Simple chemical sensor designed to detect exhaled biomarkers in patients with idiopathic pulmonary fibrosis (IPF)
Grade 9
Presentation
Problem
Introduction
Pulmonary Fibrosis - What is it? Who does it affect?
Pulmonary Fibrosis (IPF) is a lower respiratory disease that affects 3 million people worldwide. Out of those 3 million patients, an estimated 50 000 new diagnoses happen each year, with 40 000 of them succumbing to respiratory failure within 2~3 year of diagnosis. This disease is formed in the lungs due to numerous causes, which include bacterial aggregates such as biofilms, exposure to toxins, genetic factors, and radiation. Breaking the name itself, it means lung (pulmonary) scarring (fibrosis). The primary symptom of IPF is shortness of breath, especially during exercise, but other symptoms include a dry, persistent cough, fatigue, chest discomfort, clubbing at the fingers and toes, and unexplained weight loss.
Most cases that occur are usually with patients in their mid to late 50’s. IPF affects people with a weaker respiratory function, as well as low immunity in terms of cellular degeneration. Men are more affected by IPF than woman are, as shown by countless long-term studies. Some cases of the disease progress extremely fast, causing death within 2-3 years of diagnosis. Others can be dragged across time, with the average mortality time being 5 years. It is highly uncommon to see an IPF patient surviving well in the 7-10 year range.
This project was primarily put into action to potentially resolve the recurring issues of late diagnosis in IPF cases. Since there are tens of thousands of new cases each year in just the United States alone, an intuitive, smaller sensor that helps detect cases of this terminal illness has the potential to severely change the mortality rates by diagnosing the disease as early as possible.
This graph shows the projected lifespan of an IPF patient, even with medications such as pirfenidone. IPF currently has no cure, and so the goal of this project is to reduce these numbers by increasing the time patients have knowing they have the illness.
Method
How does pulmonary fibrosis affect the lungs?
When you breathe, the diaphragm contracts and moves down, increasing space in the chest cavity. Rib muscles also move up and outwards, allowing air to be sucked into the lungs. The air travels down through the trachea, and enters the lungs. Once the air enters the lungs, it proceeds to travel through the bronchi (splitting between the lungs) and then through the bronchioles (smaller branches), leading to the alveoli (all respiratory organs shown in Figure 2.1).Within the alveoli is where the gas exchange occurs. Alveoli look like small sacs of grapes, with capillaries running around their surface (2.1). Healthy alveoli are shown in figure 2.0, left side, where fibroblast cells are not aggregated and average O2 and CO2 exchange occurs. Both types of alveolar cells are not damaged, and there is no sign of scarring. In IPF, however, we can observe that the fibroblast cells have been activated, alveolar matrix tissue is thick and has signs of scarring, and that O2 and CO2 exchange is minimal, if not negligent. The scarred alveolar matrix tissue and activated fibroblasts act as a barrier for the oxygen and CO2, not allowing either to pass effectively. When this happens, red blood cells in the capillaries cannot absorb the fresh O2 through diffusion, and instead the hemoglobin bond with more CO2 cells. With more CO2 than O2, the IPF patient may be sleepy most of the time until their eventual passing, where CO2 is entirely blocked and O2 cannot enter the capillary from the alveolus. The right side of 2.0 can show this very procedure quite clearly, but the state of the alveolus in 2.0 is of premature death in IPF patients. We can see how IPF patients use oxygen particularly ineffectively.
This image shows the relative amounts of scar tissue in IPF and emphysema affected lungs. (A), (B), (C), (D), (E) and (F) all show the lateral and anteroposterior views of control subjects. But in (B) and (E), we can see small black dots throughout the entire lung. These show lower attenuation in the lungs, meaning more damage to the air sacs (emphysema). Figure 2.2, (B) and (E) show the acute destruction of alveoli, and (C) and (F) show the residual scarring of the alveoli (IPF). Additionally, all of the images show the areas of diaphragm movement in the lungs when the patient breathes. (A) and (D) are both the control subjects within this CT derived diaphragm motion. Note that “dsStar” shows the relative magnitude of the diaphragm motion vector, and in both emphysema and IPF, there is reduced diaphragm motion than in the control subjects.
Although emphysema affects the alveolus primarily, IPF still affects that same area, and both produce uncannily similar symptoms and problems. This image shows how IPF affects the lungs and the diaphragm, and how most interstitial lung disease issues usually affect the entire respiratory system (not just the lungs).
Fibroblast Cells and Lung Matrix Tissue Aggregation
In IPF, a complex interplay between aberrant fibroblast activation and excessive extracellular matrix (ECM) deposition drives the progressive scarring of lung tissue. Fibroblasts, normally responsible for tissue repair, become dysregulated and excessively produce ECM components such as collagen (a strong protein), fibronectin (a glue-like protein), and proteoglycans (proteins bonded covalently to carbohydrates, found in connective tissue). This excessive ECM deposition leads to the formation of dense, fibrotic nodules (scar-like lumps) that disrupt the lung's architecture and significantly impair gas exchange. Furthermore, the aggregated ECM can create a stiff and inflexible environment, further enhancing fibroblast activation and perpetuating the scarring of tissue. This continuous cycle results in the progressive decline in lung function of IPF patients.
