How does VEGF-A protein isoform development and identification with mass spectrometry impact drug use and treatment diagnosis for Wet AMD?
Grade 10
Presentation
Problem
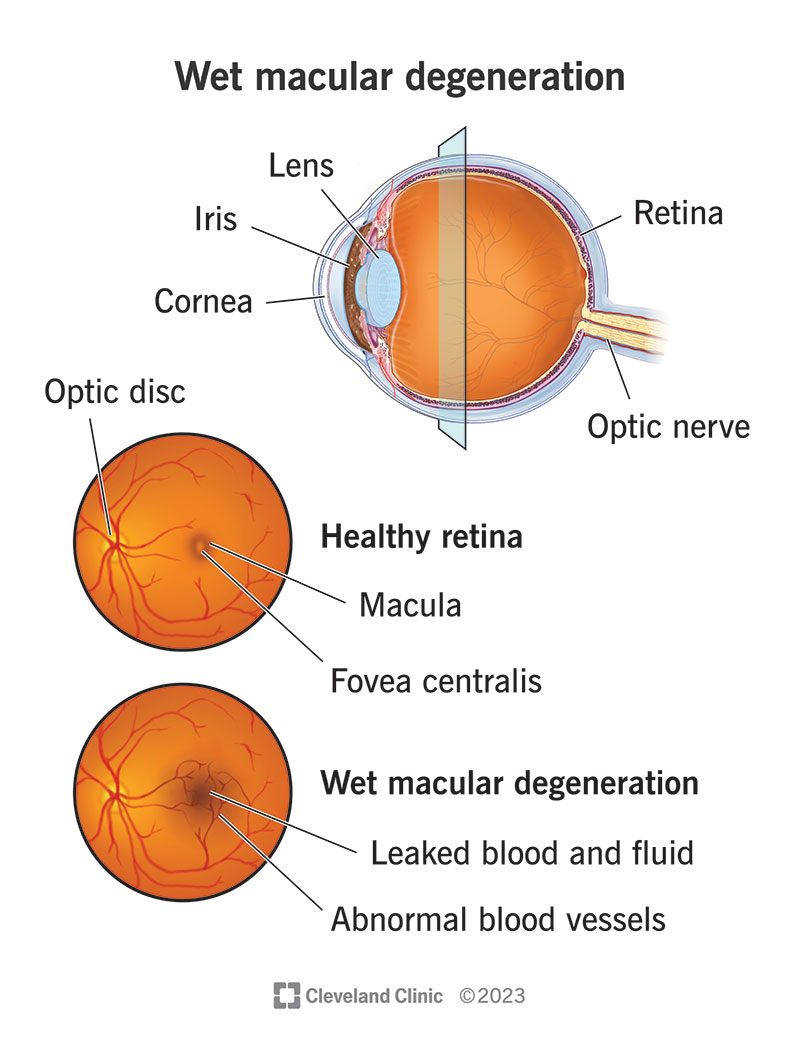
Wet age-related macular degeneration is one of the world's leading causes of rapidly developing blindness in those over 50. There are two types of AMD, dry and wet, but although wet AMD is less common than dry AMD, it is much more serious and causes vision loss faster. Wet AMD is characterized by the macula's proliferation of blood vessels in the fundus of the eye. The macula is a part of your retina that is responsible for your central vision, and in Wet-AMD, the blood vessels that proliferate can be weak and often leak blood or other fluids, causing scarring and damage to the macula, as shown in Figure 1. This project focuses on utilizing mass spectrometry and protein sequencing to identify isoforms of VEGF-A (the protein that facilitates neovascularization) and investigate the differences in characteristics between angiogenesis caused by different isoforms to optimally treat this disease.
(Figure 1 shows the appearance differences between a healthy eye and an eye with WAMD). Ref [1].
A main reason blood vessels may have varying characteristics is VEGF. VEGF stands for Vascular Endothelial Growth Factor and is a protein vital to blood vessel growth. VEGF is a protein that undergoes a complex biological process called alternative splicing, in which the exons of its genetic composition may be skipped or altered, resulting in isoforms of the protein that can have different traits that may change the presentation of the oncoming blood vessel. Wet AMD is usually treated with anti-angiogenic or anti-VEGF drugs or, in some specific cases, laser eye treatment. Anti-VEGF drugs target the vascular endothelial growth factor protein (VEGF). The inhibition of VEGF through drugs is a method that has been present for some time, initially becoming prominent in cancer treatment, and in recent years, various successful applications in ophthalmology have been found.
Method
Abstract
This research study investigates the utilization of mass spectrometry protein sequencing and its possible applications in optimizing intraocular treatment for AMD. It delves into how protein isoforms in blood vessels that leak onto the macula and degrade visual acuity can be biomarkers for differing presentations in nAMD. Specific isoforms can vary in characteristics due to missing parts of the genetic code, which can translate to blood vessels with differentiation. Anti-VEGF drugs (usually administered IVT) can be altered or optimized to target isoforms with high specificity, which has shown benefits in lowering treatment burden and lowering side effects for patients. Another aspect this research project observes is the identification of anti vs pro-angiogenic isoforms and how those can be strategically inhibited through identification for optimization. This research strives to enhance understanding of the impact of biological and physiological markers in nAMD and attempts to understand how current issues within ocular research can be addressed through personalized treatment.
1- What is RNA sequencing/mass spectrometry
Refs- [42],[43],[45],[68],
Mass spectrometry (MS) is a powerful technique to detect, quantify, and identify molecules and proteins by analyzing their mass-to-charge (m/z) ratio. Initially, its application in biological sciences focused on measuring atomic weights and determining the natural abundance of specific isotopes. The introduction of advanced macromolecule ionization techniques, such as electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI), has revolutionized the study of protein structure using MS. These ionization methods have also enabled the generation of protein mass "fingerprints," which can be compared to database entries of proteins and peptides, allowing for the identification of unknown targets [42, 68]
The main focus of this project's MS is determining proteins, their structure, and interactions to showcase the differences between isoforms of VEGF and how differences in interaction structure and binding can physiologically or biologically affect Wet-AMD. When MS is used, it is critical to analyze the goal of your research, to determine the type of instrumentation, fragmentation method and analysis strategy best suited to your sample.
1.1- How does MS work and how can data be extracted from MS?
Refs- [42],[43],[44],[48],[49],[68],[69],
Mass spectrometry is a popular method for protein sequencing, analyzing the amino acid sequence of a sample. It includes bottom-up proteomics and top-down proteomics. Bottom-up sequencing involves breaking down the protein into small fragments, while top-down sequencing analyzes the entire protein molecule. Protein sequencing by mass spectrometry is used for protein identification, post-translational modifications (PTMs) analysis, protein quantification, protein-protein interactions, drug discovery, and biomarker discovery [44]. Protein sample preparation involves clean and uncontaminated vessels, reagents, gloves, and head covers. Enzymatically digesting proteins into peptides before mass spectrometry sequencing improves detection accuracy. Methods such as fractionation, IMAC, and antibody binding can be used to minimize non-phosphorylated peptide content [68,69].
Mass spectrometers are made of 3 main parts, an ion source, a mass analyzer, and an ion detector, all of which collect data into information processing software, as shown in the image from ref [42]. Samples can be placed as a gas, liquid, or solid into a spectrometer and then vaporized and ionized. The process begins with ionization, where sample molecules are converted into charged ions. These ions are introduced into the mass analyzer within the mass spectrometer's vacuum chamber and then separated based on their m/z ratios. Following separation, the detector measures the abundance of each ion, and the data is processed and analyzed using specialized software [42, 43, 44].
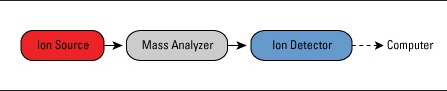
Figure 1.1- [42].
Over recent years, mass spectrometry research has revolutionized our understanding of polypeptides and proteomics. Various methods are used in mass spectrometry for protein sequencing, such as the novel orbitrap mass spectrometer, liquid chromatography-MS, and ESI [42].
MS-MS can be used and applied in many ways, but this project will focus on 2 main applications of MS-MS in protein sequencing. Top-down, and bottom-up. These two ways of identifying protein composition are generally differentiated by how the protein is processed before MS detection. Bottom-up (shotgun) protein analysis involves proteins being digested and broken down into smaller peptides, analyzing proteolytic peptide mixtures. Top-down MS involves inact proteins that are directly analyzed without prior digestion, typically using high-resolution MS [68].
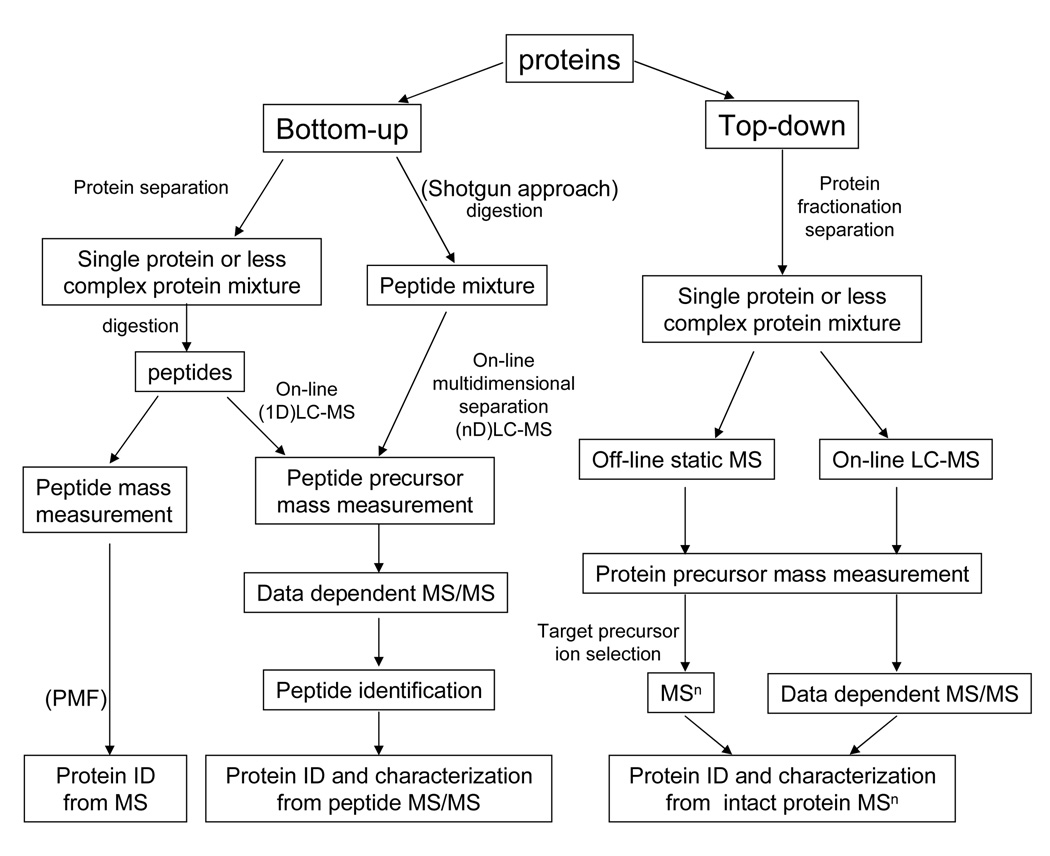
Figure 1.2 [68], shows the protein sequencing process comparing top-down and bottom-up techniques.
