All About Slime!
Grade 6
Presentation
Hypothesis
Problem:
Which household item activates glue to make the best slime? Our definition of “best slime” is slime that is physically and visually the consistency of gak slime (or slime made using borax).

Photo 1: Leina & Aliana and Gak Slime
Videos of Gak Slime:
Video of Gak Slime 1: https://www.youtube.com/watch?v=R0wX2katmRk
Video of Gak Slime 2: https://www.youtube.com/watch?v=WpkymSDlouk
Hypothesis:
Aliana: magic baking powder or laundry detergent would work because most slimes are made of laundry detergent and magic baking powder makes food bind. I thought either of these manipulated variables (magic baking powder & laundry detergent) when mixed with the controlled variable of glue would form slime.
Leina: I thought vinegar would work because it has a lot of chemicals in it that might be able to form slime. I thought one of those chemicals would combine with the glue and form slime.
Hypothesis after research: Combined Hypothesis: Once we did further research, we realized that we need 3 different ingredients:
1) water +
2) a manipulated variable that contains boric acid. When boric acid is mixed with water it becomes borate ion ->
3) Borate ion then reacts with the third ingredient, which is glue containing polyvinyl acetate =
4) causing a chemical reaction producing slime

Photo 2: Gak Slime
Research
Research:
- Research from multiple websites (all link can be found on the "Citations" tab
- Activator: a substance that starts a chemical process. it is usually powdered borax dissolved in water, liquid starch, eye drops, or contact lens solution.
- All slime requires two things: polyvinyl alcohol + borate ion
- Polyvinyl alcohol in washable glue is attracted to the borate ion from borax. This attraction makes the two molecules form long chains and tons of those chains together form slime.
- Borax and boric acid are different chemicals, in the presence of water they change into borate ion. This is why you add water to borax powder.
- Gak slime or Glue slime: glue and borax slime
- Glue contains polyvinyl acetate which is a liquid polymer. Borax links the polyvinyl acetate molecules to each other, creating one large flexible polymer.
- Slime forms when the borate ions (neutral pH) in the slime activator (sodium borate, borax powder, or boric acid) mix with the PVA (polyvinyl acetate) glue and forms this cool stretchy substance. This is called cross-linking! The glue is a polymer and is made up of long, repeating, and identical strands or molecules. how does slime form? You need two things: polyvinyl alcohol (a main ingredient in washable school glue) and borate ion (which you can get from borax, sodium tetraborate, or boric acid). What happens is that the polyvinyl alcohol in washable school glue is attracted to the borate ion from borax or boric acid (depending on whether you are using borax, liquid starch, certain brands of laundry detergent). Even though Borax (from, well, Borax and also from Sta Flo liquid starch) and Boric Acid (from certain laundry detergents) are different chemicals, in the presence of water they change into Borate Ion. This is why you add water to borax powder if you are preparing slime using a borax-based recipe.
- borate ions (activator) + polyvinyl acetate (glue) = slime
- Adding baking soda to your slime recipe helps it have more form and firmness.
- If your slime is too oozy-gooey, add another pinch of baking soda to help it firm up. Continue adding baking soda a pinch at a time until the slime is your preferred consistency. If it’s too firm, add warm water, a teaspoon at a time
- Borate ion is created when bicarbonate of soda (baking soda) is mixed with contact lens solution. It’s super important to make sure that the solution you’re using contains the ingredients boric acid and sodium borate.

Fun Facts:
- Slime in nature is called mucus, and you have some in your nose right now!
- Manipulating slime and measuring ingredients can strengthen fine motor skills in kids
- Slime was a toy product manufactured by Mattel, sold in a plastic trash can and introduced in February 1976
- If you store the container in the fridge, you can get slime to last as long as a month without drying out or molding

Variables
VARIABLES
Controlled variables:
- Clear glue: amazon basics (1 gal/ 3.8L)
- Clear glue: ¼ cup
- Measurement of household items and borax solution: ¼ teaspoon
- Time: 15 stirs, wait 3 minutes, observe visually, repeat x 2 times. Then observe visually and physically 1 hour later

Manipulated Variables: ¼ teaspoon of each
- Bausch + Lomb renu fresh (contact lens solution)
- Magic Baking Powder
- Compliments White Vinegar
- Kirkland UltraClean Premium Laundry Detergent Free & Clear (no dyes/ perfumes)
- Arm & Hammer Baking Soda
- OxiClean Max Efficiency Laundry Stain Remover
- Boric Acid (powder)
- Borax Solution (½ teaspoon boric acid into ¼ cup water)

