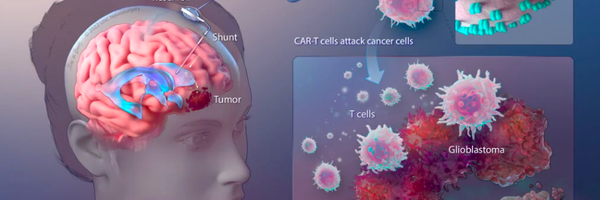
Life Saving Discovery for Brain Cancer Through Multi-Targeting CAR-T Therapy
Grade 11
Presentation
No video provided
Problem
Lack of Effective Treatments for Glioblastoma
Glioblastoma (GBM) remains one of the most difficult cancers to treat due to its heterogeneity, resistance to treatment, and frequent recurrences. Despite advances in surgery, radiotherapy, and chemotherapy, these therapies fail to achieve long-lasting remission because GBM cells are highly infiltrative and often resistant to conventional treatments.
Difficulty in Targeting GBM Antigens
The diverse and mutable nature of GBM antigens complicates the development of effective therapies, particularly for single-target CAR T-cell therapies. The most commonly targeted antigen, EGFRvIII, may not be present in all tumour cells or can be downregulated over time, leading to treatment failure. This antigen variability necessitates exploring additional or multiple antigens to enhance therapeutic efficacy.
Challenges with CAR T-cell Therapy and Antigen Escape
CAR T-cell therapies for GBM face limitations, particularly due to antigen escape, where tumours alter their surface markers to evade detection. Multitargeting CAR T-cell approaches could overcome this by targeting multiple antigens simultaneously, addressing both tumour heterogeneity and reducing the chance of antigen loss. However, designing CAR T-cells capable of effectively targeting a combination of antigens while avoiding toxicity to normal tissue remains a major hurdle.
Method
1. Selection of Target Antigens
- EGFRvIII, IL-13Rα2, and GD2 were selected based on their high expression in glioblastoma and neuroblastoma, tumor specificity, and role in cancer progression.
2. Comparison of CAR-T Cell Targeting Strategies
- Single-target, dual-target, and multitarget CAR-T approaches were analyzed to determine their effectiveness in overcoming tumor heterogeneity and reducing off-target effects.
3. Analysis of Tumor Resistance and CAR-T Exhaustion
- Research on antigen loss, immunosuppressive environments, and CAR-T cell exhaustion was reviewed to assess strategies that enhance persistence and therapeutic efficacy.
4. Evaluation of Potential Clinical Applications
- A dual-target CAR-T model incorporating EGFRvIII and IL-13Rα2, with GD2 as an additional target, was proposed to improve tumor response rates and minimize immune escape.
Research
Glioblastoma Multiforme (GBM)
Glioblastoma multiforme (GBM) is recognized as one of the most aggressive primary brain cancers, affecting more than 4 individuals per 100,000 annually in Canada. This highly malignant disease originates from the uncontrolled growth of astrocytes, which are crucial glial cells responsible for supporting and protecting brain tissue. GBMs are characterized by rapid growth and invasive behavior, primarily within the cerebral hemispheres. However, they can also spread to less common areas such as the cerebellum, brainstem, and spinal cord. Despite advances in treatment options, including surgery, radiotherapy, and chemotherapy, the prognosis for GBM patients remains poor, with an average survival of 14.6 months and frequent recurrence. In recent years, emerging immunotherapies, such as tumour neo-antigen vaccines, modified T cells, oncolytic viruses, and immune checkpoint inhibitors, have shown promising potential in improving treatment outcomes for this challenging condition.
Symptoms
The symptoms of a brain tumour like GBM largely depend on its location, with some tumours causing noticeable symptoms early on, while others may remain asymptomatic until they grow significantly. Gliomas, including GBMs, typically result in symptoms due to both the physical mass of the tumour compressing surrounding brain tissue and the direct invasion of healthy tissue. Common symptoms of GBM include persistent headaches, nausea, vomiting, and seizures. As the tumour grows, specific neurological deficits may arise depending on its location within the brain. For example, a tumour affecting the cerebellum may cause muscle weakness, coordination issues, and balance difficulties. Tumours near the sensory cortex can lead to numbness or impair the ability to recognize objects through touch. When the frontal lobe is involved, changes in personality, behavior, and memory may occur, while tumours in the temporal lobe can impair speech, emotions, and hearing. If the occipital lobe is affected, partial or complete vision loss may result. The symptoms vary greatly based on the tumour’s specific location and extent of spread.
Figure 1. Illustrates the GBM tumour exerting pressure on the frontal lobe, resulting in the manifestation of headaches and associated symptoms
Admin. (2021, March 19). Glioblastoma multiforme | Altair Health. Altair Health. https://altairhealth.com/glasser-center/glioblastoma-multiforme/
Diagnosis
Diagnosing glioblastoma involves a multimodal approach to comprehensively assess the brain’s condition. The process typically begins with imaging techniques such as Computed Tomography (CT) scans and Magnetic Resonance Imaging (MRI), both of which are crucial for determining the location, size, and features of tumours. A key part of diagnosis is the biopsy, where a small sample of tumour tissue is examined under a microscope to identify the type, grade, and specific molecular characteristics that influence prognosis and treatment options. Additionally, neurologic exams assess overall brain function, helping to detect abnormalities or deficits caused by the tumour. Genomic analysis further explores the genetic makeup of the tumour tissue, revealing mutations that inform personalized treatment strategies. These diagnostic methods, including advanced imaging and invasive procedures like biopsies, provide doctors with in-depth insights into glioblastoma, enabling them to tailor treatment plans based on the tumour’s unique characteristics. While MRI imaging is key for a definitive diagnosis, the biopsy is essential for confirming the tumour’s presence and guiding personalized therapeutic interventions based on its molecular profile.
Figure 2. MRI spectroscopy of normal brain. The NAA peak is the most prominent (If the amount of NAA is more than choline, that would suggest a normal brain, the opposite raises suspicion of a tumour).
P Thakkar, J. P. T., Paolo Peruzzi, P., & C Prabhu, V. (n.d.). Glioblastoma multiforme – symptoms, diagnosis and treatment options. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme#:~:text=Glioblastoma%20(GBM)%2C%20also%20referred,not%20spread%20to%20distant%20organs
Figure 3. Glioblastoma as visualized through Magnetic Resonance Imaging (MRI) scans.
Lobera, A., MD. (n.d.). Glioblastoma (Multiforme) imaging: practice essentials, computed tomography, magnetic resonance imaging. https://emedicine.medscape.com/article/340870-overview
Glioblastoma (GB) - Brain Tumour Foundation of Canada. (2019, November 19). Brain Tumour Foundation of Canada. https://www.braintumour.ca/brain_tumour_types/glioblastoma-gb/#:~:text=The%20incidence%20of%20glioblastoma%20(GB,Brain%20Tumour%20Registry%20of%20Canada
Osmosis from Elsevier. (2023, September 24). Glioblastoma (Year of the Zebra) [Video]. YouTube. https://www.youtube.com/watch?v=waliaz_0-54
Major Current Therapeutic Options for This Grade IV Tumour:
Surgery
- Glioblastomas (GBMs) are primarily treated through surgery, with the goal of removing as much tumour tissue as possible while preserving critical brain regions that are essential for neurological function. However, due to the infiltrative nature of GBMs, it is impossible to completely remove the tumour, as the tumour cells invade other brain areas. Surgeons employ advanced techniques, such as intraoperative mapping and neuronavigation, which use computer-assisted guidance to identify critical brain structures and tumour boundaries.
- Additionally, innovative methods, like fluorescence-guided surgery using agents such as 5-aminolevulinic acid (5-ALA), help make tumour cells more visible under specific light wavelengths, allowing for more precise removal. Surgery plays a crucial role in reducing tumour volume, targeting resilient cells at the tumour’s core that may resist radiation or chemotherapy, and relieving intracranial pressure. This can improve both the lifespan and quality of life for patients.
Figure 4. Glioblastoma microenvironment and development of ideal fluorescent probes for fluorescence-guided surgery. Created with BioRender.com.
Chirizzi, C., Pellegatta, S., Gori, A., Falco, J., Rubiu, E., Acerbi, F., & Bombelli, F. B. (2023). Next‐generation agents for fluorescence‐guided glioblastoma surgery. Bioengineering & Translational Medicine. https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/btm2.10608
Radiation
- Radiation therapy is a key step in glioblastoma treatment, typically following surgery and wound healing. The primary goal is to eliminate any remaining tumour cells embedded in nearby healthy brain tissue. Traditional external beam radiation therapy targets infiltrating tumour cells, though it inevitably damages surrounding normal tissue. Radiation treatment consists of multiple sessions where “fractions” of standard-dose radiation are administered to the tumour and its surrounding tissue. Since healthy cells tend to recover between sessions, while tumour cells have less capacity for repair, repeated radiation sessions over 10 to 30 treatments can further damage the tumour.
- Newer methods of radiation therapy, such proton therapy or intensity-modulated radiation therapy (IMRT), are intended to improve the way radiation is delivered. With the help of IMRT, radiation dose modulation can be adjusted more precisely, improving tumour conformance and reducing radiation exposure to nearby healthy tissues. By concentrating energy at the tumour site, proton therapy reduces damage to neighbouring healthy tissue and provides a more focused kind of radiation therapy. These developments could potentially improve radiation therapy's efficiency in treating glioblastoma.