1.2.1- Bottom-up MS
Refs- [68],[69],[71],[45]
Bottom-up protein analysis refers to a mass spectrometry (MS) technique that characterizes peptides released from proteins through proteolysis (protein breakdown). Typically, trypsin is used to digest these proteins before MS analysis. Trypsin is a recombinant protease enzyme specifically designed for analytical protein digestion [68, 71]. When bottom-up MS is performed on a mixture of proteins, it is called shotgun proteomics or shotgun MS. Shotgun MS has one of two potential workflows in MS; the second is known as "sort then break," which uses off-line protein fractionation and separation before protein digestion, alongside “peptide mass fingerprinting” (PMF) [68]. Shotgun proteomics provides an indirect measurement of the peptides present in proteins. Samples are usually ionized and undergo liquid chromatography-tandem mass spectrometry (LC-MS/MS), the technique most commonly used in bottom-up MS. The resulting tryptic peptides from proteolysis are acidified to confer a positive charge. The resultant peptide ions are then injected into a high-performance liquid chromatography (HPLC) column, sending them to the mass spectrometer over an extended duration (typically 60–120 minutes) [69, 45]. The mass spectrometer consists of two analytical stages. At any moment, the first stage (MS1) identifies a series of peaks with varying m/z ratios. Dominant peptides in MS1 are fragmented using inert gases like nitrogen or argon. This effectively cleaves a peptide into two fragments through collision-induced dissociation, powered by kinetic energy. The cleavage site may vary. When the cleavage products are displayed in the second stage of the tandem mass spectrometer (MS2), a series of peaks corresponding to all N-terminal fragments are shown, superimposed over all C-terminal fragments. The difference in mass-to-charge ratio between neighbouring peaks indicates the amino acid situated between the cleaved peptide bonds, as each peptide possesses a unique residue mass (except leucine and isoleucine, which share the same mass). In practice, the amino acid sequence is identified through pattern matching between the MS2 spectrum and spectra predicted from a database of all tryptic peptides coded by the relevant genome. The MS1 and MS2 results are subsequently searched by algorithmic software such as SEQUEST or MASCOT against a database of proteins derived from genomic sequencing to identify the peptides [45]. The resolution and peak capacity of the multi-dimensional separation techniques are vital to the success of the analysis. Among these techniques, multi-dimensional protein identification technology (MudPIT) is now widely implemented and has been applied to analyses of complete cell lysates, organisms, tissue extracts, sub-cellular fractions, and other sub-proteomes [45,69,68].
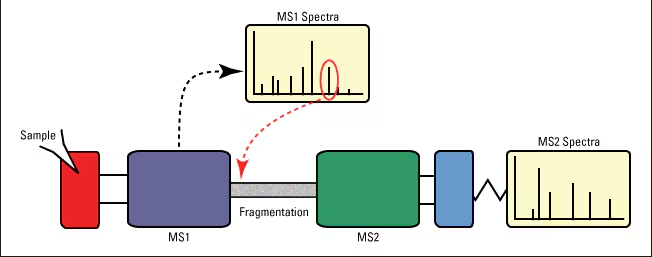
Above is figure 1.2.1 describing the process in which shotgun MS occurs. [42].
1.2.2- Top-down MS
Refs- [68],[69],[72],
In contrast, another strategy, termed ‘top-down’ proteomics, is used to characterize intact proteins. The top-down approach has some potential advantages for PTM and protein isoform determination and has achieved notable success. Intact proteins have been measured up to 200 kDa, and a large-scale study has identified more than 1,000 proteins by multi-dimensional separations from complex samples. However, the top-down method has significant limitations compared with shotgun proteomics due to difficulties with protein fractionation, protein ionization and fragmentation in the gas phase [69]. The main advantages of the top-down approach include the detection of degradation products and sequence variants, which help to resolve inference issues (e.g., isoforms, proteoforms), as well as the determination of post-translational modifications (PTMs) for protein stoichiometry and dynamics analysis. [68, 72].
2- How can samples be acquired (LCM)
Refs-[70],[73],[74],[75]
In the case of WAMD, endothelial cell samples can be obtained from retinal tissue. VEGF is present in platelets and endothelial cells, and endothelial cells line all blood vessels. Laser Capture Microdissection (LCM) is a precise technique that enables the isolation of specific cells or microscopic structures from heterogeneous tissue sections using a focused laser under microscopic visualization [74]. This method ensures minimal contamination from surrounding tissues, making it ideal for proteomic analyses. To acquire ocular samples suited for mass spectrometry (MS)-based protein sequencing, ocular tissue sections are prepared, and regions of interest, such as blood vessels or endothelial cells, are carefully excised using LCM, evidence has shown that transcriptome-wide analysis of blood vessels is possible through LCM [75]. The isolated samples can then undergo protein extraction and digestion for bottom-up or top-down proteomics, allowing for detailed characterization of the vascular proteome. Unlike enzymatic digestion methods, which may alter or degrade proteins, LCM preserves protein integrity and spatial organization, ensuring a more accurate representation of the ocular endothelial proteome. Additionally, LCM is compatible with archived tissue samples, making it valuable for studying disease-associated changes in ocular vasculature over time. Its precision, ability to maintain protein structure, and compatibility with proteomics workflows make LCM the preferred method for MS-based protein sequencing of ocular vascular tissues [73,74,75].
Research
Wet AMD is an ocular disease caused by weak blood vessel proliferation that breaks and leaks onto the macula of an eye, often causing vision loss. This project focuses on utilizing mass spectrometry and protein sequencing to identify isoforms of VEGF-A (the protein that facilitates neovascularization) and investigate the differences in characteristics between blood vessels composed of different isoforms. Additionally, it explores how identifying and understanding these isoforms can help us optimize treatment in WAMD.
1-What is wet-age-related macular degeneration?
Refs- [1],[2],[3],[16],[6],[4]
Wet age-related macular degeneration (W-ARMD) is an ocular disease characterized by abnormal blood vessel growth above the retina, the part of the eye responsible for central vision. Choroidal neovascular membranes (CNVM) are abnormal outgrowths from the capillaries of the choroid [3]. This abnormal growth can disrupt existing retinal tissue, causing blood and fluid to leak into the macula, resulting in scarring and damage. Although this disease is asymmetrical, it typically affects both eyes simultaneously [1,6]. The clinical presentation and symptoms of W-ARMD can be virtually nonexistent, and when reported, they are usually mild. For instance, loss of central visual acuity, distorted vision, and disturbances in the perception of colours and contrast may occur [3]. Within the macula, degeneration can lead to scotomas (blind spots) that later stabilize, and patients often report blurring in their central vision [3]. Upon examination, the eye of someone with W-ARMD may show retinal pigment epithelial detachment (PED), a split between the retinal pigment epithelium (RPE) and Bruch's membrane caused by lipid deposition. This split can fill with blood, drusenoid material, fibrovascular tissue, or a combination thereof [16]. Hemorrhages in the subretinal space are also seen in W-ARMD and can sometimes cause scotomas. RPE hypertrophy, atrophy, and drusen (yellow deposits under the retina made of lipids and proteins) are also often present [3]. Pathological alterations like drusen buildup, thickening of Bruch's membrane, fluid accumulation, pigment epithelial detachment, and retinal detachment expand the gap between the choriocapillaris and the retina. This increase reduces the oxygen supply to photoreceptors, causing hypoxia in retinal cells. The low oxygen environment elevates the hypoxia-inducible factor, a transcription factor that enhances the expression of the VEGF gene, resulting in heightened permeability and angiogenesis [4].
The diagnosis of WAMD can be determined through combinations of optical tomographies and angiographies. Most will begin with a routine eye exam, and for further detail, will dilate your eyes to conduct a slit lamp examination. Non-invasive diagnostic exams such as fluorescein angiographies and OCTs (optical coherence tomography) can also be utilized. A fluorescein angiography is an effective test where an ophthalmologist injects a yellow dye (fluorescein) into your arm. It travels through your blood vessels while a special camera captures images of the highlighted vessels to observe the presence of abnormal blood vessels. An OCT employs a machine to acquire high-resolution images of the retina and macula. These two methods are the most common for ophthalmologists and retinal specialists in diagnosing WAMD. Another tool that can be used more easily is an Amsler grid, a simple grid used to identify the location of scotomas or blind spots in a patient's field of view. (Figures 1.2 and 1.3 consecutively) [2].

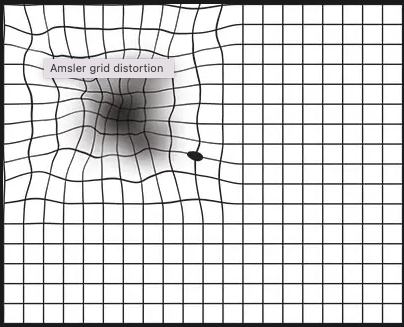
Figure 1 shows what a normal Amsler grid looks like, and 1.1 shows what a distorted Amsler grid would look like to someone with vision loss, scotomas, and blurred and bent lines. Ref- [2]
2- Protein structure and amino acids
Refs- [51],[76]
Amino acids are the essential building blocks of proteins and nitrogenous backbones for various biological compounds like neurotransmitters and hormones. Chemically, an amino acid consists of an amino group (-NH2) and a carboxyl group (-COOH). Only α-amino acids are used in proteins, where the amino and carboxyl groups are attached to a chiral (central) carbon atom. The unique side chain, also known as an R-group, of each amino acid determines its individual properties and influences the protein's overall structure. This can all be demonstrated below in Figure 1 [51].
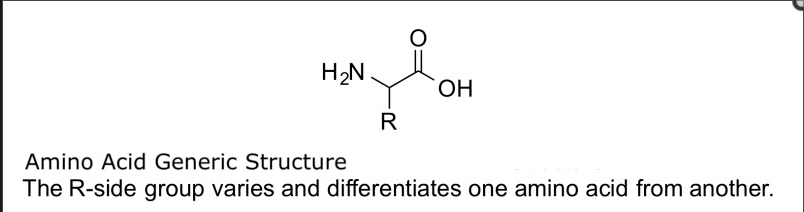
Figure 2 from ref [51] shows the basic chemical structure of a-amino acids with the H2N being the amino group, a carboxylic acid group (COOH-), a central carbon atom, and the R (side chain).
Side chains generally differentiate amino acids from each other, and combinations of different amino acids with different side chains form proteins with distinct characteristics. While hundreds of amino acids exist in nature, only about 20 are used to synthesize the proteins found in humans and most organisms. Among these 20, 9 are essential (must be obtained through diet): phenylalanine, valine, tryptophan, threonine, isoleucine, methionine, histidine, leucine, and lysine. Others, like arginine, tyrosine, and histidine, can become conditionally essential or semi-essential during periods of growth or recovery.
Proteins are polymers of these amino acids, linked by peptide bonds to form long chains. The order in which amino acids are arranged—the primary structure—contains the information necessary to fold into a specific three-dimensional shape, which is organized into secondary structures like α-helices and β-sheets, further folding into tertiary and sometimes quaternary structures. Various interactions, including hydrogen bonds, hydrophobic interactions, disulphide bonds, and ionic bonds stabilize these higher levels of structure. [51]
Proteins' hierarchical structure breakdown [76]
Primary Structure – The linear sequence of amino acids, determined by the DNA sequence of a gene.
Secondary Structure – Localized folding patterns like α-helices and β-sheets, stabilized by hydrogen bonding.
Tertiary Structure – The 3D folding of a single polypeptide, influenced by disulphide bonds, hydrophobic interactions, and ionic bonds.