The aggregation of ECM in IPF is not merely a passive process. Fibroblasts themselves play a crucial role in organizing and remodeling the ECM. They actively secrete matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which regulate ECM turnover. However, in IPF, the balance between MMPs and TIMPs is disrupted, favoring ECM accumulation. Additionally, fibroblasts can interact with other cell types, such as macrophages and epithelial (skin tissue) cells, to amplify the fibrotic response. These complex interactions contribute to the formation of dense, fibrotic tissue that is resistant to degradation and ultimately leads to irreversible lung damage.
What are Volatile Organic Compounds?
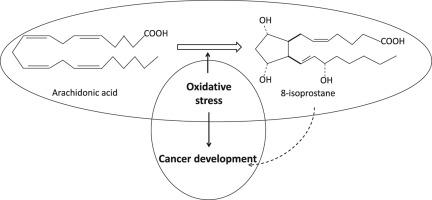
This compound is created when free radicals oxidize fatty acids found in the lung tissue through respiration. For reference, a free radical is a highly reactive and uncharged molecule that has an unpaired valence electron (meaning it is highly reactive).
8 isoprostane (C20H34O5) is shown above as a free radical. Many interstitial lung diseases have an aberrant lung function of some sort that result in the production of certain chemicals.
It is important to know that every single VOC contains at least one carbon molecule, deeming them easily reactive.
Significant research is currently being done to determine various biomarkers, most of them falling under the category of VOCs. Volatile Organic Compounds are specific permutations of organically produced molecules that are produced by countless things. These things could be foods, machinery, and anything organic, like plants. These compounds are easily formed and released into the atmosphere quite excessively, and since they evaporate relatively easily as well, are found in air composition. Excessive exposure to these chemicals can give some sickness symptoms, like nausea or headaches, but they are produced by the human body as well.
8-Isoprostane, a molecule formed from fat damage due to harmful free radicals (oxidative stress), serves as a reliable indicator of this stress within the body. Elevated levels of 8-isoprostane are strongly associated with several diseases, including Idiopathic Pulmonary Fibrosis (IPF). In IPF, a chronic inflammatory condition causing lung scarring, oxidative stress plays a significant role. Individuals with IPF exhibit markedly higher 8-isoprostane levels in their breath, blood, and lung fluid compared to healthy individuals, making it a potential biomarker for both diagnosis and disease monitoring. This heightened 8-isoprostane presence suggests its value in early IPF detection, which is crucial for timely treatment and potentially slowing disease progression.
Among these volatile organic compounds, there are two that stand out the ones produced by scarred, IPF-affected lungs. These compounds are known as 8-isoprostane and isoprene. 8-isoprostane is part of the F2-IsoP chemical family, while isoprene is an ordinary VOC. For the sake of the design, we shall choose to use 8 isoprostane as the target chemical.
Complications from IPF
- Pulmonary hypertension - High blood pressure in the arteries of the lungs. This makes it harder for the heart to pump blood through the lungs
- Acute Exacerbation - This is a sudden worsening of symptoms, such as shortness of breath, cough, and fever.
- Respiratory Failure: As the lungs become increasingly scarred, they may not be able to provide enough oxygen to the body.
- Heart Failure: In some cases, the strain on the heart from pulmonary hypertension can lead to heart failure.
- Lung Cancer: People with IPF have a slightly increased risk of developing lung cancer.
- Infections: People with IPF are more susceptible to lung infections, such as pneumonia.
- Pneumothorax: This is a collapsed lung, which can occur when air leaks into the space around the lung.
- Thromboembolism: This refers to blood clots that form in the veins, which can travel to the lungs and cause a pulmonary embolism.
- Pulmonary Embolism - A blood clot that stops blood flow to an artery.
Current methods of Detection
Currently, CT (Computerized Tomography) scans, X-ray scans, and Echocardiograms (ECHO) are the most common methods of diagnosing IPF. Exhaled Breath Condensate (EBC) tests are there as well but are not as common as ECHO and CT scans. These breath condensate tests are known either as GC/MS (Gas Chromatography/Mass Spectrometry) or ELISA (Enzyme-Linked Immunosorbent Assay).
Both of the former detection methods focus more on imaging and soundwave testing to create clear and distinct captures of the lungs for certain diagnoses. But both of the latter detection methods work through careful analysis of the exhaled breath in IPF patients. 4 types of detection methods, 2 types of target areas, and only one place to get them done: a lab or clinical room. Some types of breath detection are quite useful, such as ELISA and CT scans, but creating a chemical sensor that produces a colorimetric change would be the most efficient choice to proceed with.
Although a chemiresistor has already been used to differentiate people with lung cancer from people without, the same design can be changed to create a diagnostic tool for a separate disease. Different chemical substrates and some design changes can result in a different diagnosis.
Computerized Tomography (CT) Scan
The first known method of IPF testing is the most common. It is known as a CT scan, meaning computerized tomography. They are more specific than X-rays, combining computer precision and X-rays together to create detailed images of the human body. Since the patient is in a circular machine, the scan creates cross-sections of every place in the target area. These individual cross-sections can be compiled together to create a 2D full image (look left) as well as a 3D image of the target area. With CT scans, a specialist can screen many things, including bones, muscles, organs, blood vessels (veins, arteries, capillaries), and even fat. This is how a diagnosis is completed; scarring of the lung tissue is clearly visible through the detail of the CT scan images. As seen below, the lower images show a 3D CT scan (left) and a 2D CT scan (right). With the left scan, white and gray patches are visible, where the fibrosis has completely taken over the alveoli. The right image shows another IPF-affected lung but in a 2D version.