Figure 5. Glioblastoma stem cells (GSCs) possess the unique ability to self-renew, trigger tumour initiation, and exhibit regression when subjected to radiotherapy.
Yousuf Ali, M., R. Oliva, C., & M. Noman, A. S. (2020, September 3). Radioresistance in Glioblastoma and the Development of Radiosensitizers. https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.mdpi.com%2F2072-6694%2F12%2F9%2F2511&psig=AOvVaw0TgUj9lVvHF729FxtQLqsw&ust=1704591430693000&source=images&cd=vfe&opi=89978449&ved=0CBMQjRxqGAoTCIjFvpfQx4MDFQAAAAAdAAAAABCmAQ
Rediotherapy
- In order to minimize radiation exposure to nearby healthy brain tissue, radiosurgery, a precision radiation treatment, uses specialized devices to target tumour areas with high accuracy. Radiosurgery is an essential treatment for glioblastoma in many cases, especially when there are recurrent tumours even if it is not as prevalent as it formerly was. Advanced imaging modalities like as Positron Emission Tomography (PET) scans and Magnetic Resonance Spectroscopy (MRS) also support radiosurgery by offering comprehensive information about the metabolic activity and features of the tumour, which helps with target delineation and treatment planning
- Because GBM is an invasive and diffuse illness, it can be difficult to efficiently target every tumour cell, which contributes to the restricted use of radiosurgery in initial treatment. Its importance in recurrent tumours frequently stems from its capacity to target exact regions with radiation, successfully treating localized recurrences while avoiding needless radiation exposure to adjacent healthy brain tissue. To manage this aggressive brain cancer, complete multimodal therapy are necessary, as the overall effectiveness of radiosurgery as a stand-alone treatment for GBM remains restricted. Optimizing therapeutic advantages and improving outcomes may be possible by incorporating radiosurgery into a multimodal therapy plan that is customized for each patient.
Chemotherapy
- Patients undergoing chemotherapy are given specialized medications designed to combat malignant cells. The current acknowledged standard of therapy for glioblastoma, involves the use of temozolomide as one of the primary drugs. This drug is often taken every day while receiving radiation therapy. During the maintenance phase, it is continued for six cycles, each lasting 28 days. Every cycle starts with the five days of temozolomide treatment, followed by a 23-day rest period.
- Temozolomide is a member of the alkylating agent class of drugs and is an essential component of the chemotherapy treatment. It functions by causing damage to the DNA of rapidly dividing cells (cancer cells), which eventually results in their death or inhibits them from proliferating and replicating its DNA. This drug is a standard treatment for newly/early diagnosed GBM. Temozolomide is effective because it can cross the blood brain barrier, allowing it to reach and target the tumour cells, where the drug methylates (adding a chemical group called methyl) the DNA strand.
Figure 6. Treatment with TMZ causes DNA methylation, leading to cell arrest. If DNA damage repair is successful, the cell recovers to the proliferating pool, or undergoes apoptosis otherwise.
Sorribes, I. C., Handelman, S. K., & Jain, H. (2020). Mitigating temozolomide resistance in glioblastoma via DNA damage-repair inhibition. Journal of the Royal Society Interface, 17(162), 20190722. https://royalsocietypublishing.org/doi/10.1098/rsif.2019.0722
Immunotherapies
- Immunotherapy leverages the body’s immune system to target and fight cancer cells, and it has shown considerable promise for brain tumours like glioblastoma. Among the most innovative strategies is Chimeric Antigen Receptor (CAR) T cell therapy, which involves modifying a patient’s T cells to express receptors that bind to specific tumour antigens. Although preclinical trials have shown promising results, the clinical trials have not yet demonstrated clear advantages. In this therapy, T cells are extracted, modified, and then reintroduced into the patient’s body to better target and eliminate GBM cells. Other immunotherapeutic approaches, such as immune checkpoint inhibitors and tumour neo-antigen vaccines, are also being explored to improve treatment outcomes for glioblastoma patients.
P Thakkar, J. P. T., Paolo Peruzzi, P., & C Prabhu, V. (n.d.). Glioblastoma multiforme – symptoms, diagnosis and treatment options. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme#:~:text=Glioblastoma%20(GBM)%2C%20also%20referred,not%20spread%20to%20distant%20organs
Fernandes, C., Costa, A., Osório, L., Lago, R. C., Linhares, P., Carvalho, B., & Caeiro, C. (2017). Current standards of care in glioblastoma therapy. In Codon Publications eBooks (pp. 197–241). https://www.ncbi.nlm.nih.gov/books/NBK469987/
Challenges Faced in Immunotherapy / Limitations:
1. Blood-Brain Barrier (BBB)
- A protective barrier that controls the flow of substances from the bloodstream into the brain is called the blood-brain barrier (BBB). One of the biggest obstacles to getting therapeutic agents—such as CAR-T cells to the brain is the blood-brain barrier (BBB). While the BBB is essential for maintaining a stable brain environment, its selective permeability makes it difficult to administer treatments effectively. To overcome this obstacle, novel approaches must be investigated, such as the modification of CAR-T cells or the use of cutting-edge delivery techniques like nanoparticles to aid in their entry into the central nervous system.
Figure 9. Illustrating the challenges posed by the Blood-Brain Barrier in primary brain tumours, including glioblastoma.
Dubois, L. G., Campanati, L., Righy, C., D’Andrea-Meira, I., De Sampaio E Spohr, T. C. L., Porto-Carreiro, I., Pereira, C. M., Balça-Silva, J., Kahn, S. A., DosSantos, M. F., De Almeida Rabello Oliveira, M., Ximenes-Da-Silva, A., Lopes, M. C., Faveret, E., Gasparetto, E. L., & Moura‐Neto, V. (2014). Gliomas and the vascular fragility of the blood brain barrier. Frontiers in Cellular Neuroscience, 8. https://www.frontiersin.org/articles/10.3389/fncel.2014.00418/full
2. Tumour Heterogeneity
-
Primary brain tumours, particularly glioblastomas, exhibit heterogeneity in the expression of tumour-associated antigens (TAAs). Different cells within the tumour have varying protein, phenotype, and genetic material profiles, making it challenging to identify a single antigen that can be targeted by CAR-T cells to treat the entire tumour.
-
This can lead to antigen escape because cancer cells may mutate and change the antigens on their surface, or some cells may not have the antigen at all, making them unrecognizable to the CAR-T cells designed to target the original cancer.
3. Immunosuppressive Microenvironment
- Primary brain tumours often establish an immunosuppressive microenvironment, creating a challenge for effective CAR-T cell infiltration. This microenvironment involves various immune cells and signaling molecules, such as inhibitory cytokines like transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10), which collectively hinder the body’s immune response against the tumour.
- Within this setting, immune-suppressive factors released by tumour cells contribute to an environment that restricts the activity of immune cells, making it difficult for them to mount a robust anti-tumour response. Within the immunosuppressive microenvironment of primary brain tumours, tumour-associated macrophages (TAMs) play a critical role by acting as the “first responders” in the body’s immune system. Attracted to the tumour site by signals released from tumour cells, TAMs have the ability to detect specific molecules or markers on the surface of tumour cells, facilitating the recognition of abnormal cells. This recognition typically triggers an immune response, activating other immune cells to join the fight against the tumour. In their immunosuppressive state, TAMs send signals to other immune cells that discourage immune cell activity, often involving immunosuppressive molecules such as TGF-beta or IL-10, instructing them to stay away from the tumour site or dampen their activity. This immunosuppressive behaviour of TAMs presents a significant challenge, as it hinders the CAR-T cells’ ability to function effectively.
4. CAR-T Cell Exhaustion
- CAR-T cell exhaustion presents a substantial challenge in maintaining effective and enduring anti-tumour responses. This state, marked by a gradual decline in cytotoxic functions, is notably influenced by prolonged exposure to tumour antigens and the suppressive microenvironment within tumours.
- The design of CAR-T cells is crucial; weak structures may induce ligand-independent tonic signalling, contributing to exhaustion. Additionally, the cytokine composition during in vitro expansion and the milieu of the tumour microenvironment play pivotal roles in shaping CAR-T cell exhaustion.
- Ligand-independent tonic signalling refers to the activation of CAR-T cells without the presence of specific antigens, potentially leading to premature exhaustion. The composition of cytokines, essential for the expansion of CAR-T cells in vitro, needs meticulous consideration, as imbalances or prolonged exposure can cause exhaustion. The tumour microenvironment, enriched with immunosuppressive factors like inhibitory cytokines, regulatory T cells, and myeloid-derived suppressor cells, further compounds the exhaustion challenge.
5. Acquired Resistance
- Acquired resistance in glioblastoma multiforme (GBM) poses a significant challenge to the effectiveness of chimeric antigen receptor (CAR) T cell therapies. Gliomas, including GBM, are characterized by substantial genetic, epigenetic, and environmental intratumoral heterogeneity. This intratumoral heterogeneity, coupled with the dynamic nature of the tumour microenvironment, contributes to the development of acquired resistance.
- One notable aspect is the loss or downregulation of the targeted tumour-associated antigens (TAs) following treatment. For example, in the case of EGFRvIII, a common target in GBM, previous studies have observed escape mechanisms where a considerable proportion of patients experiencing disease recurrence had lost the expression of EGFRvIII.
