Analysis of Iron(III) chloride as Catalyst in Efficiency and Effectiveness
Grade 11
Presentation
Hypothesis
Increasing the concentration of iron(III) chloride will enhance the effectiveness and efficiency of the decomposition of hydrogen peroxide by acting as a catalyst, and a a result leading to a faster reaction rate while potentially maintaining catalyst stability.
Research
Research Question: How does varying the concentration of iron(III) chloride affect the effectiveness, efficiency, and stability of its catalytic role in the decomposition of hydrogen peroxide
When iron(III) chloride reacts with hydrogen peroxide, it forms complexes that help break down the hydrogen peroxide into water and oxygen. Researchers like De Laat and Gallard have found that these complexes decompose slowly, with a rate constant of about 2.7 × 10⁻³ s⁻¹, which is important for understanding how fast the reaction happens (De Laat and Gallard, 1999). The decomposition process involves a series of steps where iron(III) ions help create reactive radicals that speed up the reaction. According to Perez-Benito, the reaction rates decrease when the solution becomes more concentrated with other ions, and the energy needed for the reaction to occur is quite high, around 146 ± 10 and 88 ± 21 kJ mol⁻¹. The reaction starts when iron(III) ions combine with hydrogen peroxide to form an intermediate, which then breaks down into water and oxygen while releasing iron(II) ions (Haber and Weiss). Another theory suggests that an iron-oxygen complex is formed during the reaction, which reacts with more hydrogen peroxide to produce water and oxygen while regenerating iron(III) ions (Kremer and Stein). Scientists have studied this reaction under different conditions, such as varying pH levels and concentrations of hydrogen peroxide and iron(III) chloride. Experiments have shown that the reaction follows a predictable pattern, with results closely matching theoretical models, although there can be some variation depending on the exact conditions (Kwan). The reaction is a first order reaction defined by Rate=k[Fe3+]^(m)*[H2O2]^(n) sincer hydrogen peroxide volume is held constant(De Laat and Gallard, 1999).
The decomposition of hydrogen peroxide (H₂O₂) into oxygen (O₂) and water (H₂O) is normally slow because it has a high activation energy. The iron(III) chloride (FeCl₃) catalyst speeds up the reaction by participating in a redox cycle rather than breaking down itself. The key steps are:
-
Fe³⁺ (iron(III) ions) react with H₂O₂, producing Fe²⁺ and oxygen radicals:
"H2O2+Fe3+→Fe2++O2∙−+2H+H_2O_2 + Fe^{3+} \rightarrow Fe^{2+} + O_2^{\bullet-} + 2H^+H2O2+Fe3+→Fe2++O2∙−+2H+".(Laat & Gallard, 1999,2726 )- Here, Fe³⁺ facilitates the oxidation of H₂O₂, generating reactive oxygen species.(Laat & Gallard, 1999,2726)
-
Fe²⁺ is then regenerated by reacting with another H₂O₂ molecule, producing water and Fe³⁺:
"H2O2+Fe2+→Fe3++OH−+OH∙H_2O_2 + Fe^{2+} \rightarrow Fe^{3+} + OH^- + OH^{\bullet}H2O2+Fe2+→Fe3++OH−+OH∙".(Laat & Gallard, 1999,2726 )- This step restores Fe³⁺, meaning the catalyst is not consumed but rather cycles between Fe²⁺ and Fe³⁺.(Laat & Gallard, 1999,2726)
Variables
Independent Variable:
• The manipulated variable is the amount of Iron(III) chloride solution added. By dispensing different increments of the catalyst (at a concentration of 0.43 M).
Dependent Variable:
• The dependent variable is the rate of hydrogen peroxide decomposition. This can be monitored by measuring the evolution of gas over time once the catalyst is introduced.
Controlled Variables:
• Volume and concentration of hydrogen peroxide: Using 3 ml of a 3% solution in every trial maintains consistency.
• Environmental conditions: Maintaining constant temperature, pressure, and minimal light interference ensures that the reaction conditions remain the same across all trials.
• Measurement techniques: Consistent use of a stopwatch for timing and standardized methods for measuring gas evolution contribute to the reliability of your results. The evolution of gas will be measured using a burette with 1 mL increments, while solution volumes will be measured using multiple graduated cylinders of increment 0.2 mL.
