Comparing the efficiencies of different hydroxides in carbon capture: sodium, calcium, and lithium hydroxide.
Grade 10
Presentation
No video provided
Hypothesis
If the three hydroxide compounds are dissolved within distilled water, all at the same number of moles, and left open to the air to capture carbon dioxide, then sodium hydroxide will theoretically capture most carbon dioxide because it has one of the weakest bonds between the three compounds. All three hydroxides are ionic, but due to calcium hydroxide having a larger charge magnitude of 2+/1-, it will be harder to disassociate, slowing the carbon dioxide capture process, leaving sodium and lithium hydroxide. Additionally, sodium is a larger ion than lithium. Smaller ions have stronger electrostatic attractions as the nucleus of protons is closer to the anions, strengthening its bond, and resulting in it being more difficult to dissolve. Therefore, it is reasonable to assume sodium hydroxide will theoretically capture the most carbon dioxide: because of its weaker attractions, it will dissolve faster within water, allowing better carbon dioxide capture.
Research
Introduction (1a):
Carbon dioxide capture is more important than ever today. Our carbon footprint increases as our population, human settlement, and car production increase. This increase in carbon dioxide contributes to the enhanced greenhouse gas effect, where the re-emitted radiation that bounces off of the Earth gets trapped. Increased radiation inside the atmosphere then raises global temperatures, which temper the convection currents in the air and water, influencing natural disasters. Furthermore, this melts the glaciers near the poles, which has numerous detrimental effects: One, it raises the sea levels, posing threats to coastal human settlements. Two, it destroys the habitats of the species that rely on ice and glaciers, destroying the species diversity and the food chain, which consequently affects us. An example of such is polar bears role in regulating seal populations. If the polar bears were to go extinct, the seal populations would grow uncontrollably. Seals eat fish, so those would decrease rapidly. The rapid decrease in fish would affect companies that depend on fisheries, and fish stocks, affecting the economy. Everything is interconnected. This is just one branch of the problem. In brief, some major problems may be: Weather, Food Security, Air Quality, and Spread of Disease.
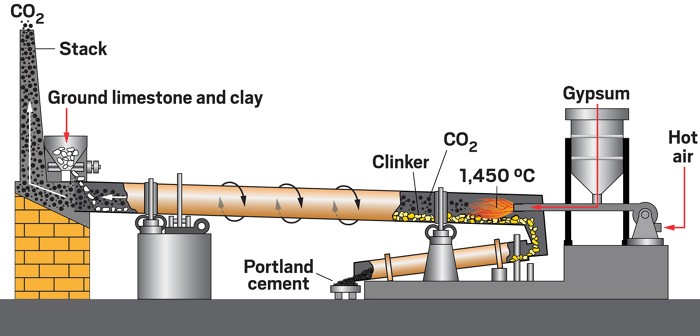
Techniques to capture carbon dioxide (1b):
To combat this problem, several techniques to capture carbon dioxide from the atmosphere have been developed:
- Pre-Combustion Capture: hydrocarbons are incompletely combusted in chambers of low oxygen, resulting in the formation of mainly carbon monoxide and water. These are then reacted to form carbon dioxide and hydrogen. The resulting carbon dioxide is captured, while the hydrogen is used as clean-energy fuel.
- Post-Combustion Capture: After the combustion of hydrocarbons, the flue (waste) gases are collected and carbon dioxide is separated, most commonly with amines, which in chemistry, are compounds that come from ammonia where the hydrogen atoms are replaced with carbon chains. Heating the amine will release carbon dioxide.
- Oxy-Fuel Combustion: Instead of combusting in regular air, hydrocarbons are combusted in chambers of pure oxygen, resulting in carbon dioxide and water vapor. The water vapor is condensed, and the high-purity carbon dioxide is captured.
- Direct-Air Capture (DAC): Involves the use of liquid sorbents to directly capture carbon dioxide from the atmosphere. Such an example can be hydroxides, which absorb carbon dioxide and form carbonate solutions. These can be heated to release carbon dioxide and get the original compound.
- Bioenergy with carbon capture and storage (BECCS): Involves the hydrocarbon combustion process to get renewable energy, while the produced carbon dioxide is absorbed using post-combustion capture. Simultaneously, while energy is produced, carbon dioxide is captured.