- This phenomenon has been documented both in response to EGFRvIII-targeted peptide vaccines and EGFRvIII-specific CAR-T cell administration. The emergence of cancer cells that no longer express the targeted TA poses a significant hurdle, as these cells can evade CAR-T cell-mediated killing, thereby allowing the disease to progress with an altered phenotype.
Figure 10. CAR-T cell therapy faces several limitations, encompassing challenges such as the immunosuppressive tumour microenvironment (TME), constrained access through the blood-brain barrier (BBB), on-target off-tumour toxicity, cytokine release syndrome, tumour lysis syndrome, and the potential for selective antigen loss.
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Strategies to Enhance the Efficiency of CAR-Based Immunotherapy Against GBM:
1. Locoregional Administration
- Locoregional administration methods, such as intratumoral or intracavitary injection are targeted delivery methods for therapeutic agents, enhancing the precision and efficiency of CAR-T cell therapy. In this context, CAR-T cells administered directly into or in close proximity to the tumour, offer distinct advantages over systemic delivery. The approach aims to concentrate therapeutic cells precisely where needed, minimizing systemic circulation and potential off-target effects. This precision is crucial for engaging with heterogeneous tumours, ensuring CAR-T cells effectively target cancer cells expressing specific antigens. Additionally, locoregional administration optimizes cytokine release, such as IL2, within the tumour microenvironment. This localized cytokine production fosters sustained CAR-T cell activity, addressing challenges associated with maintaining T cell function in the immunosuppressive tumour microenvironment. The strategy exemplifies a significant advancement in refining the delivery and performance of CAR-T therapy.
2. Combination Therapies
- Scientists are exploring the integration of CAR-T cells with glioblastoma-targeted oncolytic viruses. These engineered viruses not only selectively infect and eliminate glioblastoma cells but also contribute to an inflammatory response, rallying immune cells for a concerted assault on the tumour. To enhance CAR-T cell infiltration into the glioblastoma microenvironment, enzymes are utilized, clearing a path for therapeutic impact. Moreover, antibodies also aid in the treatment by neutralizing and blocking immunosuppressive cells, amplifying the potency of CAR-T cells in tackling glioblastoma.
- Nanoparticles enhance CAR-T cell delivery through their ability to navigate the blood-brain barrier (BBB) and improve overall therapeutic efficiency. Their small size and surface modifications facilitate BBB penetration, ensuring CAR-T cells reach the central nervous system. Surface engineering enables targeted delivery to specific receptors on tumour cells or the BBB, enhancing precision. Nanoparticles also offer protective encapsulation, shielding CAR-T cells from degradation and providing sustained release, optimizing therapeutic impact. This approach minimizes off-target effects, concentrating treatment at the tumour site.
- A primary tactic involves utilizing PD1 and CTLA4 inhibitors to counteract the inhibitory signals contributing to CAR-T cell exhaustion. This approach aligns with current clinical practices, although the application of immune checkpoint inhibitors with CAR-T cells remains a relatively uncharted territory. Furthermore, understanding the distinctive characteristics of exhausted CAR-T cells, such as their epigenetic changes and transcriptomic abnormalities, introduces layers of complexity to intervention strategies. Additionally, targeting specific factors within the tumour microenvironment (TME) responsible for driving exhaustion are being explored. The TME, enriched with immunosuppressive elements like inhibitory cytokines, regulatory T cells, and myeloid-derived suppressor cells, poses a formidable challenge to CAR-T cell function.
3. Engineering CAR Constructs to Induce or Secrete Active Cytokines
-
One approach to enhance CAR-T cell antitumour efficiency involves engineering the CAR constructs to induce or secrete active cytokines. Cytokines, like interleukins, are crucial in regulating immune responses. CAR-T cells designed to express additional cytokines can have improved activity and persistence within the tumour microenvironment. For instance, IL13Rα2 CAR-T cells engineered to produce interleukin-15 (IL-15) demonstrated greater anti-glioma activity in preclinical models. This modification enhances the cytotoxic effects of CAR-T cells on cancer cells and promotes their prolonged survival and persistence, contributing to a sustained antitumour response.
4. Disrupting Immunosuppressive Immune Checkpoint Molecules
- Immune checkpoint molecules often hinder CAR-T cell effectiveness by weakening their activity within the tumour microenvironment. Strategies have been developed to disrupt these signaling pathways. For example, CAR-T cells can be engineered to secrete antibodies against programmed cell death ligand 1 (PD-L1), a molecule that suppresses immune responses. Genetic modifications, such as CRISPR/Cas9-mediated knockout of genes associated with checkpoint molecules like PD-1 and Lag3, have been explored. Innovative designs, like incorporating a PD-1 ectodomain linked to the transmembrane and cytoplasmic domains of CD28, aim to convert immunosuppressive signals into co-stimulatory ones.
Targeted Immunotherapy For GBM Potential Targets:
1. Human Epidermal Growth Factor Receptor-2 (HER2)
-
In about 80% of glioblastoma (GBM) cases, the human epidermal growth factor receptor 2 (HER2) is significantly overexpressed, making it a key target for potential therapies. However, HER2 is not exclusive to GBM—it is also found in healthy tissues like the gastrointestinal tract, lungs, and ovaries, which complicates efforts to design effective treatments. Targeting HER2 in GBM requires a strategy that minimizes damage to these normal tissues while still effectively eliminating tumour cells.
-
Studies have shown variability in HER2 expression levels across GBM cases. Liu et al. found that 76% of primary GBM cell lines exhibited HER2 positivity, while Ramezani et al. observed that HER2 expression was more common in high-grade astrocytomas (55%) compared to low-grade ones (26%). Research by Mineo et al. suggested that HER2 expression was higher in primary GBM compared to secondary GBM (which develops from lower-grade gliomas). Patients with high HER2 levels tend to have shorter survival times, indicating that HER2 overexpression may be linked to a more aggressive disease course.
-
Despite HER2 being a promising target, there are major challenges in using HER2-directed treatments for GBM. The primary issue is safety, HER2 is present in normal tissues, so therapies must be carefully designed to avoid excessive toxicity. Another challenge is the tumour microenvironment of GBM, which makes it resistant to conventional therapies, including immunotherapies like PD-1/PD-L1 inhibitors. GBM tumours contain immunosuppressive cells, such as macrophages, that limit the effectiveness of immune-based treatments.
Current Research to Improve HER2-Specific CAR-T Therapy
- HER2-specific chimeric antigen receptor (CAR) T cells are designed to recognize and attack HER2-expressing GBM cells. These CAR-T cells are engineered with a HER2-binding domain, allowing them to selectively target tumour cells while ignoring healthy tissues (as much as possible). However, early clinical trials have shown that finding the right balance between tumour specificity and safety is difficult.
- Advancements in CAR-T Cell Structure Researchers are modifying CAR-T cell designs to improve their effectiveness against GBM. Some key strategies include:
• Adding co-stimulatory domains (like CD28 or 4-1BB) to enhance T cell survival and function.
• Combining CAR-T therapy with PD-1 blockade to prevent immune suppression from the tumour microenvironment.
• Using third-generation CAR-T cells, which include multiple signaling domains for stronger activation.
• Engineering CAR-T cells to secrete cytokines, like IL-12, to boost immune responses within the brain. - Early preclinical studies showed that HER2-specific CAR-T cells derived from GBM patients could successfully kill tumour cells, including CD133-positive cancer stem cells. However, safety concerns emerged when a patient treated for HER2-positive colon cancer developed a cytokine storm, leading researchers to modify the CAR design by using a lower-affinity HER2-binding antibody and adjusting the co-stimulatory domains.
In a clinical study involving 17 GBM patients, these modified HER2-specific CAR-T cells resulted in no major toxicity. One patient achieved a partial response, and several others experienced stable disease for up to 29 months, suggesting potential benefits.
Luksik AS, Yazigi E, Shah P, Jackson CM. (2023 Feb 23). CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10000604
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Testa, U., Castelli, G., & Pelosi, E. (2024). CAR-T cells in the treatment of nervous system tumors. Cancers, 16(16), 2913.
https://doi.org/10.3390/cancers16162913
2. Interleukin-13 Receptor Subunit Alpha-2 (IL13Ra2)
-
IL13Rα2 is a tumour-associated receptor that is found in about 75% of glioblastoma (GBM) cases but is rarely present in normal tissues, making it an appealing target for CAR-T therapy. Unlike IL13Rα1, which participates in IL-4 signaling, IL13Rα2 lacks an intracellular signaling domain, functioning primarily as a decoy receptor. Despite its inability to transmit signals, IL13Rα2 plays a role in tumour progression by promoting tumour growth, invasion, and survival. Its restricted expression pattern being largely absent from normal brain tissue except in the testis makes it a strong candidate for targeted therapy, reducing the risk of off-target effects.
Luksik AS, Yazigi E, Shah P, Jackson CM. (2023 Feb 23). CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10000604
-
Chimeric antigen receptor (CAR) T cells were administered to a patient with recurrent multifocal glioblastoma; CAR-T cells were engineered to target interleukin-13 receptor alpha 2 (IL13Rα2), a specific protein associated with GBM. Over the course of 220 days, multiple infusions of these CAR-T cells directly into the brain via two separate routes: infusions into the resected tumour cavity followed by infusions into the ventricular system. Following this treatment, a remarkable outcome was observed, a complete regress of all brain and spine tumours as well as an increase in certain immune cells and cytokine levels in the fluid surrounding the brain and spinal cord.
