water electric conductivity
Dehab Sultan
Grade 5
Presentation
No video provided
Hypothesis
I don't think water will conduct electricity. Pure water is a poor conducter of electricity.
Electricity is a form of energy that is generated by the movement of charged particles such as electrons.
Research
Pure water is not aconductor of electicity,because it does not have enough ions.The particles in pure water are always moving but they are not charged.
water conducts electricity when it contains dissolved substance that create ions.
Ions are atoms or molecules that have gained or lost electrons allowing them to carry electrical charges.
Variables
Procedure
Experiment 1
* Palace the electrodes in to the pure water,making sure they don't touch each other.
* Connect the electrodes to the light bulb and battery.
* observe the light bulb is OFF.
Experiment 2
* Mix the sugar in to the water until it is fully dissolved.
* Place the electrodes in the solution making sure they don't touch each other.
* Connect the electrodes to the light bulb and battery.
* Observe the bulb is OFF.
Experiment 3
*Mix the salt in to the water until it is fully dissolved,
* Place the electrodes in the solution making sure they don't touch each other.
* Connect the electrodes to the light bulb and battery.
* Observe the light bulb is ON.
Observations
During experiment 1,the light bulb is OFF,because no charged ions.
During experiment 2,the light bulb is OFF,because no charged ions.
During experiment 3,the light bulb is ON,because it has charged ions.
Salt in solid state is not conductor of electricity.the ions fixed in lattice and cannot move.
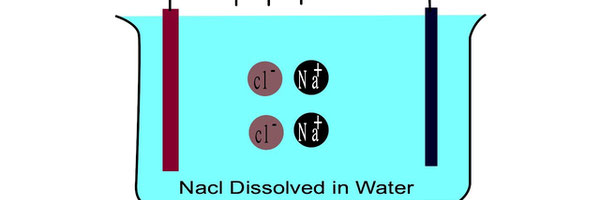
when salt dissolved in water ions can now move and conduct electricity.
Analysis
Conclusion
When salt is dissolved in water,the water molecules pull the salt apart into charged ions.
These ions are free to move and carry electricity through the water.
When a battery with positive and negative poles is placed in water,the positive ions areattracted to the negative pole and the negative ions are attracted to the positive pole this creates a closed circuit then the bulb is on.
Application
Sources Of Error
Citations
Acknowledgement
Attachments
No Log Book Provided
View Extra Attachment: report/data/exhibit/etc(may download a file)