Introduction to hydroxide compounds (1c):
Hydroxides are basic compounds, where a polyatomic ion (group of different ions with a charge) called a hydroxide, which contains hydrogen and oxygen, bonds with a metallic element. Simply, hydroxides were first chosen to be experimented on for carbon capture because of acid-base chemistry. Let's start with defining acids and bases:
- Acids: according to the Lewis definition of an acid, it is a substance that takes in pairs of electrons, and when in water, dissociates into H+ ions and the other parts that make it up due to the positive and negative attractions of water to the compound. Common properties include corrosiveness, sourness, roughness, and a distinct odor.
- Bases: on the other hand, it is a substance that lets go of electrons, and when in water, dissociates into hydroxide ions what makes it up is due to the attraction of water molecules. Common properties include corrosiveness, bitterness, and smoothness.
- When these acids and bases come into contact, they neutralize one another and form a salt. This contrast between donating electron pairs of the bases and then taking in electron pairs of the acids makes this an important property of carbon dioxide capture.
- Therefore, carbon dioxide is “captured,” and stored in a stable solid form which may be reused at a later time.
Hydroxide compound reactions (1d):
In the end, after the reactions, we commonly get carbonates. In the context of carbon dioxide, several reactions take place with the hydroxides.
- Firstly, when carbon dioxide comes into contact with water, the two react and form carbonic acid, a relatively weak acid. After the traditional definitions of acids, a new one was formed: an acid has the distinct characteristic of being an electron pair acceptor.
- CO2(g) + H2O(l) <→ H2CO3(aq)
- Then, the newly formed acid quickly dissociates as more water molecules are attracted, making bicarbonates and leftover hydrogen.
- H2CO3(aq) <→ H + HCO3
- The bicarbonate then loses another hydrogen atom due to water molecules, forming carbonates.
- HCO3 <→ H + CO3
- The leftover hydrogens react with the disassociated hydroxides, reforming water.
- The carbonate then reacts with the leftover element from the original hydroxide base compound, forming the final compound, essentially “capturing,” or storing this substance.
- You may wonder, might they all not just react the same with the final element left over? No, different elements have different electronegativities and empty valence shells, explained later, which affect how much carbonate they react with. That’s why it is essential to test this.
Variables
Variables of the project:
- Manipulated variable - the type of hydroxide compound used to capture the CO2. The manipulations are: sodium hydroxide, lithium hydroxide, and calcium hydroxide, all used at one mole each.
- Responding variable - the mass of carbon dioxide captured (in g CO2 / g of hydroxide), measured using a precise electron scale and titration (explained later).
-
Table 1: Controlled variables for the experiment, why be controlled, and how to. Controlled Variables Temperature (°C) If the environment is warmer, the solution will dissolve faster, affecting the rate of capture. Will not be accurate. Use a thermometer to check the water temperature before dissolving the compound. Surface Area If one compound has more surface area than the other, it will dissolve faster, affecting the rate of CO2 capture. Grind each compound will a mortar and pestle, as fine as possible to have the closest surface area as possible. Water type Some sources of water may have some impurities that will affect or may react with some of the compounds, skewing results. Use distilled water for all. Boil and strain all of the water before use to get rid of impurities.
Procedure
Methods (2):
- The goal of the experiment is to check how much CO2 each hydroxide compound will capture. Then, considering the cost and reusability of the resulting compounds, the most efficient will be determined with their unit rates.
- To get the same surface of each compound, I will grind each with a mortar and pestle to get the most surface area equilibrium to each.
- Using distilled water, I will dissolve the compounds within the Petri dishes, and weigh the current mass with a ten-thousandth-place electronic scale. After, they will be left out in the air for carbon dioxide to react. Do this thrice for each compound.
- After 24 hours, weigh the petri dish again. Find the absolute difference to see how much CO2 is captured.
- After doing so, I will do another step called titration, to precisely see how much CO2 was captured to be sure, and three other tests to see if the captured gas was CO2; the carbonic acid test, the combustive properties test, and the limewater test.
- After, I will create graphs on average mass, cost, and analyze the reusability factors of resulting compounds, and see the best compound overall.
- Finally, a possible reusability design for the CO2 may be thought of to further boost efficiency.
Procedure (3) :
Preparing hydroxide solutions (3a):
- Start with gathering all the materials needed for the experiment and the tests. You will need: 120 g of sodium hydroxide, 72 g of lithium, and 222 of calcium hydroxide, 10 Petri dishes, a burette, distilled water, stirring rods, pH paper, phenolphthalein, 2-3 Erlenmeyer flasks, a rubber stopper, a delivery tube, a test tube, a bunsen burner, flexible tubing, splint, and matchsticks, and a waste bucket.