This is a CT scan image. In this scan, we can clearly see organs, bone, and even some fat passing through (not clear in this image). Overall, CT scans are the most useful and accurate way to detect IPF in patients, seeing as it provide extremely detailed imaging of the lung directly.
Echocardiograms (ECHO)
An echocardiogram is a noninvasive medical test that uses ultrasound waves to create moving pictures of the heart. It is a safe and painless procedure that can be used to assess the size, shape, and function of the heart. During an echocardiogram, a small probe is placed on the chest and moved around to create images of the heart. These images can show the size and thickness of the heart muscle, the function of the heart valves, and the flow of blood through the heart. Echocardiograms can be used to diagnose pulmonary fibrosis (IPF) by assessing the size and function of the right ventricle of the heart. The right ventricle is the chamber of the heart that pumps blood to the lungs. In patients with IPF, the right ventricle may become enlarged and weakened due to increased pressure in the pulmonary arteries. This can lead to a condition called pulmonary hypertension, which can be fatal. An echocardiogram can be used to measure the size and function of the right ventricle and to assess the severity of pulmonary hypertension. This information can help to diagnose IPF and to determine the best course of treatment.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA (Enzyme-Linked Immunosorbent Assay) is a versatile laboratory technique that plays a crucial role in research related to Idiopathic Pulmonary Fibrosis (IPF). While not a direct diagnostic tool for IPF itself, ELISA provides valuable insights into the disease's underlying mechanisms.
One key application of ELISA in IPF research lies in the identification and characterization of autoantibodies. IPF is often associated with an autoimmune component, where the body's immune system mistakenly attacks its own lung tissues. By employing ELISA to detect specific autoantibodies in the blood of IPF patients, researchers can gain a deeper understanding of the immunological processes involved in disease development and progression.
For IPF detection with ELISA, there are some components used to create the colorimetric reaction that we can measure. These components are the antibody (8 isoprostane), the enzyme (horseradish peroxidase), and the substrate (tetramethylbenzidine). These components are crucial towards determining whether or not IPF resides within the person. For example, in ELISA, the enzyme is attached to a inhabitable area, and then the compounds that are to be tested are placed in the same area. Since horseradish peroxidase doesn’t react with 8-isoprostane directly, tetramethylbenzidine is used instead to provide a colorimetric reaction and show the presence of 8-isoprostane. The ELISA process is the basis of my sensor.
.
In these diagrams, we can see the process of isoprostane forming into series of other chemicals after autoxidation. Since 8-isoprostane is a product of oxidative stress, it will react to reagents that can oxidize it; the reagent in question being horseradish peroxidase. For ELISA in IPF detection, we can see that once the enzyme is placed on a surface that will not nullify the effects of the reaction, the 8-isoprostane found in the breath will automatically bind to the horseradish peroxidase, and then the tetramethylbenzidine will accelerate the reaction. In this reaction, it is important to note that the horseradish peroxidase will only catalyze the effects of the reaction and acts as a bonding surface for the 8-isoprostane to be tested on. The series that are produced from the autoxidation of the 8-isoprostane from arachidonic acid forms different types of products, most of them taking the appearance of a blue or yellow gaseous substance. This is the reaction between all of the ELISA components.
Lung Biopsies
A lung biopsy is a medical procedure where a small sample of lung tissue is removed for examination under a microscope. This is crucial for diagnosing various lung conditions, including lung cancer, infections (such as tuberculosis), inflammation (like sarcoidosis), and autoimmune diseases (such as idiopathic pulmonary fibrosis). Different biopsy methods exist, ranging from minimally invasive needle biopsies guided by imaging to more invasive procedures like thoracoscopic or open lung biopsies. While essential for diagnosis, lung biopsies carry potential risks, such as pneumothorax (collapsed lung), bleeding, and infection. Needle biopsies utilize a thin needle inserted through the chest wall, often guided by imaging. Transbronchial biopsies involve obtaining tissue through a bronchoscope inserted through the airways. Thoracoscopic biopsies require a small incision in the chest for access, while open lung biopsies necessitate a larger incision. Generally, the procedure involves preparing the patient, accessing the lung tissue using the appropriate method, collecting the sample, closing the incision (if applicable), and sending the tissue for laboratory analysis.
Design Types
I spent some time developing two specific designs that could potentially fit the purpose of the problem. These two designs are a chemical sensor that acts like a COVID-19 antigen test and a modified chemiresistor. Note that both are feasible but can be made through varying complexity. Upon further evaluation, I discovered that many different chemicals are found in the exhaled breath condensate of IPF patients excluding 8-isoprostane. These compounds were hydrogen peroxide, nitric oxide, acetoin, isoprene, and ethylbenzene. All of these compounds are classified as VOCs and are also found to be significantly higher in the breath of IPF patients than control subjects. For some types of compounds, such as 8- 8-isoprostane, there are more specific chemical reagents that require lab techniques to fabricate. Even so, they can be created and embedded into a strip for testing. For the first chemical sensor, an R-Tube design was incorporated as it is an EBC collection machine.
