The Silent Crisis: How a Changing Ocean is Eating Away at New Zealand Mussel Shells
Grade 7
Presentation
Hypothesis
If New Zealand mussel shells are kept in two different solutions (normal pH simulated seawater and acidic simulated seawater) for a month, I believe that the weight of the shells in the acidic simulated seawater will decrease because the acid might break down the shell. The color of the shells might also become lighter as the acid might bleach the color. The shells in the normal pH simulated seawater will have little to no change as they are in water that is similar to the one in their habitat (ocean water).
Research
Greenshell Mussels
The common name for Greenshell mussels is New Zealand Green Lipped Mussel. Its scientific name is Perna Canaliculus.
Greenshell mussels are bivalve mollusks that have two part hinged shells and a soft body. These mussels are native to New Zealand and make their homes on rocks and solid surfaces around New Zealand’s coastline. They get their name from the bright green stripe along their shell. They often cling to solid objects or each other using threads called byssus threads. Mussels feed on phytoplankton by pumping large volume of water over their gills. Then, phytoplankton trapped on the gills is transported to mussel’s mouth and is eaten. It is called filter feeding.
Physical Appearance
Green mussels are bivalves and have two hinged shells closed by two muscles. A strong ligamnet holds the two valves together. They have elongated shells with an average length of 10-15cm. These mussels have smooth and glossy shells with greenish blue color and radiating strips. They also have a fibrous beard which helps them attach to rocks and other surfaces in the ocean.
Composition and Functions of the Shell
Composition
The main component of mussel shells is calcium carbonate which constitutes approximately 94% of the shell. It’s shell also contains small amount of inorganic matter and traces of other elements such as magnesium, manganese, iron, copper, and zinc. Calcium carbonate provides the hardness and durability to the shell.
Functions
The shell protects the soft tissues of the mussels from external factors, and helps them defend against predators. It also provides them the ability to move and feed.
Role of Mussels in an Ecosystem
Mussels play a significant role in marine ecosystems and are considered as “ecosysytem engineers” as they modify aquatic habitat, making it suitable for themselves and other marine life.
-
Mussels filter water: When mussels feed, they draw in water containing phytoplankton. They filter the water to feed on the phytoplankton. It helps clean the water. Up to 350 litres of water can be filtered by one mussel in a day.
-
Mussel shells provide habitat: Small fish, insects, and plants use mussel shells for making their nests.
-
Mussels provide food: They act as food source to people and many marine species such as snapper, blue cod, fur seals and octopuses.
-
Mussels add nutrients: Mussels add nutrients to the water through their waste which is used by the aquatic plants to make food.
pH
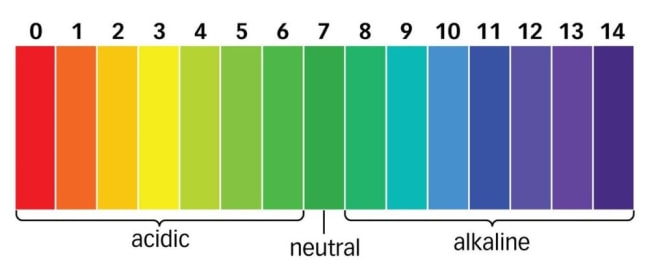
pH is the measurement of a liquid’s acidity or basicity. A pH scale is a tool for measuring acids and bases. It has numbers ranging from 0-14. A ph of 7 is considered neutral. Anything below the ph of 7 (6.9 or less) is considered acidic and anything above the ph of 7 (7.1 or more) is considered alkaline (basic).
What is optimum pH for mussel survival?
Mussels thrive best in alkaline environments ranging from the pH of 7.5 to 9.3. This pH range helps them grow efficiently by providing them nutrients required for their growth and reproduction.
Ocean Water
Seawater constitutes the oceans and it covers more than 70 percent of earth’s surface. Sea water contains 96.5% water, 2.5% salts, and small amounts of other substances including dissolved organic and inorganic matter and some dissolved gases. Average ocean water is slightly alkaline, around the ph of 8.1.