Figure 7. Regression of Recurrent Multifocal Glioblastoma, Including Spinal Metastases, after Intraventricular Delivery of IL13Rα2-Targeted CAR-T Cells.
Brown, C. E., Alizadeh, D., Starr, R., Weng, L., Wagner, V., Naranjo, A., Ostberg, Ms, B., Kilpatrick, J., Simpson, J., Kurien, A., Sj, P., Wang, X., Tl, H., D’Apuzzo, M., Ja, R., Mc, J., Me, B., Chen, M., . . . Badie, B. (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. The New England Journal of Medicine, 375(26), 2561–2569. https://doi.org/10.1056/nejmoa1610497 https://www.nejm.org/doi/full/10.1056/nejmoa1610497
Current Research to Improve IL13Rα1-Specific CAR-T Therapy
-
IL13Rα2 differs structurally from IL13Rα1 in that it binds IL-13 with high affinity but does not form functional signaling complexes. This distinction is crucial for CAR-T therapy because it allows for the development of highly specific targeting strategies. To ensure that engineered CAR-T cells selectively bind IL13Rα2 without mistakenly attacking IL13Rα1-expressing cells in normal tissues, researchers have modified the IL-13 ligand used in CAR constructs. Specifically, the E13Y mutation reduces the ability of CAR-T cells to interact with IL13Rα1, enhancing specificity for IL13Rα2. This refinement was necessary because early versions of IL13-based CARs had unintended interactions with IL13Rα1 in the lungs, raising safety concerns.
-
To improve the effectiveness of IL13Rα2-targeted CAR-T therapy, researchers have explored different delivery methods. A 2024 Phase I trial tested IL13Rα2-CAR-T cells in 65 patients with recurrent GBM, comparing three administration routes: intratumoral (ICT), intraventricular (ICV), and a combination of both. The study found that direct delivery into the tumour site or ventricles led to better outcomes compared to systemic infusion. Additionally, higher doses of CAR-T cells (200 × 10⁶ cells) were well tolerated and more effective, establishing a potential optimal dosing strategy for future trials.
-
Another major challenge in GBM treatment is the immunosuppressive tumour microenvironment, which can limit the effectiveness of CAR-T therapy. To address this, scientists have begun engineering CAR-T cells resistant to dexamethasone, a corticosteroid commonly used in GBM patients to reduce swelling. By using zinc finger nucleases to disrupt the glucocorticoid receptor gene, researchers created dexamethasone-resistant IL13Rα2-CAR-T cells that retained full functionality even in the presence of the drug.
-
Other strategies to enhance CAR-T cell activity include the development of mutant IL-13 ligands (C4 and D7 variants), which further reduce IL13Rα1 binding while maintaining strong affinity for IL13Rα2. These modifications help improve CAR-T cell trafficking, ensuring that they concentrate in tumours rather than accumulating in healthy tissues.
-
Novel single-chain variable fragments (scFvs) with enhanced affinity for IL13Rα2 have been developed, leading to improved tumour cell targeting and killing in glioblastoma models. These advancements are crucial for making IL13Rα2-targeted CAR-T therapy more effective, selective, and resistant to immune suppression, potentially improving outcomes for GBM patients in the future.
Testa, U., Castelli, G., & Pelosi, E. (2024). CAR-T Cells in the Treatment of Nervous System Tumors. Cancers, 16(16), 2913. https://www.mdpi.com/2072-6694/16/16/2913
3. Disialoganglioside GD2
-
GD2 is a disialoganglioside that is highly expressed on various tumours, including neuroblastoma, small cell lung cancer, and some brain tumours like glioblastoma (GBM). In contrast, its expression in normal tissues is relatively limited, primarily restricted to peripheral nerves, the brain, and certain mesenchymal stem cells. This makes GD2 an attractive therapeutic target, but its presence in normal tissues raises safety concerns when designing treatments.Studies have shown that GD2 is consistently overexpressed in glioblastoma, making it a promising target for immunotherapy. Research indicates that GD2-positive GBM cells exhibit aggressive characteristics, including increased tumour invasiveness and resistance to standard treatments. GD2 expression is also linked to stem-like tumour cells, which are thought to contribute to tumour recurrence and treatment resistance.
-
Despite GD2’s potential as a therapeutic target, challenges remain. The main concern is on-target, off-tumour toxicity, as GD2 is present in healthy neural tissue. This raises the risk of side effects such as neuropathic pain and neuroinflammation. Additionally, the tumour microenvironment in GBM is highly immunosuppressive, making it difficult for immune-based therapies to effectively target and eliminate cancer cells.
Current Research to Improve IL13Rα1-Specific CAR-T Therapy
-
GD2-targeting chimeric antigen receptor (CAR) T cell therapy has emerged as a promising strategy for GBM treatment. These CAR-T cells are engineered to recognize and attack GD2-expressing tumour cells, with the goal of improving survival and reducing recurrence. However, safety and efficacy challenges must be addressed.
-
Recent advancements in GD2-specific CAR-T therapy focus on improving both tumour specificity and persistence. Key strategies include:
• Modified co-stimulatory domains (e.g., 4-1BB and CD28) to enhance T cell activation and survival in the immunosuppressive GBM environment.
• Dual-targeting CAR-T cells that recognize GD2 and other glioblastoma markers to prevent tumour escape mechanisms.
• Armored CAR-T cells engineered to secrete cytokines like IL-15 or IL-12 to improve immune responses within the brain. -
Preclinical studies have shown that GD2-CAR T cells can effectively target and eliminate GBM cells in vitro and in animal models. However, early clinical trials have highlighted safety concerns, with some patients experiencing neurotoxicity due to GD2 expression in normal neural tissues. Researchers are now exploring strategies such as dose modulation, regional delivery (e.g., intratumoral or intraventricular administration), and safety switches to mitigate these risks. A recent clinical study evaluating GD2-specific CAR-T therapy in GBM patients showed early signs of efficacy, with some patients achieving stable disease. However, the long-term success of this approach will depend on refining CAR-T cell persistence and overcoming the immunosuppressive tumour microenvironment.
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Testa, U., Castelli, G., & Pelosi, E. (2024). CAR-T Cells in the Treatment of Nervous System Tumors. Cancers, 16(16), 2913. https://www.mdpi.com/2072-6694/16/16/2913
4. CD70
-
CD70, a protein found in certain cancers, including glioblastoma (GBM), contributes to immune evasion by promoting T cell death. Its presence on GBM cells serves as an immune escape mechanism, allowing the tumour to avoid recognition and attack by the immune system. This evasion strategy is achieved through the constitutive expression of CD70 on GBM, creating an environment that hampers the immune response, particularly by inducing T cell death. To counter this immune evasion, studies have investigated CD70-specific CAR-T cells, which have shown the potential to recognize and eliminate CD70+ GBM tumours in preclinical models without inducing toxicity. This targeted immunotherapeutic approach aims to disrupt the immune evasion tactics employed by GBM.
5. Epidermal Growth Factor Receptor Variant lll (EGFRvlll)
-
Glioblastomas (GBMs) frequently exhibit abnormal activation of the epidermal growth factor receptor (EGFR), with about 40% of cases showing high EGFR expression. Nearly half of these cases involve a mutated form known as EGFRvIII, which results from a deletion in the extracellular domain. This mutation causes continuous receptor activation, driving tumour growth. Unlike normal EGFR, EGFRvIII is almost exclusively found in GBM cells, making it a promising therapeutic target while minimizing harm to healthy tissues.
-
However, EGFRvIII expression varies across GBM tumours, meaning some cancer cells may lack the mutation and evade treatment. Additionally, tumours expressing EGFRvIII create an immunosuppressive environment by recruiting polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), which weaken immune responses and contribute to resistance against immunotherapies.
Current Research to Improve EGFRvlll-Specific CAR-T Therapy
- EGFRvIII-targeted CAR-T therapy is designed to recognize and eliminate tumour cells carrying this mutation. However, early clinical trials have faced significant setbacks. In one study involving 10 patients with recurrent GBM, CAR-T cells successfully reached the tumours, but most patients showed no meaningful improvement. Only one maintained stable disease for 18 months, while the rest experienced disease progression. Another trial combining CAR-T therapy with IL-2 infusion extended survival beyond a year for some patients, but median survival remained just 6.9 months, with severe side effects, including hypoxia, reported in two cases.
- To improve outcomes, researchers have explored dual-targeting strategies. One method involves engineering CAR-T cells to attack both EGFRvIII and normal EGFR using a bispecific T-cell engager (BiTE). In a small trial, this approach initially caused rapid tumour shrinkage, but the effects were short-lived due to limited CAR-T persistence. Another strategy combines CAR-T therapy with PD-1 inhibitors to counteract tumour-induced immune suppression. Although initial trials showed no major toxicities, patient survival remained low, and T cells exhibited signs of exhaustion after treatment.
- Newer research is focused on increasing CAR-T cell persistence and reducing toxicity. A recently developed therapy, GCT102, enhances EGFRvIII targeting while limiting cytokine-related side effects. Preclinical studies suggest it effectively kills GBM cells, and further clinical trials are underway to determine its impact in patients. Despite the challenges, EGFRvIII remains a promising target for glioblastoma treatment. Current research continues to refine CAR-T strategies by improving durability, overcoming immune resistance, and developing combination approaches to enhance long-term effectiveness.