Quaternary Structure – Some proteins consist of multiple polypeptide chains, held together by noncovalent interactions like hydrogen bonds and ionic bonds. [76]
3- VEGF (All types but focusing on VEGF-A and its receptors)
Refs-[7],[20],[21],[26],[27],[4],[22],[24],[3],[28],[78],[79],[8]
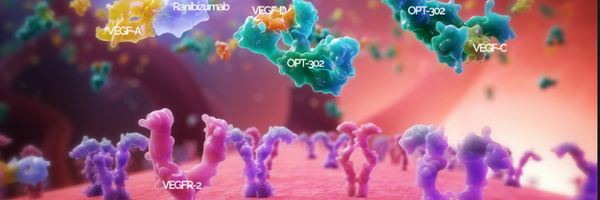
Vascular endothelial growth factor A is a potent mitogen (a protein) secreted for pathological and physiological angiogenesis and stems from the VEGF family of proteins consisting of VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PlGF (placenta growth factor) [3]. VEGF-A regulates blood vessels by binding to one of two tyrosine kinase receptors [7, 21]. It is necessary to study the protein receptors to understand what stimulates angiogenesis. These receptors are tyrosine kinases and are a family of enzymes that catalyze angiogenesis by acting as signalling pathways [20]. A kinase is an enzyme that transfers phosphates using ATP to activate or inactivate a protein or sugar in phosphorylation (the addition of a phosphate) [79]. Both tyrosine kinases are made of the same general structure. They contain a 7D Ig-like domain in the extracellular region of the receptor, and transmembrane, that splits the cytoplasmic domain and the ECM, a KI and a kinase domain (KD) which is the kinases that undergo phosphorylation and finally the C-terminal tail, the end of the receptor [27]. The first tyrosine kinase of VEGF, VEGR1, is a kinase-impaired RTK, and VEGFR2 is a highly active kinase [21]. VEGFR-1 mediates VEGF-induced chemotaxis (the directed migration of a cell in response to a chemical stimulus) and inflammation and operates through ligand trapping. At the same time, VEGFR-2 is the primary mediator of the mitogenic, angiogenic and permeability-enhancing effects of VEGF and stimulates signalling pathways [8, 28]. Both receptors are required for angiogenesis because activation of VEGFR2 is necessary for proper signalling [21]. The third receptor is mainly responsible for lymphangiogenesis, which is unrelated to the angiogenesis that often occurs in wet AMD [24]. Gene products PLGF and VEGF-B are known to bind to VEGFR1.VEGF-C and VEGF-D bind to VEGFR2 and VEGFR3, and VEGF-A binds to VEGFR1 and VEGFR2 [24].
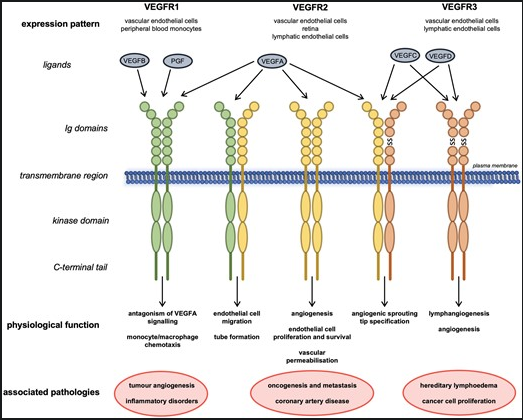
Figure 3 from reference [24] shows the ligands that bind to each receptor. More specifically, VEGF-A, the most common variant of VEGF, binds to both VEGR1 and VEGFR2. As said above, the ligands that bind to separate receptors are shown as well, Eg, how VEGF-B and PGF are variants that only bind to VEGFR1. VEGFRs associate as receptor tyrosine kinase homo- or heterodimers. Dimerization forms a protein complex from two subunits, as shown in Figure 3.1 [76]. The receptors were initially thought to be separate monomers that form to bind to a ligand; they were later shown to be pre-formed inactive dimers, likely activated by the kinases (KD) and phosphorylation. Receptor tyrosine kinases (RTKs) function through the protein kinase domain located in the intracellular region of each RTK monomer. Ligand binding to the extracellular region results in the selective trans-autophosphorylation of tyrosine residues [21] as shown in Figure 3.1.
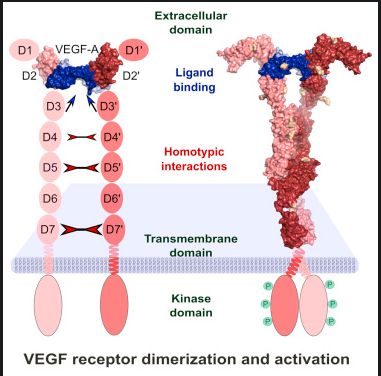
Figure 3.1 shows how the binding of VEGF as a ligand makes an inactivated dimer into an activated one, resulting in phosphorylation of the protein kinase domain [78].
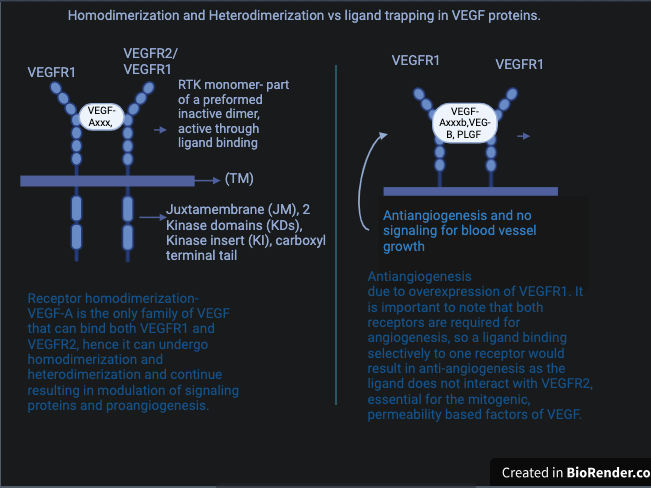
VEGF trapping is a defined condition when VEGFR-1 is activated by a ligand that only causes receptor homodimerization and results in no binding of VEGFR-2. VEGFR-1 cannot promote biological responses such as cell migration, cell proliferation and intercellular calcium release alone. This is why both receptors are required for angiogenesis. So, VEGFR1's ability to stimulate biological responses is limited to its heterodimerization capabilities with VEGFR2. VEGF trapping or ligand trapping negatively affects VEGFR1 and halts angiogenesis. The kinase activity of VEGFR-1 is about 10-fold weaker than VEGFR-2, which may be why VEGFR-1 operates through heterodimerization and ligand trapping. However, both receptor heterodimerization and homodimerization result in angiogenesis, as in both cases, activation of signals allows for the release of VEGF and cell proliferation [28, 21]
The ligands that bind VEGFR2 (VEGF-A, VEGF-C and VEGF-D) are released by cells under hypoxic conditions and direct angiogenesis [26, 4]. Of these ligands, VEGF-A exhibits the highest binding affinity for VEGFR2 and is considered the most potent angiogenic agent. W AMD is a disease that stems from ischemia or inflammation. These hypoxic or ischemic conditions occur and signal VEGF A angiogenesis, which leads to further abnormal blood vessel growth and more damage. Although this information is known, the cause of Wet AMD and the initial angiogenesis is unknown. The furthering of abnormal blood vessel proliferation may even cause adverse effects or secondary diseases, which is why anti-VEGF A treatments are used in Wet AMD. Excess VEGF-A increases the leakiness of blood vessels and the risk of advanced WAMD, which may be caused by ligand trapping from VEGFR1 [4]. Not only that, but VEGF-A is a protein that undergoes a cellular process called alternative splicing, which occurs in the pre-mRNA phase of a protein. Alternative splicing results in various isoforms where different exons of the genetic code are skipped, alternated, or changed, which may result in different physiological and biological properties [28, 22].
3.1- Co-receptors, HSPGs, Integrins, Cadherins
Refs- [28],[30],[31],[32],[34],[35],[77],[21]
The VEGF protein has various coreceptors that can influence angiogenesis. While VEGF receptors (VEGFR-1, VEGFR-2, and VEGFR-3) are the primary mediators of VEGF signalling, their activity is significantly influenced by co-receptors [21]. These co-receptors do not have intrinsic kinase activity but play a crucial role in modulating VEGF function by enhancing receptor binding, stabilizing ligand interactions, and directing specific cellular responses for certain variants of VEGF. Below is a detailed breakdown of major VEGF co-receptors and their roles.
VEGF Co-Receptor Roles
| Co-Receptor | Primary Role | Key Ligands | Major Functions |
|---|---|---|---|
| Neuropilin-1 (NRP-1) [32] | Enhances VEGFR-2 signaling | VEGF-A165 | Promotes angiogenesis and cancer progression |
| Neuropilin-2 (NRP-2) [32,77] | Enhances signalling | VEGF-165, VEGF-145 | Supports lymphatic vessel formation and |
| Heparan Sulfate Proteoglycans (HSPGs) | Increases VEGF availability | Multiple VEGF isoforms | Enhances ligand-receptor interactions |
| Integrins (αvβ3, α5β1) | Regulate endothelial adhesion/migration | VEGF-A | Promote angiogenesis and vascular stability |
| Cadherins (VE-Cadherin) | Maintain vascular integrity | VEGFR-2-associated | Control permeability and endothelial junctions |
3.1.1- Neuropillin-1 and Neuropillin-2
Neuropillin-1 (NRP-1) is the first of the coreceptors that can mediate angiogenesis. It primarily binds VEGFA-165a. Neuropillins (NRP-1 and its counterpart, NRP-2) are transmembrane glycoproteins. NRP-1's primary role is enhancing VEGFR2 signalling and NRP1/heparin-binding domain encoded by exons 7 and 8 [32]. NRP-1 has been identified as a receptor that mainly binds VEGF A165, among other VEGF families like VEGF-B and PLGF. The role of NRP2 in endothelial cells (ECs) is not well understood. Findings in [77] reveal that NRP2 enhances Rac-1 mediated adhesion and migration of ECs on fibronectin (FN) matrices, differing mechanistically from NRP1 and operating independently of β3 integrin (ITGB3) expression or VEGF stimulation. Additionally, we present evidence of a regulatory interaction between NRP2 and α5 integrin (ITGA5) within ECs; depleting NRP2 results in an increase in ITGA5 expression and alters the organization of ITGA5 in cells.
3.1.2- Heparan sulfate proteoglycans
Heparan binding is a very important factor in differentiating isoforms from each other due to its critical role in the permeability and signalling of blood vessels. Isoforms that bind HSPGs usually have longer amino acid sequences like 165, 189, and 206. Except for VEGF-A-121, all isoforms are thought to bind the polysaccharide heparin. Heparan sulphate (HS) is a complex polysaccharide found on cell surfaces and within the extracellular matrix, playing a crucial role in vascularization. Structurally, HS consists of repeating disaccharide units that can undergo extensive sulfation, resulting in diverse sulfation patterns. These patterns are essential for the binding and regulation of VEGF isoforms. This binding enhances receptor dimerization and activation, leading to downstream signalling pathways that promote angiogenesis [31, 35].
3.1.3- Integrins
Integrins serve as key modulators of VEGFR-2 activity, balancing normal vessel maintenance and promoting angiogenesis when necessary. Their ability to either suppress or enhance VEGF signalling. Specific integrins bind to the extracellular domain of VEGFR-2 and augment receptor signalling. Integrins of the β 3 subfamily specifically bind to the extracellular domain of VEGFR-2 resulting in increased receptor activation upon VEGF stimulation [77]
3.1.4 - Cadherins
VE-cadherin, a type found in endothelial cells, helps regulate VEGFR-2 signalling, permeability, and vascular integrity. β-catenin modulates the interaction between VEGFR-2 and VE-cadherin. VE-cadherin prevents excessive activation at high cell density through its association with Phosphatase PTP1/Dep1/CD148. Additionally, VE-cadherin suppresses signalling through PI3K (ras pathway) (Phosphoinositide 3-kinase), which is involved in cell survival, MAPK (Mitogen-activated protein kinases) that regulate proliferation, and PLCγ-1 (Phospholipase C gamma-1), important for pro-angiogenic signalling, mainly from receptor 2 [28]. Phospholipase Cγ1 (PLCγ1) breaks down phosphoinositides to produce the second messengers' inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 elevates intracellular Ca2+ levels, while DAG activates protein kinase C (PKC), triggering a range of cellular responses. Multiple pieces of evidence suggest that PLC-γ1 activation is crucial for angiogenesis and VEGFR-2 signal transduction. For instance, a genetic study reveals that the absence of PLC-γ1 in mice leads to greatly reduced vasculogenesis [21, 28, 24].