Ocean Acidification
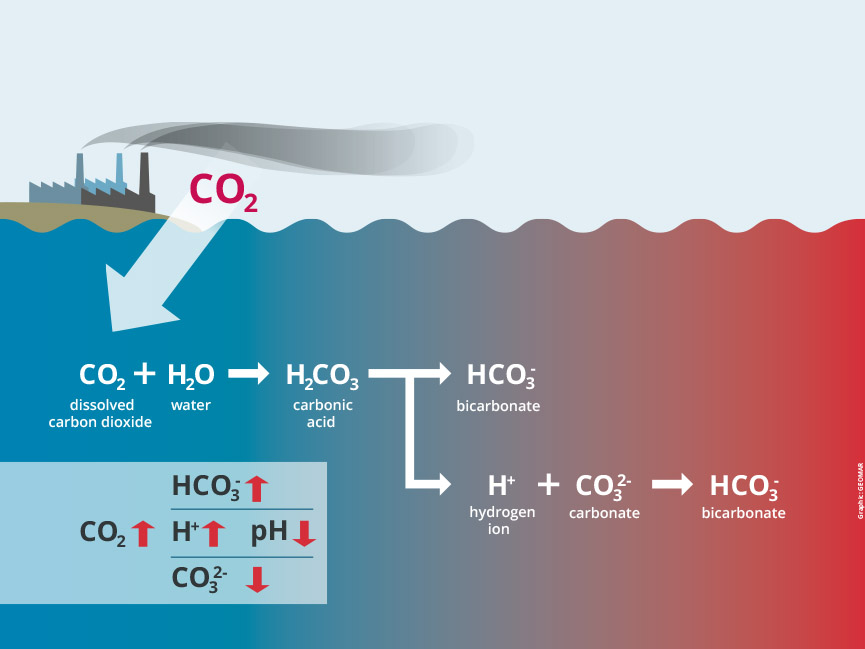
Ocean acidification means decrease in pH of the ocean over an extended period of time caused mainly due to the absorption of carbon dioxide from the atmosphere. Oceans absorb 30% of carbon dioxide present in the air. Then, carbon dioxide combines with water to make carbonic acid. The carbonic acid is a weak acid and it breaks down into hydrogen ions and bicarbonate ions. The hydorgen ions decreases the pH of water and thus the water becomes acidic. The amount of carbon dioxide has increased in the air due to burning of fossil fuels and land use change. Due to increase in the carbon dioxide in the air, more and more carbon dioxide is absorbed by the sea making it more and more acidic. The mussels needs carbonate ions present in the sea water to form their shells.This increased acidity decreases the availability of carbonates as the hyrogen ions combine with carbonates to form bicarbonate ions , thereby reducing its availability for the mussels.
Ocean Acidification and Real Life Problems
Corals in the Caribbean and cold-water reefs off of Scotland and Norway are facing the problem of ocean acidification. Many species and corals are threatened as the waters can hold so much carbon dioxide that due to corrosive conditions, shelled creatures like mussels and sea orchins, dissolve in the water. This affects the food source for birds, fish, and other marine animals.
California Mussels and Lower pH
One of the studies conducted at University of California, San Diego has shown that shells of California mussels are weakening due to the acidic conditions present in the nearby Pacific ocean. The acidic conditions are eating away the shells of California mussels and these mussels are secreting calcite more than they were secreting 60 years ago.The study has concluded that if the mussels disappear, 303 living species who are dependent on them in some way will be impacted and some of them even can become extinct.
Variables
|
Controlled Variables |
Temperature of the water and surroundings, the amount of water taken, the duration for which the shells kept in the water, and the type and the number of shells |
|
Independant Variable |
The pH of the simulated seawater |
|
Dependant Variable |
The weight, texture, and appearance of the shells |
|
Uncontrolled Variable |
Amount of carbon dioxide that enters the jars naturally |
Procedure
1. First take 18 frozen New Zealand green mussels.
2. Thaw the mussels for 24 hours.
3. Clean the meat from the mussels.
4. Let them dry for 12 hours.
5. Take six glass containers.
6. Clean them thoroughly and dry them.
7. Label the three containers as jar A and label another 3 containers as jar B with a permanent marker(using sticky notes).
8. Preparartion of sea water
a. Weigh 35g of sodium chloride (common salt).
b. Place 35g of salt in a container and add tapwater until the total mass is 1000g.
9. Fill container A with 500g of salty water.
10. Preparation of acidic (pH 6) sea water
a. Mix 2g of vinegar in 500g of sea water in a glass container
b. Check the pH with a pH strip.
11. Fill container B with the 500g of acidic sea water (pH 6).
12. Take 3 green mussel shells and weigh them.
13. Note the weight and appearance (color & texture) of mussels in a notebook.
14. Place these mussel shells in container A.
15. Take 3 more green mussel shells and weigh them.
16. Note the weight and appearance (color & texture) of mussels in a notebook.
17. Place these mussel shells in container B.
18. Place the container A and B undisturbed for four weeks in a shady place at room temperature.
19. After four weeks take out the shells from container A and B by draining water.
20. Dry the mussel shells.
21. Weigh them by using a weighing scale and note down any difference in their weights in a notebook.
22. Note down any changes in the appearance of shells in a notebook.
Repeat step 8 to 22 for another two trials.
Note: DO NOT shut the lids of the jars so that air pressure does not build up inside the jars.