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Luksik AS, Yazigi E, Shah P, Jackson CM. (2023 Feb 23). CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10000604
Testa, U., Castelli, G., & Pelosi, E. (2024). CAR-T Cells in the Treatment of Nervous System Tumors. Cancers, 16(16), 2913. https://www.mdpi.com/2072-6694/16/16/2913
Combination Therapy with JQ1 and EGFR CAR-T:
-
Antigens such as EGFRvIII have been the subject of clinical trials that have provided insight into their safety and efficiency in treating GBM. On the other hand, the ability of CAR-T cells to identify tumour cells, endure in the tumour microenvironment (TME), and maintain their functional efficiency is necessary for the treatment of GBM. Specifically, after EGFRvIII CAR-T infusion, there is increased expression of immunosuppressive molecules like PD-L1 and IDO1 in the GBM TME, raising concerns about compromised immune responses triggered by CAR-T cells.
-
Furthermore, the study shows the significance of super-enhancers—clusters of active enhancers that regulate key genes linked to cancer. Here, BRD4 is a member of bromodomain and extraterminal (BET) protein family, it contributes to gene expression by binding to particular DNA regions. It specifically binds to DNA regions referred to as super-enhancers, which stimulates the transcription of certain cancer genes. Because BRD4 is susceptible to inhibition, compounds such as JQ1 can obstruct its activity. JQ1 influences the expression of genes connected to the development of cancer and the regulation of the immune system by preventing BRD4 from binding to DNA, suggesting possible therapeutic implications.
-
The function of active enhancers triggered by CAR-T cells was interfered with when BRD4 was inhibited by JQ1. The disruption had a major impact on the expression of genes that inhibit the immune response in the microenvironment of GBM. In xenograft models, JQ1 and EGFR CAR-T cells reduced immunosuppression, which in turn inhibited tumour growth and metastasis. The findings suggest that to overcome immunosuppression in treating GBM, a combination approach of EGFR CAR-T cells and BRD4 inhibition using JQ1 could significantly enhance the long-term effectiveness of CAR-T therapy. This proposition arises from the observed exhaustion signature in the T cells infiltrating GBM, indicating an adaptive resistance. However, agents that stimulate the anti-tumour functions of CAR-T or endogenous T cells might now help overcome this resistance and extend the efficiency of treatment beyond the initially limited success.
Figure 8. A working model for combination therapy with EGFR CAR-T cells and JQ1 in GBM
Lin Xia, Jun-yi Liu, Zao-zao Zheng, Guo-sheng Hu, Ning-shao Xia, Wen Liu. (2021, May 21). BRD4 Inhibition Boosts the Therapeutic Effects of Epidermal Growth Factor Receptor-targeted Chimeric Antigen Receptor T Cells in Glioblastoma.. https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(21)00301-4
Chimeric Arm Structure:
The extracellular antigen-binding domain is responsible for recognizing and binding to a specific tumour-associated antigen. This domain is typically derived from the variable heavy (VH) and light (VL) chains of a monoclonal antibody (mAb), which are fused together to form a single-chain variable fragment (scFv). The scFv allows CAR T cells to recognize antigens directly on the surface of cancer cells, bypassing the need for MHC presentation, which is a major limitation of natural T cell receptors (TCRs). This design makes CAR T cells highly effective against tumours that evade immune detection by downregulating MHC molecules. The choice of scFv can significantly impact CAR specificity, binding strength, and potential off-target effects.
The Hinge Region
The hinge (spacer) region provides flexibility and structural support, connecting the extracellular antigen-binding domain to the transmembrane domain. This flexibility is crucial because it allows the scFv to properly engage with antigens that may be densely clustered or positioned at varying distances from the cell surface. The length and composition of the hinge influence antigen accessibility and overall CAR function. Common hinge sources include CD8α, CD28, and IgG4, each with different structural properties that can affect stability, receptor clustering, and signal transduction efficiency.
The Transmembrane Domain
The transmembrane domain serves as the anchor that embeds the CAR into the T cell membrane. It is often derived from natural immune signaling proteins like CD3ζ, CD28, or CD8α, which contribute to the stability and dimerization of the receptor. The transmembrane domain plays a crucial role in maintaining the structural integrity of the CAR and facilitating the transmission of activation signals. Certain transmembrane domains, like those from CD28, can also enhance CAR expression and persistence in T cells, improving their therapeutic durability in vivo.
The Intracellular Signaling Domain
The intracellular signaling domain is the core component responsible for triggering T cell activation, proliferation, and cytotoxic activity against cancer cells. The primary signaling domain is CD3ζ, a key component of the natural TCR complex that initiates downstream signaling upon antigen binding. However, early-generation CARs that relied solely on CD3ζ had limited persistence and effectiveness. To enhance their function, costimulatory domains like CD28 and 4-1BB were introduced in second- and third-generation CAR designs. CD28 signaling promotes rapid T cell activation and expansion, whereas 4-1BB enhances T cell longevity and resistance to exhaustion. Some third-generation CARs, such as EGFRvIII-specific CARs, incorporate both CD28 and 4-1BB alongside CD3ζ, combining the strengths of both pathways to optimize immune response, persistence, and tumour-killing efficiency.
Figure 9. Componets of Chimeric Antigen Receptor
Looi, C., Loo, E., Lim, H., Chew, Y., Chin, K., Cheah, S., Goh, B. H., & Mai, C. (2024). Revolutionizing the treatment for nasopharyngeal cancer: the impact, challenges and strategies of stem cell and genetically engineered cell therapies. Frontiers in Immunology, 15. https://doi.org/10.3389/fimmu.2024.1484535
Monoclonal Antibodies
Trastuzumab (4D5)
- In HER2-targeted CAR-T therapy, the monoclonal antibody trastuzumab (Herceptin) targets the HER2 receptor, which is often found in high amounts on cancer cells, especially in breast cancer. When too much HER2 is present on cancer cells, trastuzumab binds to it and blocks its function, stopping the cancer cells from growing and spreading.
- In CAR-T therapy, a smaller part of trastuzumab (called the single-chain variable fragment or scFv) is used to create a construct that helps modified T-cells specifically target HER2-positive cancer cells. The key parts of trastuzumab that attach to HER2 (such as amino acids E563, D556, and K562) ensure the T-cells attack only the right cells, increasing the therapy’s effectiveness.
- Researchers have shown that trastuzumab and similar antibodies can prevent cancer cells from surviving through various experiments, including techniques like flow cytometry, competitive inhibition assays, and ADCC (antibody-dependent cell-mediated cytotoxicity) assays. These tests demonstrate that trastuzumab can shrink tumours.
- Another approach being studied is using vaccines that target HER2, which could help enhance the immune response. These vaccines work by introducing HER2 proteins or DNA to trigger immunity. However, they tend to be more effective in certain patients who have the right genetic markers.
- While T-cell vaccines are still being explored, monoclonal antibodies like trastuzumab have proven more successful in treating cancers. Trastuzumab stimulates a strong B-cell immune response, which plays a significant role in fighting tumours.
- Scientists are also identifying specific parts of the HER2 protein, known as epitopes, that are essential for trastuzumab to bind to. Some researchers have even developed synthetic peptides that mimic these regions, potentially improving the effectiveness of immunotherapies and offering better protection against cancers that overexpress HER2.
- By improving CAR-T therapy, researchers hope to treat HER2-positive cancers more effectively and with fewer side effects. Future strategies focus on targeting more tumour markers and ensuring that T-cells remain active in the body for longer, potentially making CAR-T therapy useful for a wider range of cancers.
- Ultimately, CAR-T therapies represent a promising new way to treat cancer by harnessing the body’s immune system to target and eliminate cancer cells. Thinking about how this could help others, especially in light of your personal motivation, adds even more meaning to this research.
Joan T. Garrett, Sharad Rawale, Stephanie D. Allen, Gary Phillips, Guido Forni, John C. Morris, Pravin T. P. Kaumaya; Novel Engineered Trastuzumab Conformational Epitopes Demonstrate In Vitro and In Vivo Antitumor Properties against HER-2/neu1. J Immunol 1 June 2007; 178 (11): 7120–7131. https://doi.org/10.4049/jimmunol.178.11.7120
Diermeier-Daucher, S., Ortmann, O., Buchholz, S., & Brockhoff, G. (2012). Trifunctional antibody ertumaxomab: Non-immunological effects on Her2 receptor activity and downstream signaling. mAbs, 4(5), 614–622.https://www.tandfonline.com/doi/pdf/10.4161/mabs.21003
IL13Ra2 scFv
-
The monoclonal antibody targeting IL-13 typically consists of humanized or fully humanized monoclonal antibodies, designed to bind specifically to IL-13’s critical epitopes that allow its interaction with IL-13Rα2. In terms of structure, this antibody would possess:
• Complementarity-determining regions (CDRs) that specifically recognize and bind IL-13.
• Fab regions that allow the antibody to attach to the IL-13 molecule.
• The Fc region, which could be modified to enhance immune system engagement (e.g., via antibody-dependent cellular cytotoxicity, ADCC) or other immune mechanisms that help eliminate cancer cells. -
In the case of its attachment to CAR-T cells, the monoclonal antibody’s structure must allow it to recognize and bind the IL-13 ligand on the tumour, ensuring that the CAR-T cell is directed to kill tumour cells that express the ligand or are activated by it.
