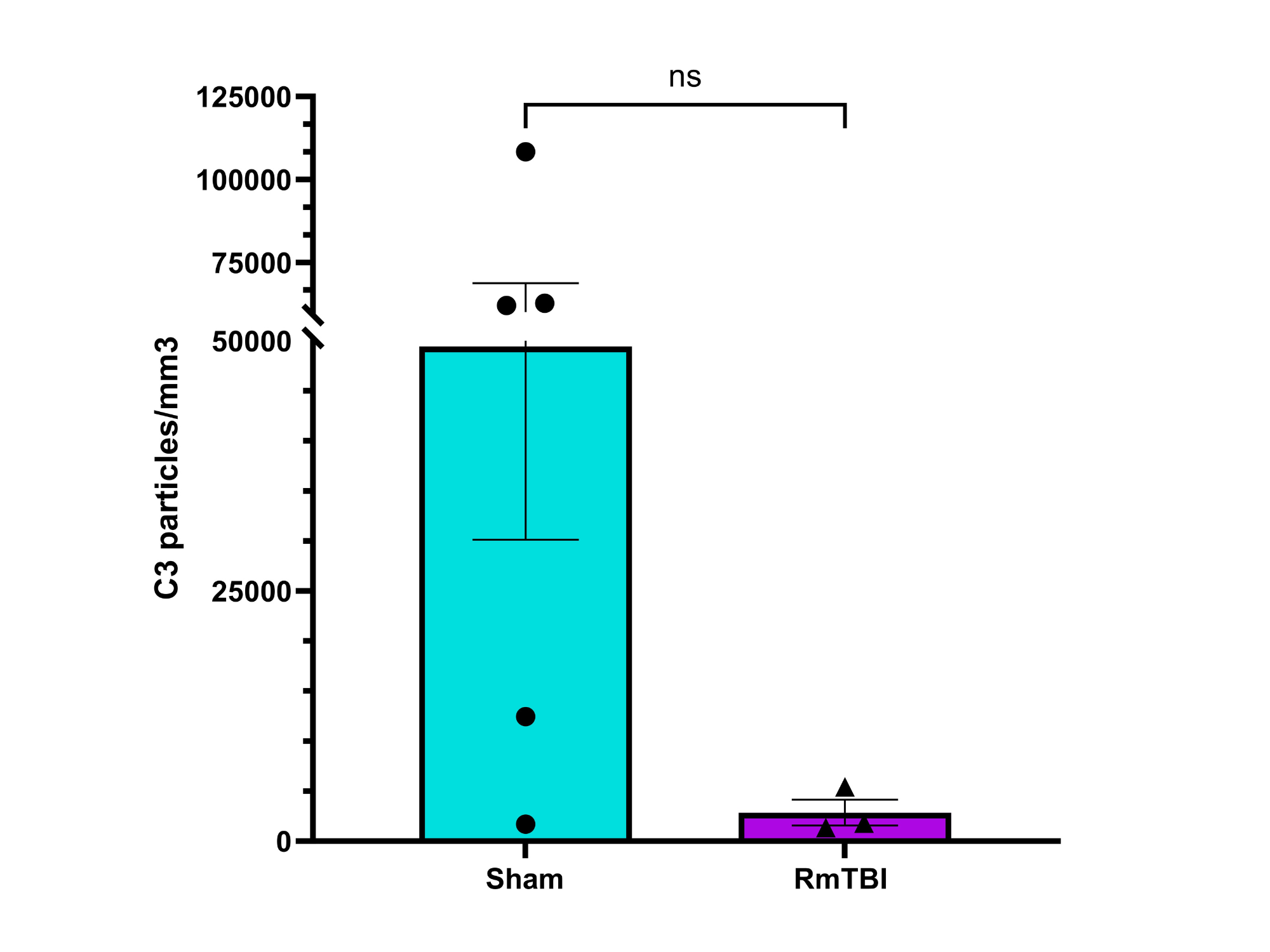
Analysis
Possible Reasons for No Statistical Significance:
-
Low power of study: sample size was too small to yield significant result
-
Sample sizes from experiment:
-
Female sham injury: n=5
-
Female RmTBI: n=4
-
Sample size was restricted by laboratory availability
-
-
-
High variability in complement C1q and C3 expression between individual mice
-
No biological effect: RmTBIs do not induce significant changes in C1q and C3 complement expression in female mice
-
Results may be different for male mice
-
-
RmTBI cascades are time-dependent: C1q and C3 complement levels may have differed based on time of euthanization/observation
-
Timeline of synaptic pruning after injury is unknown
-
-
Some complement proteins were removed by transcardial perfusion and IHC washes:
-
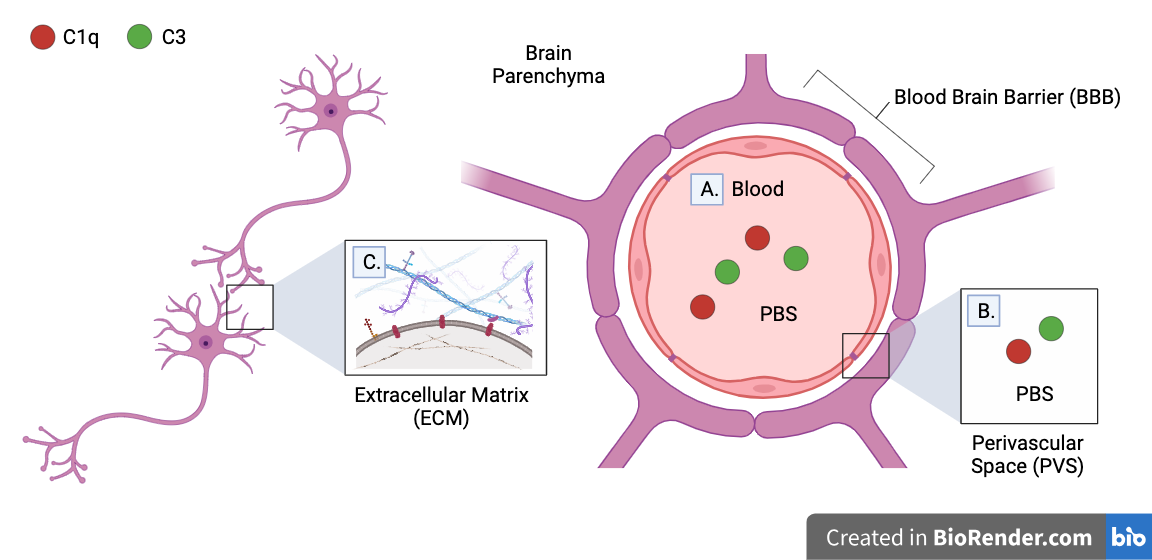
IHC is expected to detect C1q and C3 complement proteins expressed on the surface of neurons or deposited on its synapses. These proteins are thought to comprise the majority of complement proteins in the brain which play a role in synaptic pruning. However, complement proteins in other regions may not be unaccounted for.
-
Transcardial perfusion and IHC may have washed out:
-
A. Complement proteins in blood
-
B. Complement proteins in perivascular space (PVS)
-
C. Complement proteins in brain parenchyma (brain tissue) which are not bound to extracellular matrix (ECM)
-
-
A. Transcardial perfusion washes out all complement proteins in blood. However, they are not expected to play a role in synaptic pruning, as they cannot pass the blood brain barrier (BBB).
-
Note that complement proteins may pass into the brain parenchyma if BBB is damaged by injury, but no BBB damage was observed in lateral impact model used in experiment
-
-
B. Complement proteins in perivascular space (PVS) which participate in synaptic pruning may be washed out by transcardial perfusion. The PVS is the fluid-filled spaces between the brain parenchyma and the BBB.
-
C. Washes performed between IHC steps may wash out complement proteins in brain parenchyma which are not bound to the extracellular matrices (ECM) of neurons. The ECM is a collection of macromolecules attached to the outside of the cell membrane which assist in structure, environmental sensing, and cell-to-cell communication.
-
Note that although the BBB is impermeable to many particles, its thickness varies near certain blood vessels, allowing washing fluid (such as PBS) to pass into the brain parenchyma
-
-
Expression of these complement proteins may have increased after injury, but this cannot be validated given the limitations of this experiment.