The Chemical Sensor (1)
The first design I had in mind was a chemical sensor. These function by measuring and reacting to specific target chemicals that they are created to look for. If there is no reaction, it typically means that there was no presence of the target chemical in the area. If there is a reaction, it means there was presence of the chemical. This extremely fundamental concept, along with colorimetric reactions, was used to develop a sensor that could detect traces of 8- 8-isoprostane in the EBC of potential IPF patients.
The most effective way that I researched how the sensor could work would be to have a tube coated with horseradish peroxidase on the inside of the tube. This would act as the first step in the diagnostic process. The tube in general would be made of polypropylene, an extremely durable polymer that is resistant to heat and mechanical forces. A mouthpiece with a one-way valve would be the entrance point of the breath, and it would travel to one section of the tube in which the walls are coated with a small amount of horseradish peroxidase. The entrance would be as sealed a possible to ensure that the outside air does not affect the result of the test. Once the minimum required time to receive the ideal amount of EBC is reached, the patient can stop breathing into the tube. They will then need to wait a few hours (4-5) so that the 8-isoprostane in the tube can properly bind to the horseradish peroxidase. Once the patient can pull a small barrier out of the tube to allow the substrate (tetramethylbenzidine) to flow and initiate a reaction with the horseradish peroxidase, wait another 15-30 minutes for the substrate to produce a colorimetric reaction. Once the time for each reaction has been given, if the test yields any colour at all, that means there are trace amounts to large amounts of 8-isoprostane in the breath. If any colour is present, that means that the patient has IPF. They should go see a doctor immediately to confirm with CT scans, or, if the test was performed in a clinic, wait for the confirmation of the disease.
This is the entire process of the chemical “sensor”.
This is the chemical structure of tetramethylbenzidine.
Reaction that takes place within the sensor
This reaction with DNA is extremely similar to the reaction in ELISA by 8-isoprostane. The peroxidase will bind to the peroxidase such as in the image, and then the tetramethylbenzidine will be added to finalize the reaction. Once this occurs, the colorimetric production will occur, finalizing the diagnosis.
.
The Chemiresistor (2)
Chemiresistors are a class of chemical sensors that function by translating the interaction between a sensing material and a target chemical into a measurable change in electrical resistance. These sensors typically employ a sensing material, often a semiconductor, conductive polymer, or nanomaterial like graphene, that undergoes physical or chemical changes upon exposure to the target analyte.
For instance, the analyte might adsorb onto the sensing material's surface, altering its surface area and consequently affecting electron flow. Alternatively, a chemical reaction between the analyte and the sensing material can occur, modifying the material's chemical composition and, in turn, its electrical conductivity. This change in conductivity directly influences the electrical resistance of the sensor, which can then be precisely measured using electronic circuitry.
The effectiveness of a chemiresistor hinges on several critical factors. High selectivity towards the target analyte is paramount, minimizing interference from other substances in the environment. Sensitivity, the ability to detect even minute concentrations of the target chemical, is also crucial. Furthermore, rapid response times, stability over time and under varying environmental conditions, and the capacity to reversibly return to the initial state are essential for practical applications.
Chemiresistors find widespread use across various domains. They are employed in environmental monitoring to detect pollutants in air and water. In the medical field, they show promise in disease diagnosis by analyzing changes in breath or bodily fluids. Additionally, they play a vital role in food safety by monitoring food spoilage and detecting contaminants. Moreover, chemiresistors are instrumental in industrial process control, enabling the monitoring of chemical reactions and the optimization of industrial processes.
.
Here we can see the electrodes and chemiresistive film swell in the presence of the VOC in question, therefore changing the resistance of the chemiresistor. Once the VOC comes into contact with the chemiresistive film, the composite begins to swell and become enlarged, increasing the space between the particles in the sensor.
This is how the baseline resistance would be measured. The chemiresistive film is placed between and on top of two or more electrodes, therefore conducting the electrical charge throughout the entire PCB. To actually fabricate this sensor, tin oxide will be melted onto the electrodes first, then followed by an indium oxide and graphene mix and subsequent cooling of the matrix in the PCB. This way, cracking will not occur.
This image further explains the chemiresistive reaction within the sensor with a gold electrode. The gold electrode particles are covered in a carbonate substance, which reacts with a VOC and swells the substance to change the baseline resistance.
Baseline resistance: ~98 ohms
Diagnostic Resistance: Must be tested
The resulting change will be quantified using a multimeter, and within a certain breath range the patient will be diagnosed with IPF.
This is the chemiresistor function.
Importance of Detection in IPF Cases
The "so what" of this project lies in its potential to significantly impact the lives of individuals with Idiopathic Pulmonary Fibrosis (IPF). By developing a simple chemical sensor for detecting IPF biomarkers in exhaled breath, we could revolutionize early diagnosis. This non-invasive approach would offer a less burdensome alternative to current diagnostic methods, leading to earlier interventions and improved patient outcomes. Early diagnosis allows for timely treatment initiation, potentially slowing disease progression and preventing complications. Furthermore, this could lead to reduced healthcare costs associated with managing IPF, including hospitalizations and treatments for complications.
As stated before in the Introduction, exponential amounts of people lose their lives each year to IPF. With the use of this innovative design, hundreds of thousands of people would have access and know if they have an interstitial lung disease, receiving access to treatment as early as possible. Since there's no direct cure for IPF, all people diagnosed with the disease inevitably will pass away. As such, the only way to help IPF patients live longer is by diagnosing them as early as possible when there are symptoms of these terminal diseases. This way they can get treatments and medications to their disease and live their life for the next couple of years happily before they eventually pass.