Procedure
- Prepare a clean reaction vessel and fill it with 3ml of 3% hydrogen peroxide solution using a 10mL graduated cylinder and eye dropper.
- Ensure that throughout the experiment surrounding environment light interference is minimized, controlled and accounted for in calculations.
- Run a baseline trial by measuring the decomposition of hydrogen peroxide in the absence of Iron(III) chloride.
- Record environmental conditions such as pressure and temperature.
- Use a eye dropper to dispense increments of Iron(III) chloride solution at 0.43 M to a 10mL graduated cylinder.
- Begin timing the reaction with a stopwatch.
- Observe the evolution of gas.
- Repeat steps 4-8 for progressively more amounts of Iron(III) chloride solution added.
Observations
- There are significant differences between the responding variable results from the first experiment than the second. The rate of reaction decreased significantly, suggesting a change in the condition of the iron(III) chloride. As the Iron(III) Chloride was exposed to air moisture, hydrolosis occurred and thus caused the formation of oxyhydroxides.
- When the Iron(III) Chloride + Hydrogen peroxide solution was submerged under water, a cloudy brown odourless iron(III) hydroxide precipitate was formed as a result of a hydrolysis reaction.
- The iron(III) chloride solution is a brownish-black solution with a pungent odour at room temperature and standard pressure.
- The hydrogen peroxide solution is a colourless and odourless liquid at room temperature.
- The appearance of the iron-catalyzed hydrogen peroxide system is a yellowish-green mixture with a pungent odour at room temperature and standard pressure.
Analysis
Table 1: Raw and Tabulated Data of Experimental Project For Day One Experimentation
| Day One | ||||
| Trial Number | Raw Data | Tabulated Data | ||
| iron(III) chloride Solution Volume (mL ± Δ mL 0.1) | Inital Gas Volume (mL ± Δ mL 0.5) | Final Gas Volume after Thirty Seconds (mL ± Δ mL 0.5) | Amount of Gas Produced (mL ≈± Δ mL 0.71) | |
| 1 | 1.0 | 33 | 35 | 2 |
| 2 | 1.5 | 35 | 38 | 3 |
| 3 | 2.0 | 38 | 42 | 4 |
| 4 | 2.5 | 42 | 47 | 5 |
| 5 | 3.0 | 22 | 28 | 6 |
| 6 | 3.5 | 34 | 41 | 7 |
Table 2: Raw and Tabulated Data of Experimental Project For Day Two Experimentation
| Day Two | ||||
| Trial Number | Raw Data | Tabulated Data | ||
| iron(III) chloride Solution Volume (mL ± Δ mL 0.1) | Inital Gas Volume (mL ± Δ mL 0.5) | Final Gas Volume after Thirty Seconds (mL ± Δ mL 0.5) | Amount of Gas Produced (mL ≈± Δ mL 0.71) | |
| 1 | 1.0 | 43 | 44 | 1 |
| 2 | 2.0 | 35 | 37 | 2 |
| 3 | 3.0 | 24 | 27 | 3 |
| 4 | 4.0 | 30 | 35 | 5 |
| 5 | 8.0 | 37 | 43 | 6 |
| 6 | 10.0 | 46 | 53 | 7 |
- Note: Uncertainty difference for tabulated data was calculated using Pythagorean addition.
Hydrogen Peroxide: 3% Concentration, 3 mL ± Δ mL 0.1 used per trial.
Temperature recorded at approximately 23.5 °C on both days. With approximately 100.4 kPa of pressue.
.