-
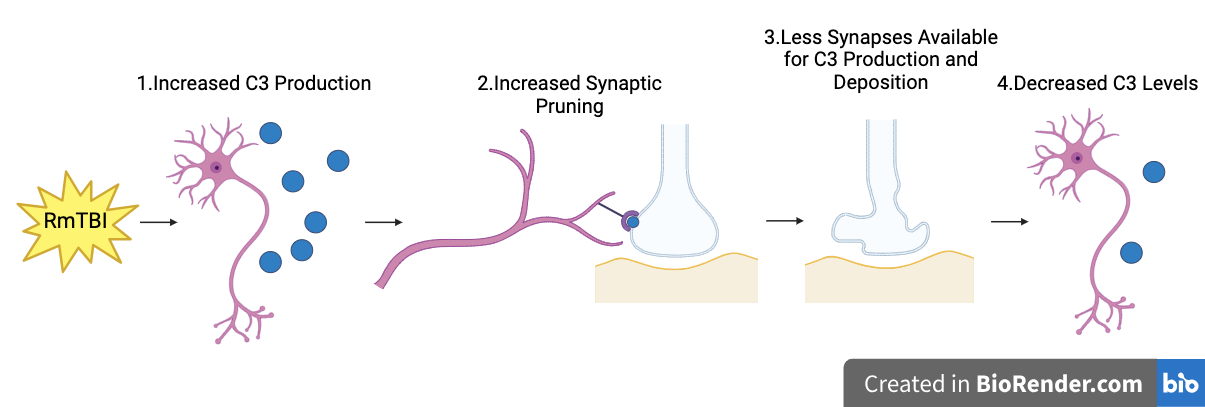
Possible Reasons for Potential C3 Downregulation:
-
Neurons downregulate C3 expression in response to injury or inflammation
-
Neuronal death after RmTBI decreases C3 expression
-
Causes of neuron death after RmTBI (part of primary and secondary injury cascades): Diffuse Axonal Injury (DAI), Wallerian Degeneration, Excitotoxicity
-
Neurons synthesize both C1q and C3 in CNS
-
-
Increased pruning of synapses decreases C3 expression
-
C3 expression may initially have increased, causing increased synaptic pruning. However, increased pruning would decrease the number of synapses available for C3 production and deposition (as synapses are part of neurons, which synthesize C3), leading to decreased C3 levels being detected by IHC over time.
-
Conclusion
No statistically significant change in expression of C1q or C3 complement proteins observed in the motor cortices of female adolescent mice after RmTBI, although there may have been a potential decreease in C3 expression
-
My hypothesis was not supported
Synaptic pruning: while complement density may not have been affected by RmTBIs, changes in microglia density following RmTBIs may still cause changes in synaptic pruning
-
It is possible that complement proteins are not responsible for changes in synaptic pruning and do not play a crucial role in the neurodegeneration caused by RmTBI
Application
-
Better understand the unique pathophysiology of adolescent and female RmTBIs, especially in regards to synaptic pruning and neuroinflammation
-
Prevent the development of long-term cognitive deficits and NDDs in the future
-
Raise awareness regarding the need for TBI research to be more representative of adolescent and females, who have previously been overlooked compared to adults, the elderly, and males. A lack of accurate representation of population demographics may be responsible for the lack of consistency between TBI studies and interfere with the applicability of their results.
-
Improve accuracy of TBI education so that the public may be better informed of the potential risks associated with injury.
-
My results indicate the complement proteins may not play a signficant role in adolescent RmTBI pathophysiology, but these findings can be used to direct future studies to investigate other mechanisms of brain injury.
-
For example, changes in microglia density have often been observed after RmTBIs and microglia may play a signifcant role in adolescent RmTBI pathophysiology.
-
-
-
Investigate the possibility of using complement inhibitors as a potential pharmaceutical TBI therapy
-
Inhibitors of C3 convertase (converts C3 into C3b, which is involved in synaptic pruning) has been proposed as a potential pharmaceutical TBI therapy
-
Despite being successful in preclinical studies, no pharmaceutical TBI therapy to this date has succeeded in clinical trials.
-
My results indicate that complement inhibitors would not be an effective pharmaceutical TBI therapy, but these findings can be used to direct future studies to investigate other proposed therapies.
-
For example, estrogen and progesterone (female sex hormones) are suspected to have neuroprotective effects and have been proposed as a potential pharmaceutical TBI therapy
-
-
Sources Of Error
Sources of Error:
-
Small sample size
-
Small sample size decreases the probability of obtaining statstically significant results by increasing the likelihood of sampling error.