3.2- VEGF-A splicing and its impact on wet AMD
Refs-[7],[22],[23],[25],[29],[32],[33],[8],[3]
Protein isoforms originate from a single gene but possess distinct amino acid sequences. The organization of the human VEGF-A genes into eight exons separated by seven introns results in at least seven VEGF-Axxx isoforms, with xxx indicating the number of encoded amino acids. These various isoforms contain between 121 and 206 amino acids, with VEGF-A165 being the most common and researched, followed by VEGF-A121 [23]. Alternative splicing is one of several processes that lead to protein variants. The alternative splicing of VEGF-A (Vascular Endothelial Growth Factor A) generates multiple isoforms that influence its function, localization, and receptor interactions, which are crucial in angiogenesis, vascular permeability, and cell survival. This process involves exons 6, 7, and 8, leading to two major isoform families: pro-angiogenic VEGF-Axxx and anti-angiogenic VEGF-Axxxb. The VEGF-Axxx isoforms, including VEGF-A121, VEGF-A165, VEGF-A189, and VEGF-A206, differ in their ability to bind to the extracellular matrix, with VEGF-A121 being freely diffusible, VEGF-A165 exhibiting partial matrix binding, and VEGF-A189/206 demonstrating strong matrix interaction [25, 23, 7, 22].
In contrast, the anti-angiogenic VEGF-Axxxb isoforms, such as VEGF-A165b and VEGF-A121b, derive from alternative splicing of exon 8b instead of exon 8a, functioning as natural inhibitors by binding VEGF receptors without activating angiogenesis and experience natural angiogenesis inhibition [22]. Proliferating a blood vessel from anti-angiogenic isoforms of VEGF can cause excess of VEGF-A, increasing protein leakiness. In ocular angiogenesis, leaky proteins from abnormal blood vessel growths cause wet AMD, as those leaky vessels exude blood, proteins, and lipids onto the macula, which causes scarring and degradation [3].
Figure 3.2 from ref [23] represents isoform structure visually:
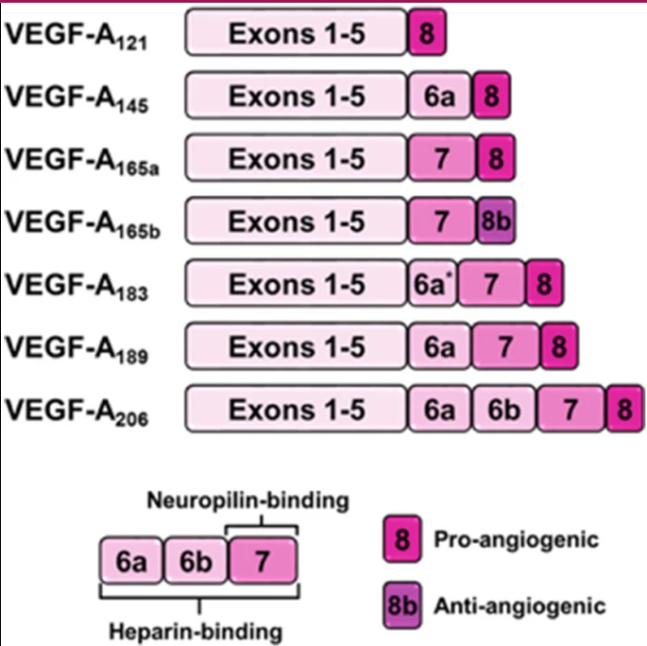
As mentioned, each isoform is broken up into 8 exons and 7 introns. Exons and introns are nucleotide sequences within a gene. Each exon codes for a certain number of amino acids. Exons 1-5 code for 115 amino acids. Alternative splicing of exon 6 results in isoforms with different lengths; for instance, VEGF-A-189 includes an additional 24 amino acids from exon 6a, while VEGF-A-206 incorporates an extra 41 amino acids from exons 6a and 6b with 6b coding for 17 amino acids. Exon 7 encodes about 44 amino acids. All of exon 8 (Both 8a and 8b) encode about 6 amino acids. Each of these exons and introns codes for different characteristics. The below table summarizes the functions of each exon:
|
VEGF-A exon |
Purpose |
Presence in isoforms |
| Exon 1 of VEGF-A | Encodes the signal peptide, directing the nascent VEGF-A protein to the secretory pathway. | Present in all isoforms, not subject to splicing. |
| Exon 2 of VEGF-A | Contributes to the formation of the receptor-binding domain, essential for interaction with VEGF receptors. | Present in all isoforms, not subject to splicing. |
| Exon 3 of VEGF-A | Continues the coding of the receptor-binding domain, playing a crucial role in receptor specificity and binding affinity. | Present in all isoforms, not subject to splicing. |
| Exon 4 of VEGF-A | Encodes a portion of the core domain and includes sites for heparin binding, influencing the protein's interaction with extracellular matrix components (coreceptors, main receptors, etc) | Present in all isoforms, not subject to splicing. |
| Exon 5 of VEGF-A | Contains gene sequences that can be alternatively spliced, affecting the heparin-binding properties and bioavailability of the isoforms. | Present in all isoforms of VEGF except VEGF-A 111. Can be spliced |
| Exon 6a/6b of VEGF-A | This exon, also subject to alternative splicing, influences heparin binding and affects the guidance of endothelial cells during angiogenesis. | 6a is present in isoforms 145, 183, 189, and 206. The only isoform that contains 6b is the largest, 206. |
| Exon 7 of VEGF-A | Encodes the heparin-binding domain, crucial for neuropilin-binding capacity, cell surface proteoglycans and co-receptors, modulating the protein's activity, localization, and distribution | Exon 7 is present in isoforms 165a, 165b, 183, 189, and 206 but not in 145 and 121, the shorter isoforms of VEGF. |
|
Exon 8a of VEGF-A
|
The terminal exon can be differentially spliced to produce pro-angiogenic isoforms, thereby regulating angiogenesis. | All isoforms ending in VEGF-Axxxa or VEGF-Axxx are pro-angiogenic isoforms and include exon 8a. |
|
Exon 8b of VEGF-A
|
The terminal exon can be differentially spliced to produce anti-angiogenic isoforms, thereby regulating angiogenesis. VEGFxxxa and VEGFxxxb isoforms only differ in the six amino acids found at their C termini. [25] | All isoforms ending in VEGF-Axxxb are antiangiogenic isoforms and include exon 8b instead of 8a. |
Based on the properties of these exons, [23, 37, 83] a protein lacking these characteristics can create varying proteins and influence angiogenesis and the attributes of an oncoming blood vessel in Wet-AMD.
Note: In practice, the isoforms of VEGF are often mainly VEGF 165 and 121, with 165 making up about 75% of cases and VEGF 121 making up 24% of the isoform prevalence [30, 84]. However, this project still observes the main prevalent isoforms, as the drug section will likely focus on VEGF 165 and 121 inhibition. It is also important to note that the other isoforms, while not as common, still exist and can be identified, which is why this project includes them as well.
3.2.1-VEGF A-165a/b
Refs- [23],[22],[8],[32],[25]
VEGF-165a is the primary isoform this research study focuses on, and it is the most common and well-researched isoform of VEGF-A [8]. The longer VEGF-A165 isoform, which also contains sequences encoded by exon 7, binds to the receptors with a similar affinity as VEGF-A121 but displays enhanced signalling potential [22]. The interaction of VEGF-A165 with neuropilin-1 is crucial for the regulation of cell migration and serves as the primary signalling output [8]. In contrast, VEGF-A165b functions as an anti-angiogenic isoform [25]. Although it binds to VEGFR-2, it induces significantly weaker receptor phosphorylation and diminished downstream signalling compared to VEGF-A165a. As a result, it suppresses endothelial cell proliferation and migration, thereby inhibiting angiogenesis [8, 32]. VEGF-A165 appears to be a critical isoform for retinal angiogenesis, not only during development but also in pathological conditions. Among the VEGF isoforms, VEGF-A165 is the principal mediator of inflammation and cellular immunity in pathological retinal neovascularization [32].
3.2.2- VEGF A-121a/b
Refs-[23],[37],[38],[32],[8]
The short form of VEGF-A, VEGF-A121, encoded by exons 1–5 and 8 [23], consists of a receptor-binding domain specific for VEGFR-1 and -2 and is the second most common isoform, a poor mitogen. This isoform comprises 121 amino acids and lacks heparin-binding domains, rendering it freely diffusible [38]. Its ability to move unimpeded from producing cells enhances its angiogenic potential compared to other isoforms [32]. VEGF-A-121 is still a weakly angiogenic mitogen for endothelial cells, promoting proliferation and new blood vessel formation. Its free diffusibility allows it to effectively reach and activate VEGF receptors on endothelial cells, facilitating angiogenesis in both physiological and pathological contexts [37, 23].
3.2.3- VEGF A-206a/b
Refs-[32],
VEGF A-206 is the longest amino acid sequence that forms a VEGF isoform, it contains all exons 1-8a/8b and extensive heparin-binding sequences, leading to its strong association with the ECM and minimal diffusion. Due to its size and binding characteristics, VEGF-A_206 is less studied, but it is believed to play a role in creating stable, localized angiogenic signals within specific tissue environments. Its restricted distribution suggests a function in maintaining vascular homeostasis rather than promoting widespread angiogenesis. [32]
3.2.4- VEGF A-189a/b
Refs-[32],[39],[23]
This isoform, comprising 189 amino acids, possesses strong heparin-binding domains, resulting in its sequestration within the ECM and limited diffusibility [39]. VEGF-A_189 can be cleaved proteolytically to release shorter, bioactive forms, thereby regulating angiogenesis in a controlled manner [32,39]. It is the first of the less common isoforms and is the second largest only to VEGF 206 [23].
3.2.5-VEGF A-145
Refs-[32],[36],[41]
Consisting of 145 amino acids, this isoform includes additional sequences that confer heparin-binding capacity, leading to its association with cell surfaces and the extracellular matrix (ECM) because it contains exons 1-6, and 8 [41]. This localization restricts its diffusion, allowing for localized angiogenic signalling. VEGF145 and VEGF165 both can bind to the KDR/flk-1 receptor on endothelial cells. Additionally, they exhibit a similar affinity for heparin. However, VEGF145 does not interact with two other endothelial cell surface receptors that VEGF165 binds, which are not recognized by VEGF121. Like VEGF165 and VEGF121, VEGF145 is secreted from its producing cells. Its binding with heparan sulphate proteoglycans in the ECM plays a crucial role in modulating its availability and activity, thereby influencing angiogenesis in specific tissue environments [36].
4- Pharmacological treatments of wet AMD
Refs- [3],[1],[5],[17],[18],[19],[40],[61],[62]
Wet AMD is treatable if detected in its early stages and is commonly addressed in one of two ways. Firstly, aims to stop or shrink new blood vessels. However, this method is less common because its effectiveness depends on the vessel's location, among other factors [61, 62]. The second form of wet AMD treatment, which is the focus of this research project, involves injections. Injections are a method of administering medication; in wet AMD treatment, these injections deliver drugs typically formulated to inhibit VEGF [5, 40]. As noted, VEGF stands for vascular endothelial growth factor, a crucial protein for your body to form blood vessels. The anti-VEGF drugs usually employed for wet AMD often fall into the category of monoclonal antibodies or MAbs. A monoclonal antibody is a lab-created drug that binds to or targets a specific antigen; in this case, that antigen is VEGF. Monoclonal antibodies that target VEGF are primarily used as therapies for metastatic cancer, where they attach to the extracellular tyrosine kinases that transmit signals for VEGF proteins (these enzymes are often genetically or epigenetically modified to benefit cancer cells). They prevent VEGF from binding to its receptor, resulting in antiangiogenesis. In cancer, reducing the number of blood vessels leads to fewer nutrients for the tumour, causing it to shrink [17, 18]. In recent years, VEGF-inhibiting monoclonal antibodies have been adapted for ocular therapy and are often administered intravitreally using a syringe. Although there are various anti-VEGF drugs, this research will focus on the most common anti-angiogenesis medications: Bevacizumab (Avastin), Ranibizumab (Lucentis), Brolucizumab (Beovu), Aflibercept (Zaltrap/Eylea), Faricimab (Vabysmo), and Pegaptanib (Macugen) [19, 5]. Note that Aflibercept and Pegaptanib are not monoclonal antibodies; Aflibercept is a recombinant fusion protein, and Pegaptanib is a polynucleotide aptamer. Both are included in this project for comparative purposes. However, all these drugs are designed to target VEGF isoforms [19], as this study investigates whether isoform identification can optimize treatments that target specific isoforms and whether isoform prevalence can influence the presentation of wet AMD.