Potential Applications
This chemical sensor could be used as a diagnostic tool commercially available to people, but it can also be a standing test to prevent misdiagnosis. For example, patients may use this “sensor” to understand if they potentially have the disease, but if a doctor is not sure of a CT scan or X-ray that points in the direction of IPF, they could utilize this as a last test to prove if the patient has IPF or not.
Think of it as another form of a COVID-19 rapid test. This revolutionized the diagnosis of the deadly and contagious disease, so the same thing can be said for the patients of IPF. They make up an extremely large population of the world, so it is imperative that their diagnosis is as accurate and as quick as possible.
The other possible use that this design could have is collecting and testing on EBC directly sourced from a patient. This way, advancements in treatments can be made through bioengineering, further pushing our knowledge of this relatively (unknown caused) disease.
There are many more diseases that it can be adjusted to detect, such as COPD, lung cancer, or cystic fibrosis (CF), another non-interstitial lung disease.
My future direction with this project will be to acquire the required materials to build this design and build it. That way, I can solidify my belief that in theory, this design will work. Further tests and analysis can also be done from there, once I collect data on how this project will work.
Analysis
Sensitivity and Measurability
One of the largest problems that occurs within this design is the ability to even collect 8-isoprostane from the breath. What this means is that the sensitivity of the horseradish peroxidase may not be enough. Normally, there are minimal errors that occur during ELISA tests, since the horseradish peroxidase is of one specific potency. However, this is because lab-used horseradish peroxidase is kept in climate controlled areas, and not exposed to many outside influences. In the image below, we can see that in the breath of IPF patients is 0.048 picograms, an exponentially small amount in the breath. The extremely low concentrations of 8-isoprostane in exhaled breath necessitate high sensor sensitivity, while the presence of numerous interfering compounds in breath can lead to inaccurate readings. Ensuring consistent and reproducible measurements across individuals and over time requires careful consideration of factors like temperature, humidity, and breathing patterns. The binding oxidation reagent must be potent enough to detect and bond with the 8-isoprostane in the breath. Currently, I have not found a concrete solution towards this problem, and further research will be required.
Another error that I have found in my project is that there are no means of measuring the exact correlation between the levels of 8-isoprostane and the severity of the disease. Like said, the only way to measure this correlation is through the use of a spectrophotometer. Within the sensor, there is no measurement tool that could be incorporated, so the only way to mitigate this error is by sending the results to a lab. That being said, there is no real need for the use of spectrophotometry. If 8-isoprostane is present in the breath, then the reaction will occur, hence giving a colorimetric product. If there is not 8-isoprostane in the breath, then the reaction will not occur, showing that the patient is not IPF affected.
Selectivity of biomarkers (Intrusivity)
Another occurrence that could potentially negate the effects of the sensor is one of unwanted biomarker intrusivity, meaning that the sensor reacts to chemicals that do not signify any real diagnosis. For example, different substrates and the chemiresistive film can still interact with alcohol or water vapour in the breath, potentially creating a false diagnosis. This happens when the biomarker in question reacts with the substrate as well, horseradish peroxidase, but another biomarker reacts with the same substrate simultaneously.
Due to horseradish peroxidase being an oxidation chemical used in ELISA, its uses are extremely broad, and the immunochemical reaction that takes place when the enzyme reacts with can happen with many others. So, to mitigate this error, tetramethylbenzidine can be used to produce a fully-fledged, unique reaction to isoprostane. Leukotrienes and proteins found in the breath as well will not react to tetramethylbenzidine because this chemical specifically reacts to F2-IsoP chemicals. However, since 8-isoprostane is in this relatively wide range of chemicals, the different isomers or series that can occur as the same chemical composition and react with the same substrates can impact the selectivity of the biomarkers. In terms of both designs, the chemiresistor will have less of an issue with this area of error since the film changes the resistance, not producing a chemical reaction. Otherwise, there is no specified way to differentiate between the different types of isoprostanes. This is another large error which currently has an ineffective solution.
Mechanical Malfunctions
Several mechanical malfunctions can hinder the performance of a chemiresistor. Loose connections between the sensing material, electrodes, and circuitry can cause intermittent electrical signals, leading to inaccurate resistance measurements. Physical damage to the sensing material, such as cracks or breaks, disrupts electron flow and significantly impacts sensitivity. Debonding of the sensing material from the substrate or electrodes results in poor electrical contact and unreliable readings. Contamination of the sensing material's surface by dust, moisture, or other substances interferes with analyte interaction and alters conductivity. Micromechanical failures within the sensor structure, like microcracks or delamination, affect its stability and performance. Finally, inadequate packaging can expose the sensor to environmental factors, degrading the sensing material and shortening its lifespan. All these issues contribute to inaccurate and unreliable sensor readings, ultimately compromising the device's functionality.
The chemical “sensor” design also has some malfunctions in its own. For example, the barrier that is designed to separate the horseradish peroxidase from the tetramethylbenzidine could break, potentially starting a premature reaction in the tube. This would cause a significant impact on the sensor accuracy and even the functionality. Any part that is at risk of breaking has the mitigation of being packaged properly and being taken care of so that the parts don't break. That is the only current solution towards a product that can break.