-
An effective sample size could be calculated using a power analysis
-
Previous studies observing female/male mice, which yielded statistically significant results, used sample sizes of around 30 for each treatment group
-
-
Some complement proteins may have been removed during transcardial perfusion and IHC
-
Complement proteins in perivascular space and complement proteins in brain parenchyma which are not bound to the extracellular matrix may be washed out by transcardial perfusion/IHC. These proteins are expected to play a role in synaptic pruning but it is unknown whether including them would signficantly effect my results.
-
-
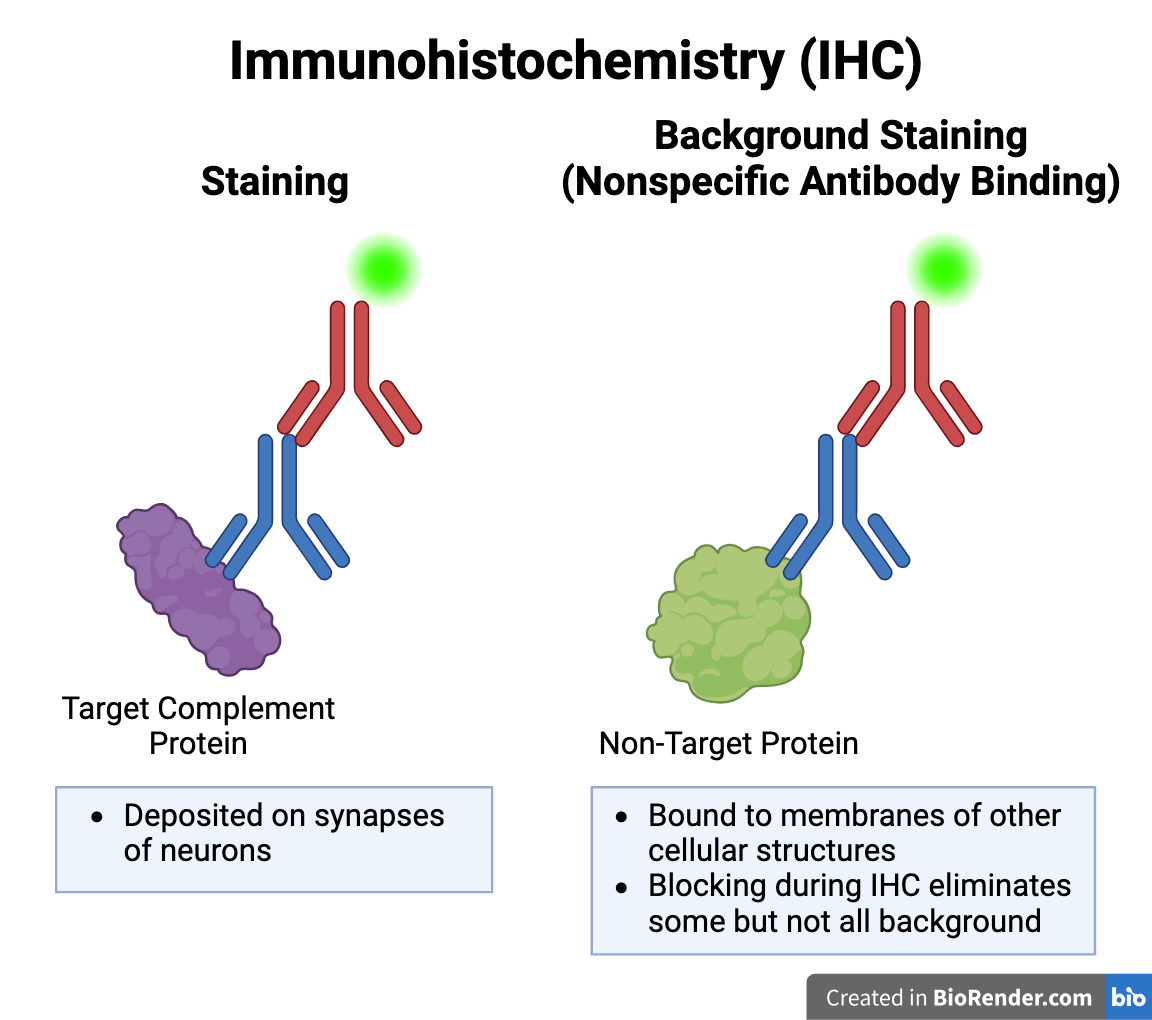
Background Staining during IHC
-
Background Staining: Error caused by nonspecific antibody binding during IHC. Primary antibody may bind to epitope (binding site) of a non-target protein which is similar in shape to that of C1q or C3. Blocking step during IHC removed some but not all background staining.