But, Anti-VEGF drugs can have adverse side effects as they block all or most present VEGF which can result in hemorrhages, clots in the arteries (with resultant stroke or heart attack), hypertension, impaired wound healing, reversible posterior leukoencephalopathy syndrome (a brain disorder), and protein in the urine. Antiangiogenesis agents that target the VEGF receptor have additional side effects, including fatigue, diarrhea, biochemical hypothyroidism, cardiac failure, and hair changes. However, these adverse effects are rather rare and often only mild side effects are present [19, 5, 40].
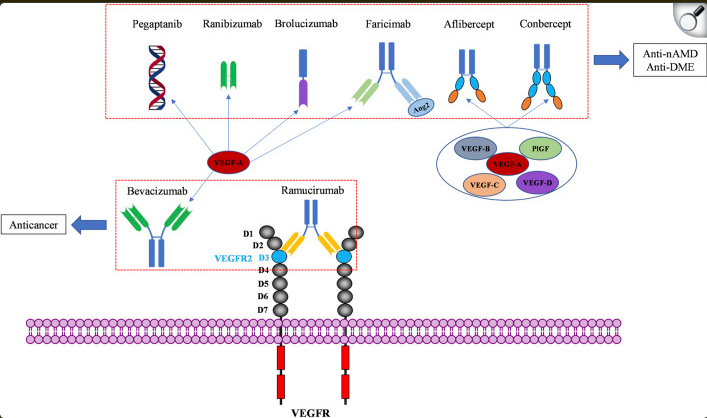
Figure 4 [19] shows the common pharmacological treatments and their structures relative to their functions.
4.1- Monoclonal antibodies
Refs-[53],[54]
Many of the anti-angiogenesis drugs examined in this study are monoclonal antibodies, and since they belong to the same category, they are likely to function in similar ways. A monoclonal antibody is a laboratory-made drug that binds to a single molecular target with high specificity. These antibodies can mark a molecule or cell for destruction by signalling to the immune system, providing targeted therapy such as chemotherapy in cancer treatment, and blocking molecules from interacting with receptors, ultimately interrupting certain biological processes to treat diseases [53]. This project focuses on inhibiting VEGF, a prominent target for monoclonal antibodies due to its high expression in cancer cells and retinal angiogenesis. When regulating VEGF, the monoclonal antibody binds to the overexpressed extracellular portion of the VEGFRs, thereby disrupting signalling pathways essential for angiogenesis. Most of the drugs listed below are murine CDRs identified by their common prefix "zumab" and are often described as humanized monoclonal antibodies [54].
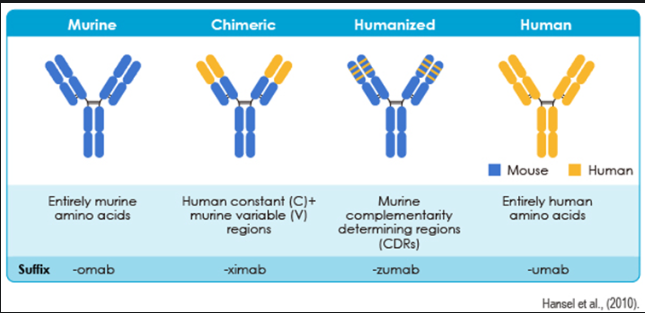
Figure 4.1 of monoclonal antibody classification and subsequent suffixes from ref [54].
4.1.1- Bevacizumab (Avastin)
Refs- [3],[19],[13],[52],[80]
Bevacizumab is the first antiangiogenic drug this study investigates. It is a full-length, humanized monoclonal antibody that binds to circulating VEGF. As the first antiangiogenic VEGF-inhibiting drug, it gained FDA approval for treating various metastatic cancers, particularly colon cancer, in 2004 [19, 52, 3, 13]. Its binding capabilities prevent interaction between VEGF and VEGFR, the receptor signalling pathway responsible for the abnormal angiogenesis that causes WAMD [19, 13]. The dosage intervals for treating WAMD can vary, but it is commonly around 5 mg/kg every two weeks for two to three treatments [3]. Since Avastin is the oldest monoclonal antibody, it remains the preferred choice for anti-angiogenic drugs, supported by relatively successful clinical trials and medical results indicating an increase in mean visual acuity and central retinal thickness [80]. After one year of treatment, Bevacizumab shows comparable effects to ranibizumab in general visual acuity [3]. Although Bevacizumab targets all isoforms of VEGF, which might appear to be the optimal approach, this could result in increased side effects due to the broad scope of inhibition, as it also targets anti-angiogenic variants of VEGF. For example, it binds both VEGFA165a and VEGFA165b equally. This could be counterintuitive, raising the risks of side effects without effectively treating the disease. Addressing this phenomenon is a key focus of this project and ties into this research. Possible clinical trials involving Bevacizumab and other monoclonal antibodies that have not successfully increased or stabilized visual acuity may have inadvertently targeted naturally antiangiogenic isoforms of VEGF; inhibiting these would not contribute to treating WAMD and may lead to diminished visual acuity, often blamed on the drug’s ineffectiveness. Targeting specific isoforms could enable treatment optimization and promote proper administration and a clearer understanding of drug diagnosis. An important aspect of Bevacizumab is that, as previously mentioned, it is a full-length antibody, setting it apart from other monoclonal antibodies, which are frequently fragments. Animal studies suggested that fragments penetrated retinal tissue better than full-length MAbs, and due to possible immune responses, the safety of full-length antibodies was questioned until 2005, when a successful clinical trial of intravitreal injections on patients with WAMD and CRVO resulted in the popularization of the drug in ophthalmology and ocular research [13].
4.1.2- Ranibizumab (Lucentis)
Refs-[3],[13],[14],[19],[81]
Ranibizumab is another monoclonal antibody, but it differs from Bevacizumab because it is a recombinant humanized IgG kappa isotype monoclonal antibody fragment derived from bevacizumab. Similarly to Avastin, ranibizumab neutralizes all isoforms of VEGF-A. Ranibizumab was also developed specifically for ocular use, alongside pegaptanib [13]. It consists solely of the antigen-binding fragment (Fab) of bevacizumab. Although significantly smaller than bevacizumab, it exhibits a stronger affinity for VEGF. It binds specifically to VEGFR2's D3 of its Ig-domain in the ECM [19]. It was specifically developed for intravitreal administration (IVT) to address neovascular ocular diseases, with its reduced size allowing for efficient diffusion from the vitreous into the retina and choroid, treating diseases like mCNV [14]. Ranibizumab is regarded as a safer alternative to bevacizumab due to its rapid systemic clearance, which many criticized, and was why so many were initially skeptical of bevacizumab's use in ocular therapies [13]. Ranibizumab's half-life is considerably shorter, lasting only 2–4 days compared to bevacizumab’s approximate three-week duration. Additionally, the absence of a fragment crystallizable (Fc) region eliminates complement-associated intraocular inflammation following IVT. In a 2006 multicenter, 2-year, double-blind, sham-controlled study, randomly assigned patients with AMD were to receive 24 monthly intravitreal injections of ranibizumab (either 0.3 mg or 0.5 mg) it was concluded that high rates of vision loss prevention and improved visual acuity, with rare serious adverse effects [3, 14]. Ranibizumab (Lucentis, Genentech, Inc.) clinical trials began shortly thereafter. The ANCHOR (Anti-VEGF Antibody for Treating Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration) and MARINA (Minimally Classic/Occult Trial of Ranibizumab for Neovascular Age-Related Macular Degeneration) studies demonstrated that over 90% of patients receiving intravitreal ranibizumab avoided significant visual loss (at least 15 letters on the logMAR chart) when treated for 24 consecutive months, regardless of the lesion’s type, size, patient age, or initial visual acuity. More than one-third experienced at least a 3-line improvement in visual acuity, and one in three doubled their visual acuity during the 24-month treatment duration. Furthermore, approximately 70% of patients maintained stable vision during this time, with gains of up to 2 lines of vision [81].
4.1.3- Brolucizumab (Beovu)
Refs-[19],[12],
Similarly to ranibizumab, brolucizumab (beovu) is a humanized single-chain antibody fragment. It has a high affinity for all VEGF-A isoforms, inhibiting ligand-receptor interactions [12]. Containing only 255 amino acids, brolucizumab has a very high permeability and solubility, making its administration fairly easy as it very efficiently reaches the retina and choroid [19]. Brolucizumab has been reported to have a higher affinity for VEGF than most other anti-VEGF treatments and is stated approximately 11 times more effective than aflibercept [19]. Approved for ocular use in 2019, a usual intravitreal injection of brolucizumab is approximately 6 mg every month for 3 months, later progressing in some patients to a 2-3 month dosing interval. The phase 3 HAWK and HARRIER studies stated that " Approximately 50% of patients could be maintained on an every-12-week brolucizumab dosage with non-inferior visual acuity (VA) outcomes compared with an every-8-week aflibercept regimen," proving brolucizumab to be a very potent angiogenic inhibitor [12].
4.1.4- Faricimab (Vabysmo)
Refs-[19],[57],
Farcimab is somewhat distinct from the other listed MaBs as it is the first humanized bispecific monoclonal antibody employed in the inhibition of VEGF for nAMD. Its heterodimeric bispecific properties allow it to bind to both VEGF and angiopoietin-2 (Ang-2). This is due to its differing light chains in each of the Fab regions, with one binding to each protein. Angiopoietin-2, or Ang-2, is also a pro-angiogenic factor, activating Tie2 receptors to promote cell survival and vascular stability. This drug can neutralize both proteins simultaneously to prevent VEGF-induced angiogenesis, restore vascular stability by reducing leakage, lower inflammation, and decrease neovascularization. In the faricimab molecule, the Fc fragment, which is the crystallizable region of antibodies binding to cell receptors and complement proteins, has also been modified to diminish undesirable immune system responses. Phase 2 trials have demonstrated Vabysmo's efficacy, said to be comparable to ranibizumab. IVT injections can be as far as 16 weeks apart, significantly lowering patient burden and clinical visits. However, some claim the high dose of faricimab injected (6 mg) may be a contributor to the decrease in the number of IVT injections, and that the extent to which Ang-2 blockade contributed to the therapeutic efficacy is still unclear, as this drug is relatively new in angiogenesis inhibition [19, 57].
4.2- Aflibercept (Zaltrap/Eylea)
Refs-[19],[3],[9],[10],[11],[55],[59]
Aflibercept is the first non-monoclonal antibody included in this project, as its popularity in ocular treatment has drastically increased in recent years, yielding excellent results in clinical trials. Aflibercept (also known as Zaltrap or Eylea) is a recombinant fusion protein made up of VEGFR-1 D2 and VEGFR-2 D3, the immunoglobulin domains of both VEGFR-1 and VEGFR-2 fused to the constant region of human IgG. It acts as a receptor decoy that binds and neutralizes both VEGF and placental growth factor ligands. It is approved for treatment in DME and AMD. The usual Aflibercept injection is 2 mg monthly or every 2 months. Aflibercept had safety and tolerability results comparable to ranibizumab. Aflibercept enhanced vision, i.e., 96%, 95%, and 95% of patients taking injections of 0.5 mg monthly, 2 mg monthly, and 2 mg every 2 months, respectively [3]. Aflibercept was approved for ocular use in 2011, stated as non-inferior to ranibizumab by the fact that it has a longer shelf life, lowering the treatment burden on patients.