Chart 1: Visually Anaylsis of Raw and Tabulated Data with Trendline Correlation and Error Bar Representation
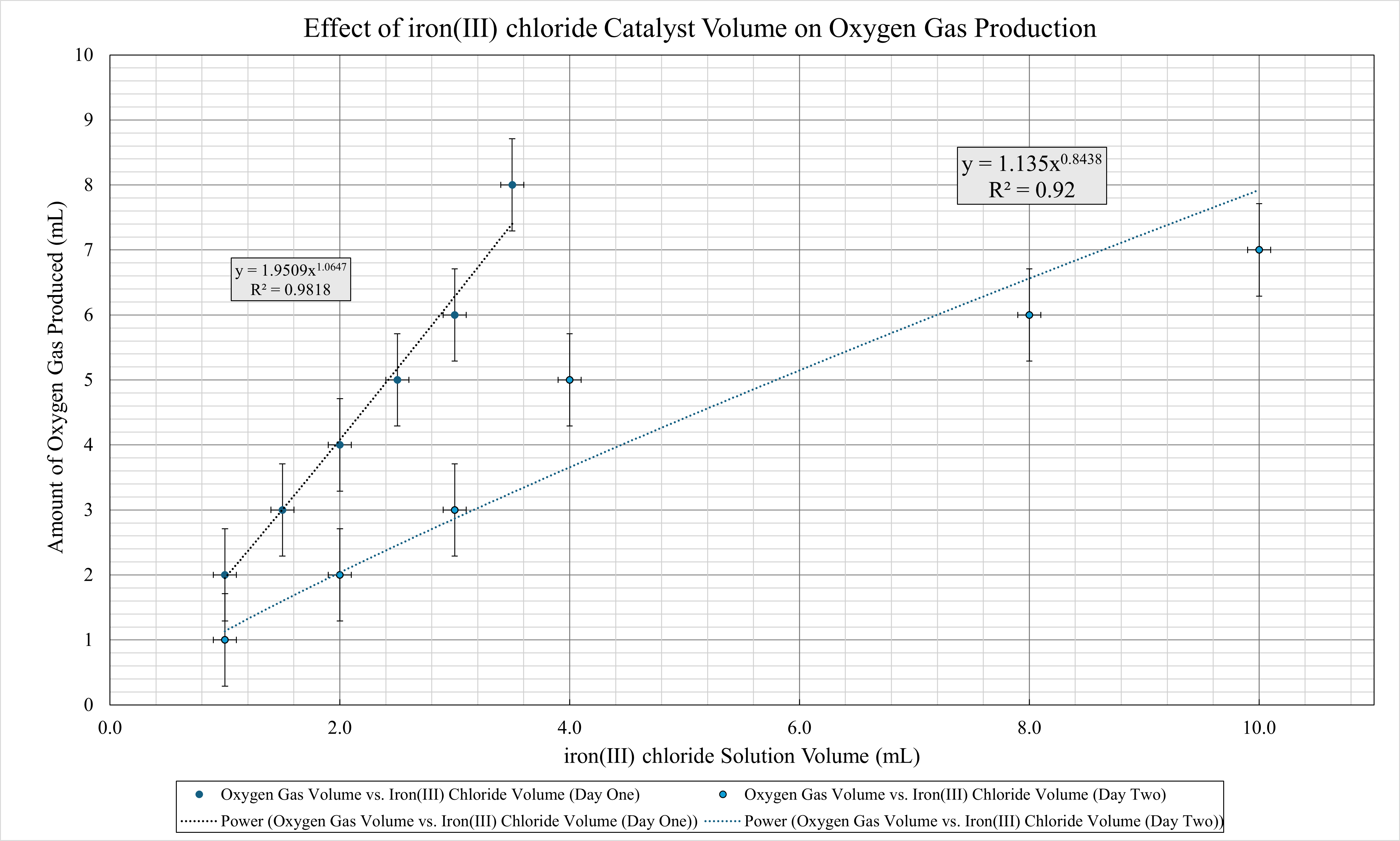
Anaylsis:
The experimental data demonstrates a clear relationship between the volume of iron(III) chloride (FeCl₃) catalyst used and the amount of oxygen gas produced during the decomposition of hydrogen peroxide (H₂O₂). As the volume of FeCl₃ increases, a corresponding increase in the volume of oxygen gas is observed. This trend suggests that higher concentrations of the FeCl₃ catalyst enhance the decomposition rate of H₂O₂, leading to greater oxygen evolution. These findings align with established research on catalytic decomposition reactions, where transition metal ions such as Fe³⁺ facilitate the breakdown of hydrogen peroxide by providing an alternative reaction pathway with lower activation energy (Laat & Gallard, 1999, 140-142)
This increase in reaction rate can be explained through reaction kinetics (Kwan, 1999, 18). In an uncatalyzed reaction, hydrogen peroxide decomposes slowly because it requires a high activation energy to break its bonds(Chung, 2021). The Fe³⁺ ions from FeCl₃ act as a catalyst by lowering the activation energy of the reaction, allowing it to proceed at a much faster rate(Kwan, 1999, 18). The catalyst achieves this by providing an alternative reaction pathway, facilitating the transfer of electrons, and promoting the formation of reactive intermediates (Macedo et al., 2020, 3-5). This process results in a higher frequency of successful collisions between reactant molecules, leading to a greater rate of oxygen gas production(Macedo et al., 2020, 3-5).