-
CAR-T cells, engineered to express a chimeric antigen receptor (CAR) that recognizes a specific target, can be designed to either recognize tumour-specific antigens or modified ligands. When the CAR-T cells are armed with the monoclonal antibody targeting IL-13, they essentially become “sensors” for the presence of the ligand (rather than just the receptor) on tumour cells.
• The CAR on the T cell binds to IL-13, which has been recognized as being present in high quantities in certain cancers. This interaction activates the T cell, leading to tumour cell recognition.
• When the T cell binds to the IL-13 ligand, it gets activated, triggering the CAR-T cell to release cytotoxins such as perforins and granzymes, which induce apoptosis in the tumour cell. -
If the tumour cells overexpress IL-13 (the ligand) but not necessarily the IL-13Rα2 receptor (or if the receptor-ligand interaction is abnormally enhanced), the CAR-T cells can still target these cells because they are recognizing the ligand, rather than the receptor. This is especially helpful in solid tumours, where tumours might modify their expression of receptors to evade immune detection but still rely on ligand-receptor signaling.
Interleukin 13 receptor alpha 2 (IL13Rα2): Expression, signaling pathways and therapeutic applications in cancer Author links open overlay panel. (2022). ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0304419X22001275
14G2 antibody
-
The 14G2a monoclonal antibody is a mouse-derived IgG2a kappa antibody that specifically targets the GD2 ganglioside, a molecule present on neuroblastoma cells. This specificity makes it a promising candidate for neuroblastoma treatment.
- Structurally, 14G2a belongs to the IgG2a subclass of immunoglobulins and possesses a kappa light chain. The antibody’s antigen-binding site, formed by the variable regions of its light and heavy chains, creates a V-shaped groove. Upon binding to GD2, this groove narrows, indicating an “induced fit” mechanism where the antibody adjusts its conformation to securely engage the antigen.
- Detailed studies have identified specific amino acids within the antibody’s binding site that are crucial for GD2 recognition. For instance, research involving peptide mimetics has shown that certain sequences can effectively mimic GD2, binding to 14G2a and inhibiting its interaction with the native ganglioside. These findings highlight the precision of the antibody-antigen interaction and provide insights into potential therapeutic applications.
- Beyond its role in marking neuroblastoma cells for immune system attack, 14G2a can directly induce apoptosis—a form of programmed cell death—in these cancer cells. Apoptosis is a regulated process where cells systematically dismantle themselves, which is crucial for eliminating damaged or unwanted cells. In studies with IMR-32 human neuroblastoma cells, treatment with 14G2a led to decreased cell survival in a dose-dependent manner, meaning higher antibody concentrations resulted in increased cell death. This apoptotic process involves the activation of caspase-3, a central enzyme that orchestrates the breakdown of cellular components during apoptosis. Caspase-3, a member of the cysteine-aspartic acid protease family, cleaves specific substrates within the cell, leading to the characteristic features of apoptosis.
- Immunoglobulins (Ig) are antibodies produced by B cells, playing a vital role in immune defense by identifying and neutralizing foreign substances. The “IgG2a” designation refers to a specific subclass of the IgG type, characterized by particular structural and functional properties. The “kappa” denotes the type of light chain present in the antibody, one of two possible types—the other being lambda. Each antibody consists of two heavy chains and two light chains, with the light chains being either kappa or lambda. The combination of heavy and light chains determines the antibody’s specificity and function.
Kowalczyk, A., Gil, M., Horwacik, I., Odrowąż, Ż., Kozbor, D., & Rokita, H. (2009). The GD2-specific 14G2a monoclonal antibody induces apoptosis and enhances cytotoxicity of chemotherapeutic drugs in IMR-32 human neuroblastoma cells. Cancer Letters, 281(2), 171–182. https://pubmed.ncbi.nlm.nih.gov/19339105/
Murray, J. L., Cunningham, J. E., Brewer, H., Mujoo, K., Zukiwski, A. A., Podoloff, D. A., Kasi, L. P., Bhadkamkar, V., Fritsche, H. A., & Benjamin, R. S. (1994). Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neuroectodermal tumors. Journal of Clinical Oncology, 12(1), 184–193. https://pubmed.ncbi.nlm.nih.gov/8270976/
DSFV-MR1 Antibody
-
The DSFV-MR1 CAR-T construct is specifically designed to enhance the targeting of EGFRvIII, a tumour-specific mutation commonly found in glioblastoma (GBM). The antigen-recognition domain of DSFV-MR1 has been meticulously engineered to improve its binding specificity and stability, allowing it to effectively distinguish tumour cellsfrom normal tissues. This is achieved through precise molecular modifications that optimize its interaction with the EGFRvIII epitope, strengthening its affinity while minimizing off-target effects.
- A key feature of DSFV-MR1 is its modified single-chain variable fragment (scFv), which has undergone targeted residue alterations to refine the epitope binding interface. Through molecular modeling studies, critical amino acid substitutions were introduced to enhance binding strength. One of the most significant changes is the replacement of Thr353 with His353, which facilitates a hydrogen bond interaction with Val507 in the EGFRvIII antigen. This modification increases binding stability, ensuring prolonged engagement of DSFV-MR1 with its target. Furthermore, an additional refinement involves the Thr403 to Arg403 substitution, which introduces another hydrogen bond with Tyr505, further reinforcing antigen recognition. These structural changes collectively enhance the overall affinity and specificity of DSFV-MR1, making it a more effective candidate for CAR-T therapy.
- Beyond these molecular refinements, computational simulations have been used to validate the structural dynamics of DSFV-MR1 in its bound state. The hydrogen bond network, as visualized through molecular modeling, reveals a highly stable antigen-receptor complex, supporting the hypothesis that these modifications significantly improve therapeutic performance. The incorporation of these affinity-enhancing mutations into the DSFV-MR1 framework suggests that this construct could offer superior tumour-targeting capabilities, potentially improving CAR-T cell persistence and efficacy in the tumour microenvironment.
- Moving forward, DSFV-MR1 will undergo further preclinical validation, including cellular assays and in vivo models, to confirm the functional benefits of its engineered antigen-binding domain. Future studies will also explore the generalizability of this approach by investigating whether similar structural modifications can enhance CAR-T therapies targeting other solid tumours. By leveraging computational design strategies, DSFV-MR1 represents a promising step toward next-generation CAR-T therapies with improved precision and durability in targeting EGFRvIII-expressing GBM cells.
Multitargeting approaches
Dual CAR T-Cells (“OR” Logic Gate)
- Dual CAR T-cells are engineered to express two separate CARs, each recognizing a different antigen. This approach follows an “OR” logic, meaning activation occurs if either antigen is present, ensuring tumour targeting even if one antigen is downregulated. This is particularly useful in cancers with high antigen heterogeneity, where tumour cells can escape therapy by losing a single antigen.
Tandem CAR (“OR” Logic in One CAR)
- A tandem CAR, also known as a bicistronic CAR, incorporates two different antigen-binding domains into a single receptor. Unlike dual CAR T-cells, which require two separate CAR molecules, tandem CARs allow T-cells to recognize multiple tumour markers through a single receptor, simplifying design and expression. This strategy has been explored for targeting glioblastoma, where tandem CARs recognizing both HER2 and IL13Rα2 have shown promise.
Dual CAR T-Cells (“AND” Logic Gate)
-
In contrast to “OR” logic designs, some CAR T-cells use an “AND” logic gate, meaning the T-cell is only activated if both antigens are recognized simultaneously. This approach enhances specificity, reducing the risk of off-target effects, particularly in solid tumours where many antigens are also found on healthy tissues. By requiring dual antigen engagement, “AND” logic CARs can prevent activation in normal cells that express only one of the two targets, improving safety. This strategy has been explored in PSMA and PSCA dual-targeting CARs for prostate cancer.

Figure 10. Dual targeting CAR-T cells and their mechanism of action
Strategies for having a more effective and less toxic CAR T-cell therapy for acute lymphoblastic leukemia. (2020). Research Gate. https://doi.org/10.1007/s12032-020-01416-3
Simon, S., & Riddell, S. R. (2020). Dual Targeting with CAR T Cells to Limit Antigen Escape in Multiple Myeloma. Blood Cancer Discovery, 1(2), 130–133. https://pmc.ncbi.nlm.nih.gov/articles/PMC8447280/
Schmidts, A., Srivastava, A. A., Ramapriyan, R., Bailey, S. R., Bouffard, A. A., Cahill, D. P., Carter, B. S., Curry, W. T., Dunn, G. P., Frigault, M. J., Gerstner, E. R., Ghannam, J. Y., Kann, M. C., Larson, R. C., Leick, M. B., Nahed, B. V., Richardson, L. G., Scarfò, I., Sun, J., . . . Choi, B. D. (2022). Tandem chimeric antigen receptor (CAR) T cells targeting EGFRvIII and IL-13Rα2 are effective against heterogeneous glioblastoma. Neuro-Oncology Advances, 5(1). https://doi.org/10.1093/noajnl/vdac185
Data
Figure 1. Illustrates the GBM tumour exerting pressure on the frontal lobe, resulting in the manifestation of headaches and associated symptoms
Admin. (2021, March 19). Glioblastoma multiforme | Altair Health. Altair Health. https://altairhealth.com/glasser-center/glioblastoma-multiforme/
Figure 2. MRI spectroscopy of normal brain. The NAA peak is the most prominent (If the amount of NAA is more than choline, that would suggest a normal brain, the opposite raises suspicion of a tumour).