4.3- Pegaptanib (Macugen)
Refs-[19],[3],[15],[8],[82]
Pegaptanib is the second non-monoclonal antibody noted in this research and serves as a prime example of an isoform-specific drug [15]. Logically, since VEGF165 is the most common, accounting for the majority of cases, Pegaptanib would specifically target VEGFA-165, and interestingly does not target VEGF165b [8]. Pegaptanib is a pegylated polynucleotide aptamer that specifically targets the C-terminal of VEGF isoforms. It received FDA approval in the same year as Avastin, 2004, for WAMD and was the first officially recognized antiangiogenic drug developed specifically for ocular pathologies [19]. A usual injection of intravitreal pegaptanib often is 0.3 mg every 6 weeks [3]. Its side effects are considerably milder compared to those of broader VEGF-targeting drugs. It was well tolerated and effective in patients with nAMD [19]. Ranibizumab and bevacizumab bind to the RBD sequence, which is common to all the VEGF isoforms, thereby blocking their binding to VEGFRs and the associated angiogenic signalling, but pegaptanib only binds the end of the protein, which is different for each isoform. This explains the more potent effect of these drugs in inhibiting endothelial cell migration, proliferation, and vascular permeability compared to pegaptanib. Pegaptanib's specificity for VEGF165 allows it to selectively inhibit pathological angiogenesis while preserving the physiological roles of other VEGF isoforms, reducing potential systemic adverse effects. Despite its efficacy, pegaptanib was eventually overshadowed by broader VEGF inhibitors, which demonstrated greater overall suppression of neovascularization [8]. Nevertheless, its development marked a significant milestone in antiangiogenic therapy, paving the way for further advancements in targeted ocular treatments. Pegaptanib was then evaluated in two concurrent, multi-center, prospective, randomized, double-blinded, sham-controlled, dose-ranging trials on patients with various types of wet AMD: the VEGF Inhibition Study in Ocular Neovascularization (VISION) trials. These studies showed that pegaptanib treatment every 6 weeks reduced vision loss by about 50% in the first year and maintained this benefit, stabilizing vision acuity later on [82]
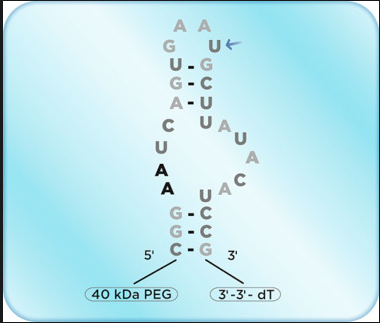
Figure 4.3- Pegaptanib nucleotide aptamer structure [8].
4.4- Combination with laser therapy
Refs-[3],[61],[62],[63]
All of the drugs listed above, more or less, can be combined with laser therapy to enhance effectiveness. Although laser therapy is used less than anti-VEGF drugs, it can be helpful in specific situations. Only people with classic CNV can be eligible for laser treatment, and only 26% of wAMD patients were found eligible through FA. The main reason why laser treatment is used less than anti-VEGF drugs is because of concerns regarding retinal damage and because it is so low in compatibility [61,62]. Laser photocoagulation can cause collateral injury to surrounding healthy tissue, which can lead to permanent scarring and vision loss. Additionally, laser therapy does not target the underlying pathological angiogenesis as effectively as anti-VEGF agents and its effects may be temporary, requiring repeated treatments, particularly in patients with large lesions or advanced disease. It can place a large burden on the patient, increased treatment administration and higher doses, increased risk of adverse effects, cost, etc. But, in some cases, laser therapy can provide a more immediate reduction in vascular leakage compared to anti-VEGF drugs, though this comes at the cost of potentially severe effects. However, many clinical trials that test in specific situations have used VEGF inhibition paired with laser photocoagulation to achieve better results and have been successful in many cases [3]. There is a combination therapy called Visudyne that works as a literal combination of anti-VEGF and laser. It is injected into the arm and then travels into the eye (similarly to a FA), and is then activated by a laser. But again, it is likely costly and very specific to the case [63].
5- Side effects
Refs-[8],[14],[19],[55],[56],[57],[58],[59],[64],[65],[10],[11],[80]
Side effects are extremely important in monitoring ocular therapies, particularly since macular degeneration is frequently associated with individuals over 50. Below is a chart of the side effects observed from Wet AMD treatments. The most common side effects are subconjunctival hemorrhages, vitreous floaters, and reduced visual acuity. Due to the infusion, most IVT injections result in a temporary increase in IOP. These often occur in the first hour after administration and usually stabilize.
| Drug / Ocular therapy | Common and uncommon side effects |
| Bevacizumab (Avastin) | Common Avastin side effects: vitreous floaters, eye redness, dry eye and itchy eyes. Uncommon side effects- endophthalmitis, retinal detachment, cataracts, photosensitivity, eye pain, ocular edema. - similar to Zaltrap. [19, 80]. |
| Ranibizumab (Lucentis) | Common Lucentis side effects: increased redness in the white of the eye, ocular pain, specks in vision/vitreous floaters) and increased IOP. Uncommon: thromboembolic events like heart attack, stroke, and death. [14, 19, 58]. |
| Brolucizumab (Beovu) | Common Beovu side effects: retinal vasculitis, intraocular inflammation, occlusive disease, subconjunctival hemorrhages, vitreous floaters, reduced visual acuity, vitreous detachment, and the most common non-ocular side effects are upper respiratory tract infection and urinary tract infection. Uncommon side effects: a significant drop in VA, arterial occlusion, severe hypersensitivity reactions, and endophthalmitis. [19, 64, 65]. |
| Faricimab (Vabysmo) |
Common Vabysmo side effects: Endophthalmitis, retinal detachment, light sensitivity, and vision loss. Uncommon side effects: thromboembolic events (heart attacks, blood clots, strokes, severe inflammation of vessels in the retina. (64 out of 1,262 patients treated with VABYSMO reported severe thromboembolic events). [19, 57]. |
| Aflibercept (Zaltrap/Eylea) | Common Zaltrap side effects are vitreous floaters, eye redness, dry eyes, and itchy eyes. Uncommon side effects- endophthalmitis, retinal detachment, cataracts, photosensitivity, eye pain, and ocular edema [10,11,55, 59]. |
| Pegaptanib (Macugen) |
Common Macugen side effects: eye irritation or discomfort, sensation of a foreign object in the eye, light sensitivity (photophobia), Swelling of the eyelid, increased tear production, and headache. Uncommon side effects- endophthalmitis, retinal detachment, vitreous hemorrhage, corneal edema (swelling), hyphema, cataract formation, and allergic reactions [8, 19, 56]. |
As shown above, many of the side effects are similar, especially between drugs that work similarly. However, each drug has advantages and disadvantages that can be considered when optimizing treatment.
5.1- abbreviations
WAMD/WARMD- wet age-related macular degeneration, nAMD- neovascular age-related macular degeneration, VEGF- vascular endothelial growth factor. PlGF- placental growth factor. PED- pigment epithelial detachment. CNVM- choroidal neovascular membranes. RPE- retinal pigment epithelium. OCT- optical coherence tomography. CRVO- central retinal vein occlusion. IVT- intravitreal injection. VEGFR1/2/3- vascular endothelial growth factor receptors. RTK- receptor tyrosine kinase. ATP- adenosine triphosphate. Ig- immunoglobulin-like domain. KD- kinase domain. ECM- extracellular matrix. ICM- intracellular. JM- juxtamembrane. KI- kinase insert. NRP-1/2- neuropilin-1/2. HSPGs- heparan sulphate proteoglycans. MaBs- monoclonal antibodies. CDR- complementarity determining regions. VA- visual acuity. FA- fluorescein angiography
Data
Conclusion
1-Discussion
VEGF is a protein vital to endothelial cell development and angiogenesis. Many biological factors contribute to the proliferation of blood vessels. Retinal neovascularization can result in many diseases, namely wet age-related macular degeneration, where a blood vessel outgrowth leaks lipids, proteins, and blood onto the macula, causing scarring and damage. This project investigates the utilization of protein analysis to identify and understand different isoforms of VEGF. Understanding a blood vessel's physiological and biological characteristics can help optimize anti-VEGF treatment. VEGF isoforms have a plethora of characteristics that differentiate them from each other. Namely, their coreceptor association. All of the isoforms of VEGF-A bind both VEGFR1 and VEGFR2. Both receptors are required for angiogenesis, and excluding VEGFR1 ligand trapping and antiangiogenic isoforms, all will proliferate and result in the formation of a blood vessel. VEGF-A165a is the most common isoform of VEGF, containing exons 1-5, 7, and 8. It binds R1/R2 with a similar affinity as VEGFA-121 but has a higher signalling potential. Its coreceptor association with NRP-1 is crucial for endothelial cell tip guidance. VEGF-121 is the second most prominent isoform of VEGF, it has only a VEGFR1/R2 specific binding domain and is a poor mitogen. Lacking heparin-binding domains renders it freely diffusible, effectively activating signalling pathways. VEGF 206, 189, and 145 are the less common VEGF isoforms, and even so have their distinct characteristics. VEGF 206, the longest amino acid sequence for a VEGF isoform, containing exons 1-8a/b. Its extensive heparin-binding domain grants it a strong association with the ECM and minimal binding. For these reasons, VEGF-206 is believed to have roles in maintaining vascular homeostasis and localization of angiogenic signals. VEGF 189, comprising exons 6a, 7, and 8, also has a strong binding domain for heparin and is sequestered within the ECM, with limited diffusion. Finally, VEGF 145, made of exons 1-5, 6a, and 8, is similar to 121, but has heparin-binding properties. Its composition allows it to associate cell surfaces and the ECM. It is said to exhibit a similar affinity for heparin as VEGF-165. Its binding with heparan sulphate proteoglycans in the ECM plays a crucial role in modulating its availability and activity, thereby influencing angiogenesis in specific tissue environments. From these facts, it can be demonstrated that differing isoforms can have distinct characteristics. Their binding to both receptors and coreceptors, their structure, diffusibility, and solubility throughout the ECM, and their role in angiogenesis play a considerable role in ocular neovascularization. A very important aspect of this research is the anti vs proangiogenic aspect of VEGF isoforms, which determines their angiogenic potential substantially. Differential splicing of VEGF exon 8b produces anti-angiogenic isoforms, thereby regulating angiogenesis. For example, VEGF-A 165b, is a variation of VEGF 165 containing exons 1-5, 7, and 8b. VEGF-A165b functions as an anti-angiogenic isoform. Although it binds to VEGFR-2, it induces significantly weaker receptor phosphorylation and diminished downstream signalling compared to VEGF-A165a. As a result, it suppresses endothelial cell proliferation and migration, thereby inhibiting angiogenesis. Treatment regimens that do not target isoforms or broadly target VEGF may end up targeting antiangiogenic isoforms of VEGF, isoforms that were already being inhibited, thereby being counterintuitive. All this does is increase the treatment burden for the patient, and increase the likelihood of side effects without reason. This is one of the reasons why a possible isoform-specific treatment could be adapted successfully. Another vital reason why this research could be adapted to ocular therapy is as mentioned, the controlling or limiting of unwanted side effects. Pegaptanib is an example of an isoform-specific drug, it targets the C-terminal (end of the protein) of VEGF proteins. Its side effects were reported considerably milder and it was much more tolerated by people with nAMD during treatment. Pegaptanib's specificity for VEGF165 allows it to selectively inhibit pathological angiogenesis while preserving the physiological roles of other VEGF isoforms, reducing potential systemic adverse effects. In a recent study, pegaptanib treatment every 6 weeks reduced vision loss by about 50% in the first year and maintained this benefit, stabilizing vision acuity in the second year. This demonstrates a clear example of the efficacy and benefits of isoform-specific ocular therapy and its potential success as an adapted therapy.