The use of polypropylene in the sensor is crucial as it mitigates the potential damage that can be done to the tube. More stronger and cheap polymers can also be used, but polypropylene has the most general durability against a large variety of things. That is why it was chosen to create the sensor.
Amount of breath being collected
One of the largest problems that occurs during an EBC test is not aequiring the amount of breath required to create a successful diagnosis. If this happens, then the diagnosis will be substantially inaccurate. A limited amount of EBC is the equivalent of an even lower amount of VOCs present in the breath. As such, the only possible ways to ensure that enough EBC is benign collected is cooperation of the patient using the device. If the patient does not breathe at specific intervals and instead breathes erratically, the results will mirror the style of breathing of the patient.
Environmental changes can also affect how much EBC will actually condense, so it is advisable to stay in a climate controlled area for the duration of the test. A cooler place could help condensing the EBC in the tube, and could produce optimal results. Humidity is also another concern. It will affect how long it would take for the breath to condense, even if there is a cooling pack that comes with the sensor.
Breathing factors, age, and hydration status will also affect the amount of EBC in the breath of the patients. For example, if the patient is breathing slowly and deeply, then the most amount of VOCs and EBC can be collected. If the patient has a higher age (most likely, as IPF affects men and women around the ages of 50-75 the most), then the amount of moisture produced in the airways will vary. Hydration is another factor. If a person is not hydrated and takes the test, the humidity of their breath will certainly be affected, producing unreliable results.
The best solution for all of these problems would be to encourage the patient to drink water 15-30 minutes prior to the test, to ensure that the breath produced is relatively saturated with humidity and water vapour. Of course, if the breath is oversaturated, then the excess water vapour could affect the chemiresistors function as well. Lower amounts of humidity and water vapour in the breath would be ideal, as it is a obstacle that can be easily solved and not affect the outcome of the diagnosis.
Difficulty breathing into the apparatus
Another problem that could occur during testing for IPF with this design is difficulty in breathing into the apparatus. If the patient has difficulty breathing into the apparatus, then there is a slight chance that the required amount of EBC will not be collected. This will therefore result in a lower accuracy, either being a false positive or negative. The reaction of 8-isoprostane requires a certain amount of chemicals in each category to produce a proper reaction; therefore, the best way to mitigate this error is by using a mouthpiece that is comfortable to breathe into. For reference, a mouthpiece like the one below could be used to enhance the comfort for a patient. This is an inhaler chamber, designed to keep the medicine from inhalers for people with asthma inside the chamber. It is used to provide a direct route from the inhaler to the mouth for more efficiency and less wastage of the medicine. The same can be used in an opposite way if we blow into a mouthpiece of this kind; the air will go through the mouthpiece and into the one-way valve of the apparatus.
Successes
- Creating a somewhat, low-cost design for a chemical sensor that will (hopefully) diagnose IPF
- Understanding and addressing shortcomings that can occur while using this design
- Designing a model that could be commercially available with a number of uses
- Exploring and understanding the usage of VOCs and how they play an important role in the detection of IPF
Shortcomings
- Sensitivity and Measurability
- Selectivity of Biomarkers (Intrusivity)
- Mechanical Malfunctions
- Inadequate Testing of Accuracy
- Amount of breath being collected
- Difficulty breathing into the apparatus
- False Diagnosis and Design Changes
False Diagnosis and Design Changes
Even with most concerns validated and the design of the device, there is always potential for false diagnosis. This cannot be avoided, even in the most advanced medical equipment and diagnostic tools. But, since this project has not yet been tested, the validity and the accuracy of the different scenarios that may occur simply broaden. Alongside that, many errors (that have been addressed in the project) have contributed to detriment the overall potential success of the design, Although each design has its flaws, careful consideration has been given as to how these flaws may be mitigated and the design of the device may be overall improved. Design changes include:
- Including a comfortable Mouthpiece
- Including a high quality one-way valve
- Using tetramethylbenzidine as well as a specified chemiresistor film made for isoprene (VOCs that are not antigens or antibodies from lipid peroxidation will not work with ELISA techniques)
- Adding dry reagent strips with horseradish peroxidase and using tetramethylbenzidine as a liquid solution to place on the 8-isoprostane bonded reagent strip
- Using electrically adaptive polymers like polypyrrole to conduct electricity when isoprene comes into contact with them, giving a measurable change
- Using things like graphene or possibly nanomaterials for better sensitivity
- Sequential substrate addition already incorporated
Inadequate Testing of Accuracy
Due to the lack of testing and my inability to create my design, there is no way to tell that this device could accurately work through the use of chemical reactions. To deem this a working prototype, I must build, test, and validate the sensor’s accuracy before I can correctly determine whether or not this design would work accurately outside of theory.
I am quite sure that this design would work in theory. Since the similar types of things have been created before with amazing accuracy, I hypothesize that either design will be quite feasible to create based on antigen and ELISA methods/technologies.
For this very reason, this is a large error within my project. The only way to mitigate this obstacle is through really testing and validating my design’s functionality. In other words, the future direction of my project would also be to test my design. For example, the simple chemiresistor from NIH.gov was shown to discriminate between patients with and without lung cancer with an average of almost 90% accuracy. This can be shown in the following diagram, through the detection of specific VOCs such as heptane (VOC present in lung cancer). Please note that this diagram shows the peak output time for the chemiresistor, and that the accuracy was tested for against real lung cancer patients.