Observations
Data Chart- Jar A
|
Weight of the Shells(g) |
Trial 1 |
Trial 2 |
Trial 3 |
|
Initial Weight |
24.6 |
26.8 |
26.3 |
|
Final Weight |
24.6 |
26.8 |
26.3 |
Data Chart- Jar B
|
Weight of the Shells(g) |
Trial 1 |
Trial 2 |
Trial 3 |
|
Initial Weight |
24.6 |
26.8 |
26.3 |
|
Final Weight |
24.6 |
26.8 |
26.3 |
Graph
Quantitative obervations: Above data clearly demonstrates that the intial and final weights of shells kept in neutral simulated sea water (Jar 1A, Jar 2A & 3A) had no difference in weight. However,the initial and final weight of shells placed in acidic simulated sea water (Jar 1B, 2B & 3B) had decreased by 0.3g, 0.2g, and 0.3g in trial 1, 2, and 3 respectively.
Qualitative observations: The skin of shells kept in the acidic water were peeled off from some spots. Additionally, the fibrous beards of the shells kept in acidic water also appeared to be gone. Both the shells in the acidic seawater and normal seawater had tiny pieces broken off of them and the shells lost their gloss after a month of being left in water.
Analysis
From the data collected in the above experiment, we can clearly infer that the acidic water has profound impact on the weight and appearance of shells of New Zealand green mussels.The weight of the shells placed in simulated acidic sea water has decreased in every trial. It happened as the acid (acetic acid) in the water reacted with the calcium carbonate present in the shells of the mussels and formed calcium acetate (dissolves in water), water, and carbon dioxide.This had resulted in the decomposition of shells, thereby reducing their weight.
Formula:
Acetic acid + Calcium Carbonate → Calcium Acetate + Carbon dioxide + Water
(soluble in water)
Additionally, the shine and beard on the shells placed in acidic water has also gone due to corrosiveness of the acid present in the water.However, some inconsistencies were also noticed in the experiment such as broken shells pieces in both acidic and neutral simulated sea water, which can be attributed to handling of shells during the experiment.
Conclusion
The objective of this experiment was to investigate the effect of acidification of oceans on the shells of New Zealand green mussels. To test it, prepare simulated sea water (water that has the same composition of water as the ocean) by mixing 35 g of salt in 1000 ml of water. Then divide the solution into two equal parts (500ml each) and label them A and B. Take 2 milliliters of vinegar with the help of a measuring cylinder and add it to jar B to prepare the acidic solution (pH=6). Mix it thoroughly. After this, take three mussel shells, weigh them and place them in jar A. Simmilarly weigh another 3 mussel shells and place them in jar B. Leave them in an area that will remain undisturbed for a month. After a month, take them out, dry them, and check the difference in weight and appearance.
In all three trials, the weight of shells in the acidic water had decreased due to decomposition of calcium carbonate present in the shells by acetic acid. Additionally, there is a significant difference in the appearance of shells. The skin of the shells is clearly gone at some places, along with their fibrous beards. All of the results of above has proved that my hypothesis, which predicted that the acidic seawater will damage and dissolve the shells of mussels, was correct.
Application
My experiment will help us to understand the impact of acidification of oceans, due to climate change, on the shells of New Zealand mussels, coral reefs, and other aquatic species with shells. Many marine life creatures such as abalones, seashells, and sea urchins also have an exoskeleton made up from calcium carbonate. The shells of all these creatures are very important for their survival as they provide them protection, shelter and mobility. If the shells disappear or get damaged, the mussels will eventually fail to survive. Due to this the animals, fish, and humans which rely on the mussels for food will also struggle. As a result of it, the entire ecosystem which depends on mussels will get imbalanced as this affects the marine and territorial food webs due to lack of food. Additionally, due to decline in the amount of marine life which depends on mussels for food, the fisheries across the world will also be impacted, thereby affecting their food supply.
Sources Of Error
The most prominent source of error in my project was the temperature as the temperature of my house changes occasionally. The temperature affects the decomposition of mussels as it can affect the rate of the reaction between acid and mussels. Also, the shells got more light then they do in the ocean which could also have impacted the final results as the amount of light might change the speed and nature of chemical reactions going on in the different jars. Furthermore, the water in which the shells were placed had only salt in it, and did not have all the minerals that are found in seawater. I could have also done more trials with a bigger experimental group, so that I could get more accurate results.To improve my experiment, I could have kept the tempurature constant by keepin it in a controlled temperature room.