1.1- Direct applications
Evidently from the context of this research, the main application of this is the potential optimization of treatment for nAMD through isoform targeting. By understanding the characteristics of blood vessels that are leaking and abnormally appearing intraocularly, treatment can be optimized. This optimization can lower the risk of side effects associated with broader inhibition of VEGF, side effects of drugs noted as severe as fatality or heart attack and stroke. The personalization of treatment can also lower the treatment burden in certain cases. Dosage intervals can be altered according to the patient and their needs. Aside from ocular therapy, the majority of this research could be taken and applied in cancer research. Throughout my research, especially when researching ocular therapies like Bevacizumab and Lucentis, I found that these treatments were also heavily adapted in oncological treatment, even sometimes derived from cancer treatment, adapted into ocular therapy. Bevacizumab is a drug originally made to treat mCRC (metastatic colorectal cancer). It does so by inhibiting the VEGF released by cancer cells to grow the tumour. Without this VEGF, the tumour cannot supply itself with nutrients and shrinks. It is a very popular oncological treatment, alongside some other anti-VEGF drugs that treat other cancers like NSCLC in the same way. These alternative applications beg the question of whether treatment personalization or optimization through isoform targeting could be possible or even more feasible in cancer treatment.
1.2- Limitations
Recognizing the limitations of a scientific idea or possible application of a technology is a very important part of research. The first possible limitation and likely the most important, is cost. Although exact costs can't be predicted, they can be expected to be quite high because, in combination with sample analysis and drug treatment, costs can pile up. It is difficult to find a definite solution to an issue like this as there are various ways that financials can be combated. For example, the coverage of certain insurance companies for the ocular therapy, the use of other analysis strategies based on the area, etc. Another issue that may arise is a low abundance of isoforms. Some VEGF-A isoforms may be present at very low concentrations, making their detection difficult without extensive enrichment or fractionation. This is combated by the use of LCM for acquiring endothelial cell samples, which can acquire small sample amounts, causing minimal damage to surrounding tissue. Other issues arise in sample processing like VEGF-A isoforms being susceptible to proteolysis, oxidation, and other post-translational modifications that may affect their detection and quantification. A possible solution for this could be adding protease inhibitors during sample preparation to preserve isoform integrity. In hindsight, this research is based mainly on facts and other real-world data, and there are only ways to strongly predict the practicality of this method and none to ensure that this process will be feasible when in practice, which is why it is important to address possible limitations for research.
Citations
[1]Wet macular degeneration. (2025, February 18). Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/wet-macular-degeneration
[2]Understanding macular degeneration. (2024, October 1). American Academy of Ophthalmology. https://www.aao.org/eye-health/diseases/amd-macular-degeneration
[3]Fernandes, A. R., Zielińska, A., Sanchez-Lopez, E., Santos, T. D., Garcia, M. L., Silva, A. M., Karczewski, J., & Souto, E. B. (2022). Exudative versus Nonexudative Age-Related Macular Degeneration: Physiopathology and Treatment Options. International Journal of Molecular Sciences, 23(5), 2592. https://doi.org/10.3390/ijms23052592
[4]What is the role of ischemia in AMD? (2023, December 7). PentaVision. https://retinalphysician.com/issues/2012/april/what-is-the-role-of-ischemia-in-amd/
[5]Prall, F. R., MD. (n.d.). Exudative (WeT) Age-Related Macular Degeneration (AMD) Treatment & management: medical care, surgical care, consultations. https://emedicine.medscape.com/article/1226030-treatment?form=fpf
[6]Comparison of Anti-VEGF treatments for wet AMD. (2024, May 23). American Academy of Ophthalmology. https://www.aao.org/eye-health/diseases/avastin-eylea-lucentis-difference
[7]Arcondeguy, T., Lacazette, E., Millevoi, S., Prats, H., & Touriol, C. (2013). VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Research, 41(17), 7997–8010. https://doi.org/10.1093/nar/gkt539
[8]Amadio, M., Govoni, S., & Pascale, A. (2015). Targeting VEGF in eye neovascularization: What’s new? Pharmacological Research, 103, 253–269. https://doi.org/10.1016/j.phrs.2015.11.027
[9]What is Eylea? (2024, September 11). American Academy of Ophthalmology. https://www.aao.org/eye-health/drugs/what-is-eylea
[10]Metastatic Colorectal Cancer (mCRC) Treatment | ZALTRAP® (ziv-aflibercept). (n.d.). https://www.zaltrap.com/
[11]Aflibercept in the treatment of patients with metastatic colorectal cancer: latest findings and interpretations - PMC. (n.d.). https://pmc.ncbi.nlm.nih.gov/articles/PMC3808572/
[12]Brolucizumab - EyeWiki. (2024, December 4). https://eyewiki.org/Brolucizumab
[13]Stewart, M. W. (2012). The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clinic Proceedings, 87(1), 77–88. https://doi.org/10.1016/j.mayocp.2011.10.001
[14]Genentech. (n.d.). What is mCNV? | LUCENTIS® (ranibizumab). Lucentis. https://www.lucentis.com/patient/mcnv/about/what-is-mcnv.html
[15]Pegaptanib: Uses, interactions, mechanism of action | DrugBank Online. (n.d.). DrugBank. https://go.drugbank.com/drugs/DB04895
[16]Pigment Epithelial Detachment - EyeWiki. (2024, August 12). https://eyewiki.org/Pigment_Epithelial_Detachment
[17]Monoclonal antibodies (mAbs). (n.d.). https://www.cancerresearchuk.org/about-cancer/treatment/targeted-cancer-drugs-immunotherapy/monoclonal-antibodies
[18]Professional, C. C. M. (2024, May 1). Monoclonal antibodies. Cleveland Clinic. https://my.clevelandclinic.org/health/treatments/22246-monoclonal-antibodies
[19]Wang, L., Liu, W., Broussy, S., Han, B., & Fang, H. (2024). Recent advances in anti-angiogenic inhibitors targeting the VEGF/VEGFR axis. Frontiers in Pharmacology, 14. https://doi.org/10.3389/fphar.2023.1307860
[20]Paul, M. K., & Mukhopadhyay, A. K. (2004). Tyrosine kinase – Role and significance in Cancer. International Journal of Medical Sciences, 101–115. https://doi.org/10.7150/ijms.1.101
[21]Rahimi, N. (2006). VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Frontiers in Bioscience, 11(1), 11. https://doi.org/10.2741/1839
[22]Delcombel, R., Janssen, L., Vassy, R., Gammons, M., Haddad, O., Richard, B., Letourneur, D., Bates, D., Hendricks, C., Waltenberger, J., Starzec, A., Sounni, N. E., Noël, A., Deroanne, C., Lambert, C., & Colige, A. (2012). New prospects in the roles of the C-terminal domains of VEGF-A and their cooperation for ligand binding, cellular signalling and vessel formation. Angiogenesis, 16(2), 353–371. https://doi.org/10.1007/s10456-012-9320-y
[23]Mamer, S. B., Wittenkeller, A., & Imoukhuede, P. I. (2020). VEGF-A splice variants bind VEGFRs with differential affinities. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-71484-y
[24]Monaghan, R. M., Page, D. J., Ostergaard, P., & Keavney, B. D. (2020). The physiological and pathological functions of VEGFR3 in cardiac and lymphatic development and related diseases. Cardiovascular Research, 117(8), 1877–1890. https://doi.org/10.1093/cvr/cvaa291
[25]Peach, C. J., Mignone, V. W., Arruda, M. A., Alcobia, D. C., Hill, S. J., Kilpatrick, L. E., & Woolard, J. (2018). Molecular Pharmacology of VEGF-A isoforms: binding and signalling at VEGFR2. International Journal of Molecular Sciences, 19(4), 1264. https://doi.org/10.3390/ijms19041264
[26]King, C., & Hristova, K. (2019). Direct measurements of VEGF–VEGFR2 binding affinities reveal the coupling between ligand binding and receptor dimerization. Journal of Biological Chemistry, 294(23), 9064–9075. https://doi.org/10.1074/jbc.ra119.007737
[27]Cébe-Suarez, S., Zehnder-Fjällman, A., & Ballmer-Hofer, K. (2006). The role of VEGF receptors in angiogenesis; complex partnerships. Cellular and Molecular Life Sciences, 63(5). https://doi.org/10.1007/s00018-005-5426-3
[28]Shibuya, M. (2011). Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signalling in angiogenesis: a crucial target for anti- and Pro-aging therapies. Genes & Cancer, 2(12), 1097–1105. https://doi.org/10.1177/1947601911423031
[29]Keyt, B. A., Berleau, L. T., Nguyen, H. V., Chen, H., Heinsohn, H., Vandlen, R., & Ferrara, N. (1996). The carboxyl-terminal domain(111–165) of vascular endothelial growth factor is critical for its mitogenic potency. Journal of Biological Chemistry, 271(13), 7788–7795. https://doi.org/10.1074/jbc.271.13.7788
[30]Whitaker, G. B., Limberg, B. J., & Rosenbaum, J. S. (2001). Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signalling potency of VEGF165 and VEGF121. Journal of Biological Chemistry, 276(27), 25520–25531. https://doi.org/10.1074/jbc.m102315200
[31]MuñOz, E. M., & Linhardt, R. J. (2004). Heparin-Binding Domains in Vascular Biology. Arteriosclerosis Thrombosis and Vascular Biology, 24(9), 1549–1557. https://doi.org/10.1161/01.atv.0000137189.22999.3f
[32]Holmes, D. I., & Zachary, I. (2005). The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biology, 6(2), 209. https://doi.org/10.1186/gb-2005-6-2-209
[33]Redirecting. (n.d.). https://www.google.com/url?q=https://ashpublications.org/blood/article/105/4/1383/20352/The-pathophysiologic-role-of-VEGF-in-hematologic&sa=D&source=docs&ust=1740915046033126&usg=AOvVaw10QNHTbAJuqv8EmSaOZeEr
[34]Robinson, C. J., & Stringer, S. E. (2001). The splice variants of vascular endothelial growth factor (VEGF) and their receptors. Journal of Cell Science, 114(5), 853–865. https://doi.org/10.1242/jcs.114.5.853
[35]Krilleke, D., Ng, Y. E., & Shima, D. T. (2009). The heparin-binding domain confers diverse functions of VEGF-A in development and disease: a structure-function study. Biochemical Society Transactions, 37(6), 1201–1206. https://doi.org/10.1042/bst0371201
[36]Poltorak, Z., Cohen, T., Sivan, R., Kandelis, Y., Spira, G., Vlodavsky, I., Keshet, E., & Neufeld, G. (1997). VEGF145 is a secreted vascular endothelial growth factor isoform that binds to the extracellular matrix. Journal of Biological Chemistry, 272(11), 7151–7158. https://doi.org/10.1074/jbc.272.11.7151
[37]Catena, R., Larzabal, L., Larrayoz, M., Molina, E., Hermida, J., Agorreta, J., Montes, R., Pio, R., Montuenga, L. M., & Calvo, A. (2010). VEGF121b and VEGF165b are weakly angiogenic isoforms of VEGF-A. Molecular Cancer, 9(1). https://doi.org/10.1186/1476-4598-9-320