All of the potential errors that could occur have been taken into careful consideration and given some thought as to how the problem could be solved. Out of all of them, accuracy is the largest due to the sensors validity not being tested.
Conclusion
We see the dangers of late, unprecedented and misdiagnosis in IPF, and how it can tear apart families. Of course, it is important to learn everything we can about this mysterious and terminal disease. But currently, we see that the only way to help families with patients who have contracted IPF is to inform them as early as we can and provide a diagnosis with the most possible accuracy that we can achieve.
Throughout this project, I have analyzed, prototyped, and modeled three feasible designs for a device that can diagnose this terminal disease simply from the exhaled breath of a potential patient. The intention of the design and device is to address the catastrophic ending a person could have when they are not diagnosed properly. It is to decrease doubt and dependency simply on traditional lab techniques and advanced X-ray scans and imaging.
My project aims to address this disease and show that a great way to survive with it is to be aware of it as soon as possible. To learn and to know that a person's end is near is hard, but at informing patients of their disease with accuracy is of the highest and most utmost importance. My project has aimed to created a possible solution that could change the lives of millions, improving their care of their disease and make their lives as normal as possible for their remaining 3 to 5 years.
Although the design could come with some shortcomings, these have been considered, deeming this design to have a high potential in the dangers of pulmonary fibrosis and (hopefully) minimal errors when diagnosing.
Citations
- Yamada, Yu-Ichi, et al. “Volatile Organic Compounds in Exhaled Breath of Idiopathic Pulmonary Fibrosis for Discrimination From Healthy Subjects.” Lung, vol. 195, no. 2, Feb. 2017, pp. 247–54. https://doi.org/10.1007/s00408-017-9979-3.
- Kang, Ji Hee, et al. “CT-derived 3D-diaphragm Motion in Emphysema and IPF Compared to Normal Subjects.” Scientific Reports, vol. 11, no. 1, July 2021, https://doi.org/10.1038/s41598-021-93980-5.
- Saintsing, Andrew, PhD. “Reversing Lung Scarring With a Shot of Stem Cells.” Drug Discover NewsDrug Discovery News, Development & Diagnostics Articles | DDN Magazine, 30 Oct. 2024, www.drugdiscoverynews.com/reversing-lung-scarring-with-a-shot-of-stem-cells-16115.
- Caldaroni, Massimiliano. “R-Tube.” COSMED, www.cosmed.com/en/products/pulmonary-function/breath-condensate-rtube..
- IPF: Stubborn Scars in Stiff Lungs. 13 Apr. 2022, misciwriters.com/2022/04/13/ipf-stubborn-scars-in-stiff-lungs.
- Sakai, Norihiko, and Andrew M. Tager. “Fibrosis of Two: Epithelial Cell-fibroblast Interactions in Pulmonary Fibrosis.” Biochimica Et Biophysica Acta (BBA) - Molecular Basis of Disease, vol. 1832, no. 7, Mar. 2013, pp. 911–21. https://doi.org/10.1016/j.bbadis.2013.03.001.
- Zhang, Yihang, and Jiazhen Wang. “Cellular and Molecular Mechanisms in Idiopathic Pulmonary Fibrosis.” Advances in Respiratory Medicine, vol. 91, no. 1, Jan. 2023, pp. 26–48. https://doi.org/10.3390/arm91010005.
- Digital, Azuro. “Pulmonary Hypertension | Chahal Cardiovascular Centre.” Chahal Cardiovascular Centre, 19 Jan. 2024, chahalcardiovascularcentre.com/services/pulmonary-hypertension.
- Echocardiogram - Mayo Clinic. www.mayoclinic.org/tests-procedures/echocardiogram/about/pac-20393856.
- “Figure 7. Exhaled Breath Condensate. (a) Diagram of the Apparatus. (B)...” ResearchGate, www.researchgate.net/figure/Exhaled-breath-condensate-A-diagram-of-the-apparatus-B-exhaled-nitrotyrosine-and_fig4_11937601.
- Fine, George F., et al. “Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring.” Sensors, vol. 10, no. 6, June 2010, pp. 5469–502. https://doi.org/10.3390/s100605469.
- Chizhov, Artem, et al. “Photoactivated Processes on the Surface of Metal Oxides and Gas Sensitivity to Oxygen.” Sensors, vol. 23, no. 3, Jan. 2023, p. 1055. https://doi.org/10.3390/s23031055.
- Yamada, Yu-Ichi, et al. “Volatile Organic Compounds in Exhaled Breath of Idiopathic Pulmonary Fibrosis for Discrimination From Healthy Subjects.” Lung, vol. 195, no. 2, Feb. 2017, pp. 247–54. https://doi.org/10.1007/s00408-017-9979-3..
- Tomić, Milena, et al. “VOCs Sensing by Metal Oxides, Conductive Polymers, and Carbon-Based Materials.” Nanomaterials, vol. 11, no. 2, Feb. 2021, p. 552. https://doi.org/10.3390/nano11020552.
- Bai, Hua, and Gaoquan Shi. Gas Sensors Based on Conducting Polymers. 7 Mar. 2007, pmc.ncbi.nlm.nih.gov/articles/PMC3756721.
- CARBON NANOMATERIALS FOR CHEMICAL AND BIOLOGICAL SENSING - D-Scholarship@Pitt. d-scholarship.pitt.edu/11772.