Responding Variable:
- Location (bottom, top, side flat)
- Bubbles (foam)
- Size (small, big)
- Amount (few, lots)
- Texture (chunky, blob, hard, rubber, stringy, sticky, watery, clumpy, thick, condensed, consistency)
- Color Change (clear cloudy)

Procedure
Procedure:
Step 1: add a ¼ cup of clear glue to 8 plastic containers and mark one manipulated variable per container and lid + control
Step 2: add ¼ teaspoon of each manipulated + control variable to the ¼ cup of glue
Step 3: stir glue + manipulated & controlled variable 15X
Step 4: wait 3 minutes, visually observe and record observation in logbook
Step 5: repeat step 2, 3 & 4 in the same containers from step 1
Step 6: wait one hour after step 5 and physically/ visually observe changes. Record observations in the logbook
REPEAT the experiment or all steps 3X


Observations
Observations: what we observed from the 3X we conducted the experiment:
Results 1


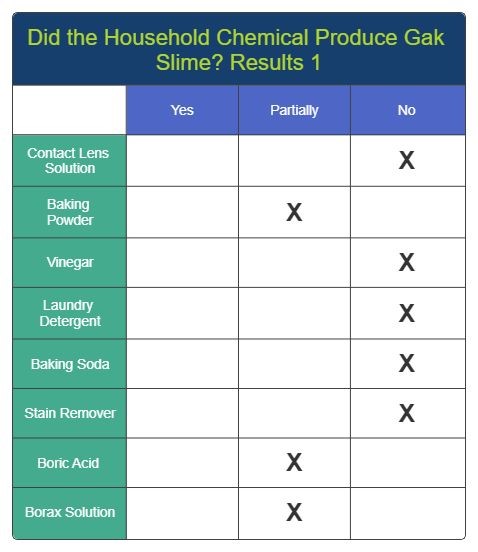
Results 2

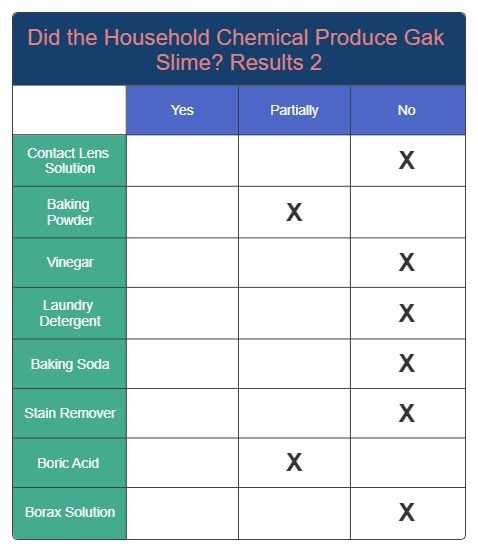
Results 3

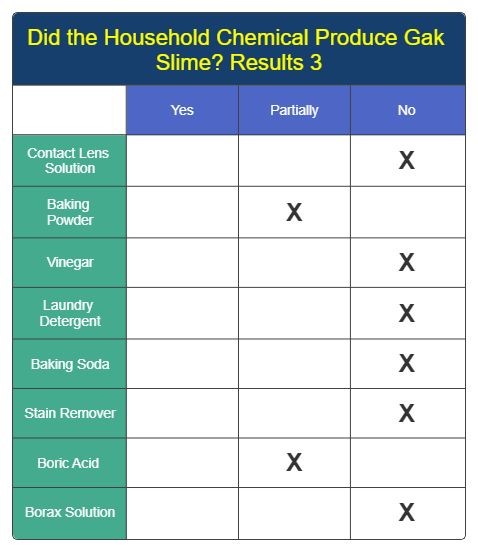
Analysis
Analysis:
- We learnt to make slime you need water, glue and borate ion with the glue containing polyvinyl acetate. Borate ions can be found in some contact lens solutions, borax powder and some laundry detergents - but not all
- We also learnt that vinegar breaks down slime instead of making it
- We learnt that stirring 30x (15x after each manipulated variable 1/4 tsp was added) total does not form slime even if all 3 ingredients are present. To make slime you have to mix it A LOT for the chemical reaction to occur which in the case of slime is the two molecules mixing to form long chains and a lot of those chains make SLIME
- We read that contact lens solution contains small amounts of boric acid and when mixed with baking powder makes slime. We decided to try it out even though our contact lens solution did not work during our initial observations. We also noticed that the contact lens solution ingredient list did not contain borate ion. However this reaction did start to produce slime on the outer edge meaning there must be some borate ion present.