[38]Cohen, T., Gitay-Goren, H., Sharon, R., Shibuya, M., Halaban, R., Levi, B., & Neufeld, G. (1995). VEGF121, a vascular endothelial growth factor (VEGF) isoform lacking heparin-binding ability, requires cell-surface heparan sulphates for efficient binding to the VEGF receptors of human melanoma cells. Journal of Biological Chemistry, 270(19), 11322–11326. https://doi.org/10.1074/jbc.270.19.11322
[39]Unique properties of 189 amino acid isoform of vascular endothelial growth factor in tumorigenesis. (2002, December 1). PubMed. https://pubmed.ncbi.nlm.nih.gov/12429975/
[40]Angiogenesis inhibitors. (2018, April 2). Cancer.gov. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/angiogenesis-inhibitors-fact-sheet
[41]Poltorak, Z., Cohen, T., Sivan, R., Kandelis, Y., Spira, G., Vlodavsky, I., Keshet, E., & Neufeld, G. (1997). VEGF145 is a secreted vascular endothelial growth factor isoform that binds to the extracellular matrix. Journal of Biological Chemistry, 272(11), 7151–7158. https://doi.org/10.1074/jbc.272.11.7151
[42]Thermo Scientific Orbitrap Fusion Mass Spectrometer Animation. (n.d.). [Video]. https://www.thermofisher.com/ca/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-mass-spectrometry.html
[43]Protein Mass spectrometry. (n.d.). https://www.sigmaaldrich.com/CA/en/applications/protein-biology/protein-mass-spectrometry
[44]Mass spectrometry-based protein sequencing - Creative proteomics. (n.d.). https://www.creative-proteomics.com/proteinseq/mass-spectrometry-based-protein-sequencing.htm
[45]Klein, J. B., & Knepper, M. A. (2018). Protein mass spectrometry is made simple. Journal of the American Society of Nephrology, 29(6), 1585–1587. https://doi.org/10.1681/asn.2018030244
[46]Ionization Source Technology Overview | Thermo Fisher Scientific - CA. (n.d.). https://www.thermofisher.com/ca/en/home/industrial/mass-spectrometry/mass-spectrometry-learning-center/mass-spectrometry-technology-overview/ionization-source-technology-overview.html
[47]Crosslinking mass spectrometry. (n.d.). [Video]. https://www.thermofisher.com/ca/en/home/industrial/mass-spectrometry/proteomics-mass-spectrometry/protein-structure-analysis-mass-spectrometry.html
[48]Liquid Chromatography Mass Spectrometry (LC-MS) information | Thermo Fisher Scientific - CA. (n.d.). https://www.thermofisher.com/ca/en/home/industrial/mass-spectrometry/mass-spectrometry-learning-center/liquid-chromatography-mass-spectrometry-lc-ms-information.html
[49]Guan, Z., & Eichler, J. (2011). Liquid chromatography/tandem mass spectrometry of dolichols and polyprenols, lipid sugar carriers across evolution. Biochimica Et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1811(11), 800–806. https://doi.org/10.1016/j.bbalip.2011.04.009
[50]Klein, J. B., & Knepper, M. A. (2018). Protein mass spectrometry made simple. Journal of the American Society of Nephrology, 29(6), 1585–1587. https://doi.org/10.1681/asn.2018030244
[51]Lopez, M. J., & Mohiuddin, S. S. (2024, April 30). Biochemistry, essential amino acids. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK557845/
[52]Bevacizumab. (2006, October 5). Cancer.gov. https://www.cancer.gov/about-cancer/treatment/drugs/bevacizumab
[53]Monoclonal antibodies. (2019, September 24). Cancer.gov. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/monoclonal-antibodies
[54]Long, G. (2023, September 1). Monoclonal Antibody Drugs for Cancer – Caltag Medsystems. https://www.caltagmedsystems.co.uk/information/monoclonal-antibody-drugs-for-cancer/
[55]Heier, J. S., Brown, D. M., Chong, V., Korobelnik, J., Kaiser, P. K., Nguyen, Q. D., Kirchhof, B., Ho, A., Ogura, Y., Yancopoulos, G. D., Stahl, N., Vitti, R., Berliner, A. J., Soo, Y., Anderesi, M., Groetzbach, G., Sommerauer, B., Sandbrink, R., Simader, C., & Schmidt-Erfurth, U. (2012). Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology, 119(12), 2537–2548. https://doi.org/10.1016/j.ophtha.2012.09.006
[56]Gragoudas, E. S., Adamis, A. P., Cunningham, E. T., Feinsod, M., & Guyer, D. R. (2004). Pegaptanib for Neovascular Age-Related Macular Degeneration. New England Journal of Medicine, 351(27), 2805–2816. https://doi.org/10.1056/nejmoa042760
[57]Genentech. (n.d.). VABYSMO Phonetic video [Video]. Vabysmo. https://www.vabysmo.com/
[58]Rosenfeld, P. J., Brown, D. M., Heier, J. S., Boyer, D. S., Kaiser, P. K., Chung, C. Y., & Kim, R. Y. (2006). Ranibizumab for Neovascular Age-Related Macular Degeneration. New England Journal of Medicine, 355(14), 1419–1431. https://doi.org/10.1056/nejmoa054481
[59]Kalishwaralal, K., BarathManiKanth, S., Pandian, S. R. K., Deepak, V., & Gurunathan, S. (2010). Silver nano — A trove for retinal therapies. Journal of Controlled Release, 145(2), 76–90. https://doi.org/10.1016/j.jconrel.2010.03.022
[60]Broader VEGF pathway inhibition for wet AMD. (2024, April 1). PentaVision. https://retinalphysician.com/issues/2024/april/broader-vegf-pathway-inhibition-for-wet-amd/
[61] Laser photocoagulation for Age-Related Macular degeneration. (2024, November 19). Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/laser-photocoagulation-for-agerelated-macular-degeneration
[62]Fighting Blindness Canada (FBC). (2025, January 28). Age-Related Macular Degeneration - Fighting Blindness Canada (FBC). https://www.fightingblindness.ca/eyehealth/eye-diseases/age-related-macular-degeneration/
[63]Macular degeneration Treatments for dry and wet AMD. (2018, September 20). Better Vision Guide. https://www.bettervisionguide.com/macular-degeneration-treatments/
[64]Witkin, A. J., Hahn, P., Murray, T. G., Arevalo, J. F., Blinder, K. J., Choudhry, N., Emerson, G. G., Goldberg, R. A., Kim, S. J., Pearlman, J., Schneider, E. W., Tabandeh, H., & Wong, R. W. (2020). Occlusive retinal vasculitis following intravitreal brolucizumab. Journal of VitreoRetinal Diseases, 4(4), 269–279. https://doi.org/10.1177/2474126420930863
[65]Motevasseli, T., Mohammadi, S., Abdi, F., & Freeman, W. R. (2021). Side effects of brolucizumab. Journal of Ophthalmic and Vision Research. https://doi.org/10.18502/jovr.v16i4.9757
[66]What is Avastin? (2024, September 9). American Academy of Ophthalmology. https://www.aao.org/eye-health/drugs/avastin
[67]Pegaptanib (intraocular route). (2025, February 1). Mayo Clinic. https://www.mayoclinic.org/drugs-supplements/pegaptanib-intraocular-route/description/drg-20070848
[68]Han, X., Aslanian, A., & Yates, J. R. (2008). Mass spectrometry for proteomics. Current Opinion in Chemical Biology, 12(5), 483–490. https://doi.org/10.1016/j.cbpa.2008.07.024
[69]Zhang, Y., Fonslow, B. R., Shan, B., Baek, M., & Yates, J. R. (2013). Protein analysis by Shotgun/Bottom-up Proteomics. Chemical Reviews, 113(4), 2343–2394. https://doi.org/10.1021/cr3003533
[70]Curran, S. (2000). Laser capture microscopy. Molecular Pathology, 53(2), 64–68. https://doi.org/10.1136/mp.53.2.64
[71]Mass spectrometry grade proteases. (n.d.). https://www.thermofisher.com/order/catalog/product/90058
[72]Quantitative Top-Down Proteomics and its Impact on Clinical Research and Basic Biology. (n.d.). [Video]. https://www.thermofisher.com/ca/en/home/industrial/mass-spectrometry/proteomics-mass-spectrometry/protein-structure-analysis-mass-spectrometry/top-down-proteomics.html
[73]Golubeva, Y., Salcedo, R., Mueller, C., Liotta, L. A., & Espina, V. (2012). Laser capture microdissection for protein and NanoString RNA analysis. Methods in Molecular Biology, 213–257. https://doi.org/10.1007/978-1-62703-056-4_12
[74]Laser Capture Microdissection | Thermo Fisher Scientific - CA. (n.d.). https://www.thermofisher.com/ca/en/home/life-science/gene-expression-analysis-genotyping/laser-capture-microdissection.html
[75]Datta, S., Malhotra, L., Dickerson, R., Chaffee, S., Sen, C. K., & Roy, S. (2015). Laser capture microdissection: Big data from small samples. PubMed, 30(11), 1255–1269. https://doi.org/10.14670/hh-11-622
[76]Levels of protein organization. (n.d.). https://comis.med.uvm.edu/VIC/coursefiles/MD540/MD540-Protein_Organization_10400_574581210/Protein-org/Protein_Organization_print.html
[77]Alghamdi, A. A. A., Benwell, C. J., Atkinson, S. J., Lambert, J., Johnson, R. T., & Robinson, S. D. (2020). NRP2 as an Emerging Angiogenic Player; Promoting Endothelial Cell Adhesion and Migration by Regulating Recycling of α5 Integrin. Frontiers in Cell and Developmental Biology, 8. https://doi.org/10.3389/fcell.2020.00395
[78]Markovic-Mueller, S., Stuttfeld, E., Asthana, M., Weinert, T., Bliven, S., Goldie, K. N., Kisko, K., Capitani, G., & Ballmer-Hofer, K. (2017). Structure of the Full-length VEGFR-1 Extracellular Domain in Complex with VEGF-A. Structure, 25(2), 341–352. https://doi.org/10.1016/j.str.2016.12.012
[79]NCI Dictionary of Cancer Terms. (n.d.). Cancer.gov. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/kinase
[80]Michels, S., Rosenfeld, P., Puliafito, C., Marcus, E., & Venkatraman, A. (2005). Systemic bevacizumab (Avastin) therapy for Neovascular Age-Related Macular DegenerationTwelve-Week results of an uncontrolled open-label clinical study. Ophthalmology, 112(6), 1035-1047.e9. https://doi.org/10.1016/j.ophtha.2005.02.007
[81]Bryn Mawr Communications. (n.d.). GLOBAL PERSPECTIVES: Verteporfin Photodynamic therapy for AMD in the Anti-VEGF era - Retina Today. Retina Today. https://retinatoday.com/articles/2010-apr/global-perspectives-verteporfin-photodynamic-therapy-for-amd-in-the-anti-vegf-era
[82]Gragoudas, E. (2004, May 1). VEGF Inhibition Study in Ocular Neovascularization–1 (VISION–1): Efficacy results from Phase II/III MacugenTM (Pegaptanib sodium) clinical trials. https://iovs.arvojournals.org/article.aspx?articleid=2407904
[83]FIG. 1. A, the structure of the VEGF splice variants. The peptides. . . (n.d.). ResearchGate. https://www.researchgate.net/figure/A-the-structure-of-the-VEGF-splice-variants-The-peptides-encoded-by-the-various-exons_fig1_14157892
[84]Nowak, D. G., Woolard, J., Amin, E. M., Konopatskaya, O., Saleem, M. A., Churchill, A. J., Ladomery, M. R., Harper, S. J., & Bates, D. O. (2008). Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. Journal of Cell Science, 121(20), 3487–3495. https://doi.org/10.1242/jcs.016410
Acknowledgement
I would like to take a moment to thank the people who aided me in the development of this project. Firstly, my parents, who I look up to so much, especially my dad, for looking over my project multiple times to make suggestions and give feedback, and my mom who pushed me and helped me when I was lacking focus and motivation. A huge thanks goes to J. Sung, my science fair coordinator, for being extraordinarily patient with me the first time I showed interest in the science fair, unsure of my idea and unable to properly form a concept. Despite that, you were supportive and were a huge help in the completion of this project. Finally, my friends, siblings and teachers in science always ask questions, inspire me, and help me look through different perspectives, to make sure my project was the best it could be. All of these people were crucial in the completion and success of this project and I am forever grateful for their patience and support.