- Chen, Robert J., et al. “Noncovalent Functionalization of Carbon Nanotubes for Highly Specific Electronic Biosensors.” Proceedings of the National Academy of Sciences, vol. 100, no. 9, Apr. 2003, pp. 4984–89. https://doi.org/10.1073/pnas.0837064100.
- Sukul, Pritam, et al. “Origin of Breath Isoprene in Humans Is Revealed via Multi-omic Investigations.” Communications Biology, vol. 6, no. 1, Sept. 2023, https://doi.org/10.1038/s42003-023-05384-y.
- “RTubeTM Exhaled Breath Condensate Collection Device.” Respiratory Research, respiratoryresearch.com/rtube.
- Carugo, Oliviero, and Kristina Djinović Carugo. “When X-rays Modify the Protein Structure: Radiation Damage at Work.” Trends in Biochemical Sciences, vol. 30, no. 4, Mar. 2005, pp. 213–19. https://doi.org/10.1016/j.tibs.2005.02.009.
- “Figure 4. Structure of One Isoprene Unit.” ResearchGate, www.researchgate.net/figure/Structure-of-one-isoprene-unit_fig1_342236262.
- Roberts, L. Jackson, and Ginger L. Milne. “Isoprostanes.” Journal of Lipid Research, vol. 50, Oct. 2008, pp. S219–23. https://doi.org/10.1194/jlr.r800037-jlr200.
- RTP Company. “Asthma Inhaler Chamber | RTP Company.” RTP Company - Your Global Compounder of Custom Engineered Thermoplastics, 1 Sept. 2021, www.rtpcompany.com/asthma-inhaler-chamber.
- RayBioTech. “Isoprostane ELISA Kit.” RayBioTech, www.raybiotech.com/human-isoprostane-eia-eia-ipf2a.
- “CAS No : 64285-73-0| Chemical Name : 3,3,5,5-Tetramethylbenzidine Dihydrochloride.” https://www.pharmaffiliates.com/en, www.pharmaffiliates.com/en/64285-73-0-3-3-5-5-tetramethylbenzidine-dihydrochloride-pa2713380.html.
- “CT-Guided Lung Biopsy.” Saint Luke’s Health System, www.saintlukeskc.org/health-library/ct-guided-lung-biopsy.
- Branch, Angie. “What Is a Competitive ELISA?” Echelon Biosciences, 6 Sept. 2024, www.echelon-inc.com/what-is-a-competitive-elisa.
- Joseph, Satheesh. “What’s an Echocardiogram? | Brookhaven Heart.” Brookhaven Heart, 6 Jan. 2016, brookhavenheart.com/whats-an-echocardiogram.
- Ponzoni, Andrea. “Metal Oxide Chemiresistors: A Structural and Functional Comparison Between Nanowires and Nanoparticles.” Sensors, vol. 22, no. 9, Apr. 2022, p. 3351. https://doi.org/10.3390/s22093351. .
- Indium(III) oxide 99.99 trace metals 1312-43-2. (n.d.). https://www.sigmaaldrich.com/CA/en/product/aldrich/289418?gad_source=1&gclid=Cj0KCQiAz6q-BhCfARIsAOezPxliqcI1TSM3BI2UpRsUP6FencfRW3Hi1jnZl1TY-IPjg-tDH9yWzWcaAovSEALw_wcB
-
The Lab Depot. (n.d.). Tetracosane. https://www.labdepotinc.com/p-23240-tetracosane?srsltid=AfmBOooEkS35xQwX70eZHRy0af7nNYYqcE-8RtXbT512MM0zaLgaKGv1
-
Indium oxide powder. (n.d.). Ossila. https://www.ossila.com/products/indium-oxide-powder?variant=50067694026968
-
Tetracosane 99 646-31-1. (n.d.). https://www.sigmaaldrich.com/CA/en/product/aldrich/t8752?gad_source=1&gclid=Cj0KCQiAz6q-BhCfARIsAOezPxlrAyE5gKqcM7LGWqqsqbtf9sKlxY4wzKSr7-YDfFtCbPkN8W_hJoUaAh2GEALw_wcB
-
China powder activated carbon for wastewater treatment Manufacturers & Suppliers & Price - Tongke. (n.d.). https://www.tongkeac.com/powder-activated-carbon-for-wastewater-treatment.html
-
Delta Adsorbents. (n.d.). Activated Carbon Bulk & bags products - Delta adsorbents. https://deltaadsorbents.com/activated-carbon-bulk-bags/?gad_source=1
-
Activated charcoal DARCO , particle size -100mesh, powder 7440-44-0. (n.d.). https://www.sigmaaldrich.com/CA/en/product/sigald/242276?gad_source=1&gclid=Cj0KCQiAz6q-BhCfARIsAOezPxk3LUsKOZk2yYiXnQryUVuuorh-pDhsT7iPgmMnfwwYBDYva5fyBXEaApuGEALw_wcB
-
Product: Indium(III) oxide (99.998%-In) PURATREM: (n.d.). https://www.strem.com/product/93-4906?gQT=1
Acknowledgement
I would like to express my heartfelt gratitude to some individuals that have helped me along through the few months that this project was in place. I would first like to thank Ms. Shoults, my science teacher, who answered every single question I presented to her and gave me helpful advice on my project. I would also like to express my gratitude towards my parents, who encouraged me to complete my project when many times it seemed impossible. Thank you.