-
Depending on the image analysis parameters (maximum and minimum particle size), background staining may have been included in the particle count.
-
Future Directions:
-
Repeat experiment using male adolescent mice and/or a larger sample size
-
Male mice have been observed to experience more severe motor deficits than female mice. As a result, they have more dramatic changes in C1q and C3 expression in their motor cortices.
-
-
Observe complement changes in other regions of the brain
-
Other proposed regions: corpus callosum (CC), thalamus, angular insular cortex (AID)
-
Changes in microglia and dendritic spine density observed in these regions after RmTBIs, likely caused by changes in synaptic pruning.
-
Males: decreased microglia and dendritic spine density in AID, increased dendritic spine density in MC
-
Females: decreased spine density in AID
-
-
-
Observe mice at different times after injury
-
Proposed times:
-
6 hours, 1 day, and 3 days after injury: times of peak peripheral immune cell recruitment - may indicate peak in RmTBI primary and secondary cascades
-
1 week, 2 weeks, and 1 month after injury: determine long-term changes in complement expression
-
-
-
Use cell markers to tag neurons and compared with IHC results to improve accuracy of results
-
Although blocking was performed to block all non-specific binding sites/epitopes, background staining may still occur. The non-target proteins detected by background staining are bound to the membranes of various cellular structures in the brain, whereas all C1q and C3 complement proteins are expected to be bound to the synapses of neurons. Comparing images from neuronal cell marker stains and IHC would allow me to normalize my complement protein counts and eliminate any signal that was background staining, improving the accuracy of my results.
-
-
Perform cell marker staining for microglia (Ionized calcium-binding adaptor molecule 1/Iba1)
-
Microglia are the resident immune cells of the brain which produce C1q complement proteins and phagocytose pathogens and synapses.
-
Changes in microglia density following a RmTBI may effect synaptic pruning.
-
Citations
Chen, Y., Chu, J. M. T., Chang, R. C. C., & Wong, G. T. C. (2022). The Complement System in the Central Nervous System: From Neurodevelopment to Neurodegeneration. Biomolecules, 12(2). https://doi.org/10.3390/biom12020337
Eyolfson, E., Carr, T., Fraunberger, E., Khan, A., Clark, I., Mychasiuk, R., & Lohman, A. W. (2022). Repeated mild traumatic brain injuries in mice cause age- and sex-specific alterations in dendritic spine density. Experimental Neurology
Eyolfson, E., Carr, T., Khan, A., Wright, D. K., Mychasiuk, R., & Lohman, A. W. (2020a). Repetitive Mild Traumatic Brain Injuries in Mice during Adolescence Cause Sexually Dimorphic Behavioral Deficits and Neuroinflammatory Dynamics. Journal of Neurotrauma
Eyolfson, E., Khan, A., Mychasiuk, R., & Lohman, A. W. (2020b). Microglia dynamics in adolescent traumatic brain injury. Journal of Neuroinflammation
Gomez-Arboledas, A., Acharya, M. M., & Tenner, A. J. (2021). The Role of Complement in Synaptic Pruning and Neurodegeneration. ImmunoTargets and Therapy
Stevens, B., Allen, N. J., Vazquez, L. E., Howell, G. R., Christopherson, K. S., Nouri, N., Micheva, K. D., Mehalow, A. K., Huberman, A. D., Stafford, B., Sher, A., Litke, A. M., Lambris, J. D., Smith, S. J., John, S. W. M., & Barres, B. A. (2007). The classical complement cascade mediates CNS synapse elimination. Cell
Veliz, P., McCabe, S. E., Eckner, J. T., & Schulenberg, J. E. (2017). Prevalence of Concussion Among US Adolescents and Correlated Factors. JAMA: The Journal of the American Medical Association
Whitford, T. J., Rennie, C. J., Grieve, S. M., Clark, C. R., Gordon, E., & Williams, L. M. (2007). Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology
Zabel, M. K., & Kirsch, W. M. (2013). From development to dysfunction: microglia and the complement cascade in CNS homeostasis. Ageing Research Reviews
Acknowledgement
I would like to thank:
-
Webber Academy Applied Science Project instructor, Dr. Beatriz Garcia-Diaz
-
My mentor, Mr. Thomas Carr, a neuroscience PhD candidate at the University of Calgary