P Thakkar, J. P. T., Paolo Peruzzi, P., & C Prabhu, V. (n.d.). Glioblastoma multiforme – symptoms, diagnosis and treatment options. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme#:~:text=Glioblastoma%20(GBM)%2C%20also%20referred,not%20spread%20to%20distant%20organs
Figure 3. Glioblastoma as visualized through Magnetic Resonance Imaging (MRI) scans.
Lobera, A., MD. (n.d.). Glioblastoma (Multiforme) imaging: practice essentials, computed tomography, magnetic resonance imaging. https://emedicine.medscape.com/article/340870-overvi
Figure 4. Glioblastoma microenvironment and development of ideal fluorescent probes for fluorescence-guided surgery. Created with BioRender.com.
Chirizzi, C., Pellegatta, S., Gori, A., Falco, J., Rubiu, E., Acerbi, F., & Bombelli, F. B. (2023). Next‐generation agents for fluorescence‐guided glioblastoma surgery. Bioengineering & Translational Medicine. https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/btm2.10608
Figure 5. Glioblastoma stem cells (GSCs) possess the unique ability to self-renew, trigger tumour initiation, and exhibit regression when subjected to radiotherapy.
Yousuf Ali, M., R. Oliva, C., & M. Noman, A. S. (2020, September 3). Radioresistance in Glioblastoma and the Development of Radiosensitizers. https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.mdpi.com%2F2072-6694%2F12%2F9%2F2511&psig=AOvVaw0TgUj9lVvHF729FxtQLqsw&ust=1704591430693000&source=images&cd=vfe&opi=89978449&ved=0CBMQjRxqGAoTCIjFvpfQx4MDFQAAAAAdAAAAABCmAQ
Figure 6. Treatment with TMZ causes DNA methylation, leading to cell arrest. If DNA damage repair is successful, the cell recovers to the proliferating pool, or undergoes apoptosis otherwise.
Sorribes, I. C., Handelman, S. K., & Jain, H. (2020). Mitigating temozolomide resistance in glioblastoma via DNA damage-repair inhibition. Journal of the Royal Society Interface, 17(162), 20190722. https://royalsocietypublishing.org/doi/10.1098/rsif.2019.0722
Figure 9. Illustrating the challenges posed by the Blood-Brain Barrier in primary brain tumours, including glioblastoma.
Dubois, L. G., Campanati, L., Righy, C., D’Andrea-Meira, I., De Sampaio E Spohr, T. C. L., Porto-Carreiro, I., Pereira, C. M., Balça-Silva, J., Kahn, S. A., DosSantos, M. F., De Almeida Rabello Oliveira, M., Ximenes-Da-Silva, A., Lopes, M. C., Faveret, E., Gasparetto, E. L., & Moura‐Neto, V. (2014). Gliomas and the vascular fragility of the blood brain barrier. Frontiers in Cellular Neuroscience, 8. https://www.frontiersin.org/articles/10.3389/fncel.2014.00418/full
Figure 10. CAR-T cell therapy faces several limitations, encompassing challenges such as the immunosuppressive tumour microenvironment (TME), constrained access through the blood-brain barrier (BBB), on-target off-tumour toxicity, cytokine release syndrome, tumour lysis syndrome, and the potential for selective antigen loss.
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Figure 7. Regression of Recurrent Multifocal Glioblastoma, Including Spinal Metastases, after Intraventricular Delivery of IL13Rα2-Targeted CAR-T Cells.
Brown, C. E., Alizadeh, D., Starr, R., Weng, L., Wagner, V., Naranjo, A., Ostberg, Ms, B., Kilpatrick, J., Simpson, J., Kurien, A., Sj, P., Wang, X., Tl, H., D’Apuzzo, M., Ja, R., Mc, J., Me, B., Chen, M., . . . Badie, B. (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. The New England Journal of Medicine, 375(26), 2561–2569. https://doi.org/10.1056/nejmoa1610497 https://www.nejm.org/doi/full/10.1056/nejmoa1610497
Figure 8. A working model for combination therapy with EGFR CAR-T cells and JQ1 in GBM
Lin Xia, Jun-yi Liu, Zao-zao Zheng, Guo-sheng Hu, Ning-shao Xia, Wen Liu. (2021, May 21). BRD4 Inhibition Boosts the Therapeutic Effects of Epidermal Growth Factor Receptor-targeted Chimeric Antigen Receptor T Cells in Glioblastoma.. https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(21)00301-4
Figure 9. Componets of Chimeric Antigen Receptor
Looi, C., Loo, E., Lim, H., Chew, Y., Chin, K., Cheah, S., Goh, B. H., & Mai, C. (2024). Revolutionizing the treatment for nasopharyngeal cancer: the impact, challenges and strategies of stem cell and genetically engineered cell therapies. Frontiers in Immunology, 15. https://doi.org/10.3389/fimmu.2024.1484535

Figure 10. Dual targeting CAR-T cells and their mechanism of action
Strategies for having a more effective and less toxic CAR T-cell therapy for acute lymphoblastic leukemia. (2020). Research Gate. https://doi.org/10.1007/s12032-020-01416-3
Conclusion
The proposed CAR-T cell therapy model, targeting EGFRvIII, IL-13Rα2, and GD2, uses a dual-targeting approach that utilizes a combination of AND-logic and OR-logic to improve specificity and efficacy. The AND-logic approach ensures that CAR-T cells will only be activated when both target antigens are present on the tumour cells, enhancing the precision of tumour targeting and reducing the potential for off-tumour toxicity. For example, the EGFRvIII mutation, commonly expressed in glioblastoma, can be effectively targeted alongside IL-13Rα2, a receptor highly overexpressed in glioblastoma and other cancers. This dual targeting minimizes the risk of immune evasion, as the tumour would need to simultaneously downregulate both antigens to escape immune detection, a far more complex task than evading a single target. Additionally, the inclusion of GD2, expressed on pediatric neuroblastomas and other tumours, provides broader coverage across different tumour types, ensuring that multiple cancer cell populations are targeted and reducing the chance of relapse.
This multitargeted CAR-T cell strategy addresses several critical issues in cancer immunotherapy, including tumour heterogeneity, CAR-T exhaustion, and resistance. By targeting multiple antigens, the therapy effectively combats tumour diversity, significantly decreasing the likelihood of immune escape due to antigen loss or mutation. Furthermore, by utilizing OR-logic for the second layer of targeting (e.g., EGFRvIII or IL-13Rα2, or GD2), CAR-T cells can be activated in the presence of at least one of these antigens, ensuring that even tumour cells with heterogeneous antigen expression are attacked. This approach reduces the chances of off-target effects while enhancing the persistence of CAR-T cells and preventing the exhaustion commonly seen in traditional therapies. The dual-targeting, coupled with the OR-logic system, is designed to improve immune response durability, minimize toxicity, and, ultimately, provide a more effective and safer treatment for patients with glioblastoma and other cancers, potentially transforming current cancer therapy paradigms and saving many lives.
Citations
Brown, C. E., Alizadeh, D., Starr, R., Weng, L., Wagner, V., Naranjo, A., Ostberg, Ms, B., Kilpatrick, J., Simpson, J., Kurien, A., Sj, P., Wang, X., Tl, H., D’Apuzzo, M., Ja, R., Mc, J., Me, B., Chen, M., . . . Badie, B. (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. The New England Journal of Medicine, 375(26), 2561–2569. https://doi.org/10.1056/nejmoa1610497 https://www.nejm.org/doi/full/10.1056/nejmoa1610497
P Thakkar, J. P. T., Paolo Peruzzi, P., & C Prabhu, V. (n.d.). Glioblastoma multiforme – symptoms, diagnosis and treatment options. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme#:~:text=Glioblastoma%20(GBM)%2C%20also%20referred,not%20spread%20to%20distant%20organs
Lobera, A., MD. (n.d.). Glioblastoma (Multiforme) imaging: practice essentials, computed tomography, magnetic resonance imaging. https://emedicine.medscape.com/article/340870-overview
Glioblastoma (GB) - Brain Tumour Foundation of Canada. (2019, November 19). Brain Tumour Foundation of Canada. https://www.braintumour.ca/brain_tumour_types/glioblastoma-gb/#:~:text=The%20incidence%20of%20glioblastoma%20(GB,Brain%20Tumour%20Registry%20of%20Canada
Chirizzi, C., Pellegatta, S., Gori, A., Falco, J., Rubiu, E., Acerbi, F., & Bombelli, F. B. (2023). Next‐generation agents for fluorescence‐guided glioblastoma surgery. Bioengineering & Translational Medicine. https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/btm2.10608
Yousuf Ali, M., R. Oliva, C., & M. Noman, A. S. (2020, September 3). Radioresistance in Glioblastoma and the Development of Radiosensitizers. https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.mdpi.com%2F2072-6694%2F12%2F9%2F2511&psig=AOvVaw0TgUj9lVvHF729FxtQLqsw&ust=1704591430693000&source=images&cd=vfe&opi=89978449&ved=0CBMQjRxqGAoTCIjFvpfQx4MDFQAAAAAdAAAAABCmAQ
Sorribes, I. C., Handelman, S. K., & Jain, H. (2020). Mitigating temozolomide resistance in glioblastoma via DNA damage-repair inhibition. Journal of the Royal Society Interface, 17(162), 20190722. https://royalsocietypublishing.org/doi/10.1098/rsif.2019.0722
P Thakkar, J. P. T., Paolo Peruzzi, P., & C Prabhu, V. (n.d.). Glioblastoma multiforme – symptoms, diagnosis and treatment options. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme#:~:text=Glioblastoma%20(GBM)%2C%20also%20referred,not%20spread%20to%20distant%20organs
Fernandes, C., Costa, A., Osório, L., Lago, R. C., Linhares, P., Carvalho, B., & Caeiro, C. (2017). Current standards of care in glioblastoma therapy. In Codon Publications eBooks (pp. 197–241). https://www.ncbi.nlm.nih.gov/books/NBK469987/
Luksik AS, Yazigi E, Shah P, Jackson CM. (2023 Feb 23). CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10000604
Lin Xia, Jun-yi Liu, Zao-zao Zheng, Guo-sheng Hu, Ning-shao Xia, Wen Liu. (2021, May 21). BRD4 Inhibition Boosts the Therapeutic Effects of Epidermal Growth Factor Receptor-targeted Chimeric Antigen Receptor T Cells in Glioblastoma.. https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(21)00301-4
Janeway, C. A., Jr. (2001). The major histocompatibility complex and its functions. Immunobiology - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK27156/#:~:text=The%20major%20histocompatibility%20complex%20(MHC,to%20the%20T%2Dcell%20receptor.