The power trendlines fitted to the data for both Day One and Day Two exhibit high coefficients of determination with R² values of 0.9818 and 0.92, respectivel), indicating a strong correlation between FeCl₃ volume and oxygen gas production. The expoential powers of 1.0647and 0.8438 respectivly suggest that the reaction follows a near-linear catalytic effect in this concentration range. The similarity between the results on different days further supports the repeatability and reliability of the experiment. However, slight variations between the two data sets may be attributed to factors such as environmental conditions, minor differences in H₂O₂ concentration, or hydroysis effects regarding FeCl₃(Inorganic Ventures, 2025).Future investigations could explore the effect of varying FeCl₃ concentration beyond the tested range to determine if the observed trend holds at higher catalyst volumes or if reaction saturation occurs.
Conclusion
The experimental investigation demonstrated a clear correlation between the volume of iron(III) chloride (FeCl₃) catalyst and the rate of hydrogen peroxide (H₂O₂) decomposition, as measured by the volume of oxygen gas produced over a 30-second period. The results indicate that increasing the FeCl₃ volume led to a greater oxygen yield, suggesting that FeCl₃ effectively enhanced the decomposition rate of H₂O₂. The power trendline fitted to the data exhibited high R² values (0.9818 and 0.92) for Day One and Day Two, confirming a strong mathematical relationship between catalyst volume and gas production. These findings align with established reaction kinetics, where transition metal ions like Fe³⁺ facilitate redox cycling, lowering the activation energy of the reaction (Flinn Scientific, 2016; Chung, 2021). Additionally, the consistency between Day One and Day Two data suggests that the catalytic effect of FeCl₃ follows a predictable trend within the tested volume range.
The accuracy of the experiment was influenced by instrumental limitations and environmental factors, alongside inherent systematic error and external random error. The use of a burette for gas collection provided precise volume measurements, with an uncertainty of ±0.5 mL, reducing significant errors in gas volume readings. Similarly, FeCl₃ volumes were measured with a graduated cylinder, introducing a ±0.2 mL uncertainty minimal.. However, potential systematic errors—such as temperature fluctuations or minor variations in H₂O₂ concentration—could have introduced slight deviations in gas production rates. In addition water formation form the decomposition of hydrogen peroxide form water molecules which at an atomic level, it would reduce the chance of collisions between Fe³⁺ and H₂O₂ molecules, directly reducing the overall rate of reaction and effecting reaction kinetics. Repeating trials and calculating standard deviations helped mitigate these uncertainties and improve data reliability within future testing. The shift in gas production from Day One to Day Two is likely due to the hydrolysis of FeCl₃, where prolonged exposure to moisture leads to the formation of Fe(OH)₃ precipitates, reducing the availability of Fe³⁺ ions for catalysis (Inorganic Ventures, 2025). This change could have lowered the effective Fe³⁺ concentration in solution, slightly decreasing the reaction rate and leading to variations in oxygen gas production. To mitigate this in future experiments, freshly prepared FeCl₃ solutions should be used, or stabilizing agents (such as controlled pH adjustments or anhydrous storage conditions) should be introduced to prevent Fe³⁺ hydrolysis over time.
The experiment maintains strong validity, as it effectively tests the relationship between catalyst concentration and reaction rate, aligning with theoretical expectations. The use of controlled variables—including constant H₂O₂ concentration, time intervals, and measurement conditions—ensured that the FeCl₃ volume remained the sole independent variable influencing the reaction. The inclusion of error bars, trendline analysis, and multiple trials further strengthens confidence in the results. While minor uncertainties exist, the experiment successfully demonstrates how FeCl₃ catalyzes the decomposition of H₂O₂, supporting well-established kinetic principles (Laat & Gallard, 1999; Kwan, 1999). Future studies could explore a broader FeCl₃ concentration range or vary reaction conditions (e.g., pH, temperature) to assess their impact on catalytic efficiency.