Conclusion
Conclusion:
- We learnt to make slime you require water, borate ion and glue containing polyvinyl acetate
- Borate ions can be found in some contact lens solution, borax powder and some laundry detergents - but not all
- We also learnt that vinegar breaks down slime instead of making it
- Stirring 30X total is not enough to form slime even if all 3 ingredients are present. To form slime you must mix it A LOT for the chemical reaction to occur which in the case of slime is the two molecules mixing to form chains and a lot of those chains make slime!

Application
Application:
- Our results show other people the best way to make slime, what ingredients are required and the best household items you can use. We also showed the importance of hand mixing in creating the slime chemical reaction. If you do not use ingredients that contain polyvinyl acetate or borate ion it will not form slime.
- If we did our experiment again, we would use contact lens solution to see if borate ion is present even though it is not listed in the ingredients on the bottle. We went ahead and tried this and found renu fresh (borate ion or acid not listed) + baking soda did indeed make slime when mixed with clear glue.
- We also would add more of the manipulated variable, maybe increasing it to half a teaspoon of each manipulated variable each time to see if the chemical reaction occurs faster. We would still use clear glue instead of white glue so we can observe visually what is happening inside of the glue.
Sources Of Error
Sources of Error:

- Measurement error: innacurracy in the measurements we made and the tools we used to measure. Glue is hard to measure and transfer. Measurement tools had to be washed and dried in between using to keep the tools the same.
- Human error: contamination for example experiment 2 the manipulated vinegar should not produce slime but there was a little bit of slime forming so we likely introduced contamination. Making a mistake that might be known or unknown to us when conducting the experiments.
- Observer bias: we researched, prepared and interpretated the experiment. We know what should happen and this bias can be transferred to our reporting of the results.
- Systematic error: completed and repeated the experiment multiple times which produces inaccuracy which can effect the things we observed and reported.
Citations


- Videos of Gak Slime:
- Video of Gak Slime 1: https://www.youtube.com/watch?v=R0wX2katmRk
- Video of Gak Slime 2: https://www.youtube.com/watch?v=WpkymSDlouk
- https://www.armandhammer.com/articles/kid-friendly-slime
- https://science-u.org/experiments/smooshy-slime.html#:~:text=When%20these%20two%20ingredients%20are,form%20the%20slime%20we%20love!
- https://science-u.org/experiments/smooshy-slime.html#:~:text=When%20these%20two%20ingredients%20are,form%20the%20slime%20we%20love!
- https://www.funathomewithkids.com/2014/08/the-science-behind-slime.html
- https://www.pbs.org/parents/crafts-and-experiments/create-your-own-blob#:~:text=Pour%20the%20glue%20into%20a,%C2%BD%20cup%20room%20temperature%20water.&text=Add%20the%20food%20coloring%20and%20mix%20your%20goo%20well.&text=In%20a%20small%20cup%20stir,the%20Borax%20is%20completely%20dissolved.&text=Add%20the%20Borax%20mixture%20to,the%20food%20coloring%20and%20stir.
- https://www.acs.org/education/whatischemistry/adventures-in-chemistry/experiments/slime.html
- https://littlebinsforlittlehands.com/how-to-make-borax-slime-easy/
- https://littlebinsforlittlehands.com/slime-activator-list/#:~:text=Slime%20forms%20when%20the%20borate,and%20identical%20strands%20or%20molecules.
- https://www.armandhammer.com/articles/kid-friendly-slime#:~:text=Adding%20baking%20soda%20to%20your,a%20teaspoon%20at%20a%20time.
- https://www.curiscope.com/blogs/blog/the-perfect-slime-recipe
- https://kansasdiscovery.org/nine-serious-fun-slime-facts/
- https://slimeobsidian.com/blogs/slime-blog/10-interesting-facts-about-slime
- https://slimeobsidian.com/blogs/slime-blog/10-interesting-facts-about-slime
- https://www.cnet.com/home/smart-home/everything-you-need-to-know-about-slime/
- https://encounteredu.com/take-action/slimy-chains
Acknowledgement
Acknowledgment:

- We would like to thank Ms. Swinton and Ms. McGrath for giving us the opportunity and having the first in-person Hawkwood School Science Fair since the pandemic where we were chosen to move on to the CYSF.
- We would like to thank our grade 6 teacher - Mrs. Harms for supporting us in taking the time out of the classroom to participate in the Hawkwood School and CYSF.
- We would like to thank our parents for getting us the materials to do the experiments and helping us with our project. Without you we would not have been able to get the board transported and filled out or learn the parts of an experiment, what they mean and how to put it all together to form our project.
- We would like to thank the kids of Hawkwood School for their excitement and interacting with our project during Hawkwood School Science Fair.
 H
H
Hawkwood School Science Fair