Z. Song, E., Wang, X., I. Philipson, B., M. O’Rourke, D., Song, H., & C. Milone, M. (2022). The IAP antagonist birinapant enhances chimeric antigen receptor T cell therapy for glioblastoma by overcoming antigen heterogeneity. Molecular Therapy Oncology. https://www.cell.com/molecular-therapy-family/oncology/fulltext/S2372-7705(22)00139-5
Admin. (2021, March 19). Glioblastoma multiforme | Altair Health. Altair Health. https://altairhealth.com/glasser-center/glioblastoma-multiforme/
Osmosis from Elsevier. (2023, September 24). Glioblastoma (Year of the Zebra) [Video]. YouTube. https://www.youtube.com/watch?v=waliaz_0-54
Maity, R., Benaoudia, S., Zemp, F. J., Lee, H., Barakat, E., Leblay, N., Ahn, S., Mahoney, D. J., Neri, P., & Bahlis, N. J. (2021). A BCL2L1 armoured BCMA targeting CAR T cell to overcome exhaustion and enhance persistence in multiple myeloma. Blood, 138(Supplement 1), 327. https://ashpublications.org/blood/article/138/Supplement%201/327/478084/A-BCL2L1-Armoured-BCMA-Targeting-CAR-T-Cell-to
Lin, Y., Mashouf, L. A., & Lim, M. (2022). CAR T cell therapy in primary brain tumors: current investigations and the future. Frontiers in Immunology, 13. https://www.frontiersin.org/articles/10.3389/fimmu.2022.817296/full
Mo, F., Pellerino, A., Soffietti, R., & Rudà, R. (2021). Blood–Brain barrier in brain tumors: Biology and clinical relevance. International Journal of Molecular Sciences, 22(23), 12654. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8657947/
Zhu, X., Li, Q., & Zhu, X. (2022). Mechanisms of CAR T cell exhaustion and current counteraction strategies. Frontiers in cell and developmental biology, 10, 1034257. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9773844/
Dubois, L. G., Campanati, L., Righy, C., D’Andrea-Meira, I., De Sampaio E Spohr, T. C. L., Porto-Carreiro, I., Pereira, C. M., Balça-Silva, J., Kahn, S. A., DosSantos, M. F., De Almeida Rabello Oliveira, M., Ximenes-Da-Silva, A., Lopes, M. C., Faveret, E., Gasparetto, E. L., & Moura‐Neto, V. (2014). Gliomas and the vascular fragility of the blood brain barrier. Frontiers in Cellular Neuroscience, 8. https://www.frontiersin.org/articles/10.3389/fncel.2014.00418/full
Maggs, L., Cattaneo, G., Dal, A. E., Moghaddam, A. S., & Ferrone, S. (2021b). CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Frontiers in Neuroscience, 15. https://www.frontiersin.org/articles/10.3389/fnins.2021.662064/full
Santomasso, B., Bachier, C., Westin, J. R., Rezvani, K., & Shpall, E. J. (2019). The other side of CAR T-Cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. American Society of Clinical Oncology Educational Book, 39, 433–444. https://ascopubs.org/doi/10.1200/EDBK_238691
CAR T-cell therapy and its side effects. (n.d.). American Cancer Society. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/car-t-cell1.html
Landry, R., Klimowicz, A., Lavictoire, S. J., Borisova, S., Kottachchi, D. T., Lorimer, I. a. J., & Evans, S. V. (2001). Antibody recognition of a conformational epitope in a peptide antigen: Fv-peptide complex of an antibody fragment specific for the mutant EGF receptor, EGFRvIII. Journal of Molecular Biology, 308(5), 883–893. https://www.sciencedirect.com/science/article/abs/pii/S0022283601946285?via%3Dihub
Cheung, J., Wazir, S., Bell, D. R., Kochenderfer, J. N., Hendrickson, W. A., & Youkharibache, P. (2023). Crystal structure of a chimeric antigen receptor (CAR) SCFV domain rearrangement forming a VL-VL dimer. Crystals, 13(4), 710.https://doi.org/10.3390/cryst13040710
Joan T. Garrett, Sharad Rawale, Stephanie D. Allen, Gary Phillips, Guido Forni, John C. Morris, Pravin T. P. Kaumaya; Novel Engineered Trastuzumab Conformational Epitopes Demonstrate In Vitro and In Vivo Antitumor Properties against HER-2/neu1. J Immunol 1 June 2007; 178 (11): 7120–7131. https://doi.org/10.4049/jimmunol.178.11.7120
Diermeier-Daucher, S., Ortmann, O., Buchholz, S., & Brockhoff, G. (2012). Trifunctional antibody ertumaxomab: Non-immunological effects on Her2 receptor activity and downstream signaling. mAbs, 4(5), 614–622.https://www.tandfonline.com/doi/pdf/10.4161/mabs.21003
Simon, S., & Riddell, S. R. (2020). Dual Targeting with CAR T Cells to Limit Antigen Escape in Multiple Myeloma. Blood Cancer Discovery, 1(2), 130–133. https://pmc.ncbi.nlm.nih.gov/articles/PMC8447280/
Schmidts, A., Srivastava, A. A., Ramapriyan, R., Bailey, S. R., Bouffard, A. A., Cahill, D. P., Carter, B. S., Curry, W. T., Dunn, G. P., Frigault, M. J., Gerstner, E. R., Ghannam, J. Y., Kann, M. C., Larson, R. C., Leick, M. B., Nahed, B. V., Richardson, L. G., Scarfò, I., Sun, J., . . . Choi, B. D. (2022). Tandem chimeric antigen receptor (CAR) T cells targeting EGFRvIII and IL-13Rα2 are effective against heterogeneous glioblastoma. Neuro-Oncology Advances, 5(1). https://doi.org/10.1093/noajnl/vdac185
Kowalczyk, A., Gil, M., Horwacik, I., Odrowąż, Ż., Kozbor, D., & Rokita, H. (2009). The GD2-specific 14G2a monoclonal antibody induces apoptosis and enhances cytotoxicity of chemotherapeutic drugs in IMR-32 human neuroblastoma cells. Cancer Letters, 281(2), 171–182. https://pubmed.ncbi.nlm.nih.gov/19339105/
Murray, J. L., Cunningham, J. E., Brewer, H., Mujoo, K., Zukiwski, A. A., Podoloff, D. A., Kasi, L. P., Bhadkamkar, V., Fritsche, H. A., & Benjamin, R. S. (1994). Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neuroectodermal tumors. Journal of Clinical Oncology, 12(1), 184–193. https://pubmed.ncbi.nlm.nih.gov/8270976/
Joan T. Garrett, Sharad Rawale, Stephanie D. Allen, Gary Phillips, Guido Forni, John C. Morris, Pravin T. P. Kaumaya; Novel Engineered Trastuzumab Conformational Epitopes Demonstrate In Vitro and In Vivo Antitumor Properties against HER-2/neu1. J Immunol 1 June 2007; 178 (11): 7120–7131. https://doi.org/10.4049/jimmunol.178.11.7120
Diermeier-Daucher, S., Ortmann, O., Buchholz, S., & Brockhoff, G. (2012). Trifunctional antibody ertumaxomab: Non-immunological effects on Her2 receptor activity and downstream signaling. mAbs, 4(5), 614–622.https://www.tandfonline.com/doi/pdf/10.4161/mabs.21003
Interleukin 13 receptor alpha 2 (IL13Rα2): Expression, signaling pathways and therapeutic applications in cancer Author links open overlay panel. (2022). ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0304419X22001275
Acknowledgement
I am grateful for the invaluable support and guidance my mentor, Shannon Snelling (University of Calgary) has offered me. I also extend my appreciation to our science fair project team members at North trail Highs School in Calgary and my family.