In conclsuion, the data indicates that oxygen production increases consistently with increasing FeCl₃ volume, suggesting that no saturation point was reached within the tested range. Theoretically, a saturation point should occur when all available hydrogen peroxide molecules have reacted, and additional FeCl₃ no longer enhances decomposition. Given that FeCl₃ acts as a catalyst, its effectiveness is limited by the concentration of H₂O₂; thus, a saturation point would be expected when the Fe³⁺ ions far exceed the available H₂O₂ molecules, leading to no further increase in reaction rate. In most Fe³⁺-catalyzed hydrogen peroxide reactions, saturation is often observed when Fe³⁺ reaches a concentration where its redox cycling becomes limited by the availability of H₂O₂ (Laat & Gallard, 1999). Future trials using FeCl₃ volumes above the tested range, 5–10 mL, could determine whether oxygen production eventually plateaus, confirming the theoretical saturation point. Theoretical estimates place the ratio saturation at 1:10 with the maximum effectiveness at 20.5 mL based on a 0.43 molarity; however, such volume could pose risks to research due to the high reaction kinetics and potential comparison of laboratory equipment, considering it unsafe and as such unable to be tested.
Application
The application of iron(III) chloride as a catalysts extends to various biological areas of study including cellular health and functions. Hydrogen peroxide and copper ions can form harmful byproducts during lipid peroxidation, when 0.25 mM of iron(III) chloride is added, the damage is reduced by 50%. The protective effects of Fe³⁺ created a special condition that required a specific protein in the erythrocyte membrane. This means that when membranes are heated or when trypsin is present, Fe³⁺ loses its ability to prevent lipid peroxidation (Nagashima et al., 1999). Aside from using iron(III) chloride to create a hydrogen peroxide redox reaction, it could also act as a catalyst for the reaction of ethylene with chlorine, forming ethylene dichloride (1,2-dichloroethane) (DLAB, 2007). In the realm of environmental science, iron(III) chloride acting as a catalyst for hydrogen peroxide is a crucial part of advanced oxidation processes, which is used to degrade organic pollutants in wastewater. Additionally, a mixture of FeCl₃ and H₂O₂ has been employed for the recovery of landfill leachate with a significant reduction in chemical oxygen demand, colour, and UV absorbance hence reducing the environmental impact of landfill runoff. (Taoufik, 2018).
Sources Of Error
A major systematic source of error is water formation as a byproduct of redox reaction. From the reaction 2H2O2→2H2O+O2, the water dilutes both of the reactants(iron(III) chloride and hydrogen peroxide) which causes the rate of reaction to slow down as the concentration of both reactants decreases. At an atomic level, it would reduce the chance of collisions between Fe³⁺ and H₂O₂ molecules, directly reducing the overall rate of reaction. However, the volume(mL) of iron(III) chloride increases for each trial, which affects the impact of water diluting the mixture as concentration changes. (Nirou Chlor Co., n.d.)
A random error would be the different rate of reaction recorded on day one and day two. The iron(III) chloride solution was put in room temperature overnight for storage, which caused hydrolysis to occur and form iron oxyhydroxides. This change to the catalysis caused the rate of reaction to decrease by 42% when compared in table 1 and table 2. (Scheck et al., 2015)
One human error that was hardly preventable occurred during the process of sealing the iron(III) chloride and hydrogen peroxide mixture, as the reaction occurred instantly, a considerable volume of oxygen wasn't captured by the cylinder. Further exacerbation occurred during the process of submerging the beakers into the container filled with water. However, the fact that this error occurred on all trials mitigated the impacts significantly as we attempted to keep a consistent time when submerging the beakers.
Instrumental errors are present in this experiment as burettes and graduated cylinders are used. The graduated cylinder has an inaccuracy ofΔ± 0.1mL, whereas the burette has an inaccuracy of Δ ± 0.5mL. This limited the collected data to an accuracy of Δ ± 0.5mL, which would affect the overall collected data. However, this would not impact the overall validity of this experiment as the data still revealed a consistent trend.