Citations
- Best, K. (2023, March 7). Mussels and Other Aquatic Animals Provide Critical Coastal Ecosystem Protections. Yale School of the Environment. Retrieved November, 2024, from https://environment.yale.edu/news/article/sea-levels-rise-mussels-provide-coastal-ecosystem-protections
- Claudi, R., Graves, A., Taraborelli, A. C., Prescott, R. J., & Mastitsky, S. E. (2011, August 31). Impact of pH on survival and settlement of dreissenid mussels. Aquatic Invasions, 7(21–28), 8. 10.3391/ai.2012.7.1.003
- Gil, L. (2018, November 27). Jeopardy at Sea: What Atoms in Clams Tell us about Ocean Acidification. International Atomic Energy Agency. Retrieved November, 2024, from https://www.iaea.org/newscenter/news/jeopardy-at-sea-what-atoms-in-clams-tell-us-about-ocean-acidification
- Gillespie, C. (2022, March 24). How To Replicate Seawater At Home. Sciencing. Retrieved November, 2024, from https://www.sciencing.com/make-sea-water-home-6368912/
- Glossary:Fossil Fuel. (n.d.). eurostat. Retrieved November, 2024, from https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary%3AFossil_fuel#:~:text=Fossil%20fuel%20is%20a%20generic,products%20and%20non%2Drenewable%20wastes
- Life of a green-lipped mussel — Science Learning Hub. (2013, June 14). Science Learning Hub. Retrieved November, 2024, from https://www.sciencelearn.org.nz/resources/733-life-of-a-green-lipped-mussel
- Liou, J. (2022, June 8). What is Ocean Acidification? | IAEA. International Atomic Energy Agency. Retrieved November, 2024, from https://www.iaea.org/newscenter/news/what-is-ocean-acidification
- Morton, B. (2025, February 3). Mussel | Mollusk Adaptation & Benefits. Britannica. Retrieved November, 2024, from https://www.britannica.com/animal/mussel
- Mussel reefs and biodiversity — Science Learning Hub. (2021, May 17). Science Learning Hub. Retrieved November, 2024, from https://www.sciencelearn.org.nz/image_maps/108-mussel-reefs-and-biodiversity
- National Ocean Service. (2024, June 16). What are phytoplankton? NOAA's National Ocean Service. Retrieved November, 2024, from https://oceanservice.noaa.gov/facts/phyto.html
- National Ocean Service. (2024, June 16). What is Ocean Acidification? NOAA's National Ocean Service. Retrieved November, 2024, from https://oceanservice.noaa.gov/facts/acidification.html
- Ocean Acidification is Transforming California Mussel Shells. (2021, January 11). UC San Diego. Retrieved November, 2024, from https://today.ucsd.edu/story/ocean-acidification-is-transforming-california-mussel-shells
- Thompson, A. (2024, September 4). Green Lipped Mussels For Dogs: Do They Really Work? dogs naturally. Retrieved November, from https://thenaturaldogstore.com/blogs/health/green-lipped-mussels-for-dogs
- Understanding Ocean Acidification | NOAA Fisheries. (n.d.). NOAA Fisheries. Retrieved November, 2024, from https://www.fisheries.noaa.gov/insight/understanding-ocean-acidification
- Water Science School. (n.d.). pH Scale | U.S. Geological Survey. USGS.gov. Retrieved November, 2024, from https://www.usgs.gov/media/images/ph-scale
-
What is Ocean Acidification? (n.d.). Biological Impacts of Ocean Acidification. Retrieved November, 2024, from https://www.bioacid.de/ocean-acidification/?lang=en
-
What is tissue concept short.pub. (n.d.). Roswell Park Comprehensive Cancer Center. Retrieved November, 2024, from https://www.roswellpark.org/sites/default/files/What_is_Tissue__amp__Why_is_it_Important.pdf
- Winters, B. (n.d.). NZ Green Lipped Mussel: A Journey Through Its Life and Role in the Mar. Lifespan NZ. Retrieved November, 2024, from https://purelifespan.com/blogs/the-unique-features-of-green-lipped-mussel-powder/nz-green-lipped-mussel-a-journey-through-its-life-and-role-in-the-marine-ecosystem#:~:text=The%20green%20lipped%20mussel%20is%20a%20filter%20feeder%2C%20drawing%20in,quality%2
- Woods, C. (n.d.). The Asian Green Mussel: Recent Introduction to the South Atlantic Bight. South Carolina Department of Natural Resources. Retrieved November, 2024, from https://www.dnr.sc.gov/marine/sertc/The%20Asian%20Green%20Mussel.pdf
Acknowledgement
I would like to thank the people who helped me with this project. First of all, I thank my parents who bought the materials I didn't have and helped me conduct my experiment. Secondly, I thank my sister, who helped me with the CYSF platform. I also thank my teachers, Mrs. Bedi and Mrs. Aulakh, who guided me through my project and helped me improve it. This project woldn't have happened without them.