Citations
References
Canadian Centre for Occupational Health and Safety. (n.d.). WHMIS - Pictograms. Retrieved March 20, 2025, from https://www.ccohs.ca/oshanswers/chemicals/whmis_ghs/pictograms.htm
Chung, D. (2021, December 6). https://edu.rsc.org/feature/investigating-activation-energies/2020172.article. educationinchemistry. https://edu.rsc.org/feature/investigating-activation-energies/2020172.article
Flinn Scientific, Inc. (2016). Catalytic Decomposition of Hydrogen Peroxide. Flinn Scientific. https://www.flinnsci.com/api/library/Download/92be0774307542f293587ce65d49b546?srsltid=AfmBOoobRlw-TpeODqcOaFFOLlsCkbOA8Etfg2mny0sJqxvy7EU0mwXo
Inorganic Ventures. (2025). https://www.inorganicventures.com/forum/chemical-stability-and-compatibility/stability-of-fecl3-iron-chloride-solution. inorganicventures. Retrieved March 15, 2025, from https://www.inorganicventures.com/forum/chemical-stability-and-compatibility/stability-of-fecl3-iron-chloride-solution
Kwan, W. P. (1999, September). KINETICS OF THE Fe(III) INITIATED DECOMPOSITION OF HYDROGEN PEROXIDE: EXPERIMENTAL AND MODEL RESULTS. Doctoral dissertation, Massachusetts Institute of Technology. https://dspace.mit.edu/bitstream/handle/1721.1/80211/44602388-MIT.pdf?sequence=2
Laat, J. D., & Gallard, H. (1999). Catalytic Decomposition of Hydrogen Peroxide by Fe(III) in Homogeneous Aqueous Solution: Mechanism and Kinetic Modeling. Environmental science & technology, 33(16), 2726-2732. http://lib3.dss.go.th/fulltext/Journal/Environ%20Sci.%20Technology1998-2001/1999/no.16/16,1999%20vol.33,p.2726-2732.pdf#page=1.84
Macedo, L. J.A., Hassan, A., Sehendo, G. C., & Crespilho, F. N. (2020, January 16). Assessing electron transfer reactions and catalysis in multicopper oxidases with operando X-ray absorption spectroscopy. nature communications, 11, 1-7. https://www.nature.com/articles/s41467-019-14210-1
Nagashima, K. (1999, May 24). Ferric(III) ions inhibits copper(II)/hydrogen peroxide-catalyzing lipid peroxidation in human erythrocyte membranes. Ferric(III) ions inhibits copper(II)/hydrogen peroxide-catalyzing lipid peroxidation in human erythrocyte membranes. https://pubmed.ncbi.nlm.nih.gov/1592156/
Pędziwiatr, P., Mikołajczyk, F., Zawadzki, D., Mikołajczyk, K., Bedka, A., & Oktan, S. K. N. (2018). DECOMPOSITION OF HYDROGEN PERODIXE - KINETICS AND REVIEW OF CHOSEN CATALYSTS. Acta Innovations, 26(2018), 45-52. https://www.proakademia.eu/gfx/baza_wiedzy/461/nr_26_45-52_2_2.pdf
Sugimoto, H., Spencer, L., & Sawyer, D. T. (1986, November 12). Ferric chloride-catalyzed activation of hydrogen peroxide for the demethylation of NN-dimethylaniline, the epoxidation of olefins, and the oxidative cleavage of vicinal diols in acetonitrile: A reaction mimic for cytochrome P-450. Proceedings of the National Academy of Sciences, 84(7), 1731-1733. https://www.pnas.org/doi/pdf/10.1073/pnas.84.7.1731
Taoufik, M. (2018, Feb 10). Treatment of landfill leachate by coagulation-flocculation with FeCl3: process optimization using Box–Behnken design. Treatment of landfill leachate by coagulation-flocculation with FeCl3: process optimization using Box–Behnken design. https://www.jmaterenvironsci.com/Document/vol9/vol9_N8/270-JMES-3904-Taoufik.pdf
Acknowledgement
We acknowledge the steadfast support from our chemistry teacher, Ms. Willoughby by provided us with the lab equipment, schedule, and environment. We truly appreciate her help and recognize the significance of her dedication to our project. We furthermore acknowledge the support provided by Strathcona Tweedsmuir School by providing us with the facility to conduct our research.