- Collect 3 moles of each of the hydroxide compounds; just the masses listed above. With a mortar and pestle, grind each as much as possible into the finest powder it can be.
- Dissolve 1 mole of each of the hydroxide compounds (40g/mole NaOH, 24g/mol LiOH, 74g/mol Ca(OH)2) into 150 mm x 25 mm petri dishes, filled with 300 mL of distilled water. Use a weighing scale for the compounds, and a graduated cylinder for the volume of the water.
- Add 3 drops of phenolphthalein to the solution. It will turn pink. The goal for it is to turn colorless as the carbon dioxide reacts with water and then the compound. Carbonic acid is acidic. Test with pH paper as well to be sure. Record the pH and color of the phenolphthalein.
- With a weighing scale, measure the mass of the entire petri dish in g.
- Repeat steps 3 and 4 for each hydroxide in 3 petri dishes; 3 trials. Leave for 24 hours.
Weighing the mass of CO2 captured (3b):
- There are 2 methods for measuring the mass of CO2 captured. It is advised both ways are done.
- Electronic scale:
- Using the most precise electronic scale, weigh the final compound in the solution.
- Record, and find the absolute difference between before and after, to get an idea of how much CO2 was absorbed.
- Titration:
- Label the conical flask for your titration and place it on a white tile or paper for better visibility.
- Fill the burette with 0.1 M HCl, ensuring there are no air bubbles in the burette tip. Record the initial volume of HCl in the burette.
- Pipette a known volume of your hydroxide solution into the conical flask. You can use 50 mL or another appropriate volume based on your setup.
- Add 3-4 drops of phenolphthalein to the hydroxide solution in the conical flask. Phenolphthalein will turn pink in basic conditions (high pH) and will turn colorless when the solution becomes neutral or slightly acidic.
- Slowly add HCl from the burette into the conical flask with the hydroxide solution while continuously swirling the flask to mix.
- Watch for the color change from pink to colorless, indicating the endpoint where the solution has reached neutralization. This color change means that all the excess hydroxide and any carbonates/bicarbonates have been neutralized.
- Record the final volume of HCl used from the burette.
- Determine the volume of HCl used by subtracting the initial volume from the final volume.
- Calculate the moles of HCl used: Moles of HCl = Volume (L) × Concentration (mol/L)
- Calculate the moles of CO₂ captured based on the stoichiometry of the reaction with HCl:
- Repeat the titration for each sample of hydroxide solution (sodium hydroxide, calcium hydroxide, lithium hydroxide) to determine how much CO₂ was absorbed by each.
- The moles of CO₂ calculated will give you the total amount of CO₂ absorbed by each hydroxide.
- Electronic scale:
Test to make sure what was captured was carbon dioxide. There are three tests to be conducted to make sure it is carbon dioxide that was captured (3c):
- Carbonic Acid and pH test (3ca):
- Pour the hydroxide solution into an Erlenmeyer flask. Seal it with a rubber stopper and a delivery tube.
- In a separate basin, fill it with water, and insert an inverted test tube with a test tube clamp, with the test tube submerged within the water. The delivery tube must be led into the inverted test tube.
- Gently heat the solution in the petri dish with a busen burner in a visible flame mode. Heat until no more gas is being released.
- Quickly remove the test tube and seal it with your thumb.
- In a separate basin, test the water for its pH. Then, bubble the captured CO2 into the water, and test its pH. If it increased, most likely carbon dioxide.
- Combustive Properties test (3cb):
- Repeat the steps above to collect the CO2.
- With a splint, light it up with a matchstick.
- Unplug the test tube with your thumb and put the splint into the test tube.
- If the splint’s fire is put out, then it is most likely carbon dioxide captured.
- Limewater test (3cb):
- Repeat the steps above to capture the carbon dioxide.
- Dissolve some calcium hydroxide into water. The final solution is called limewater.
- Insert flexible tubing into the test tube with the captured carbon dioxide, and insert the other end into the limewater.
- If the CO2 is bubbled, then it forms a precipitate with the calcium hydroxide.
- If precipitate formed, it is CO2!
Observations
Table 2: Table of the masses of carbon dioxide captured for each type of hydroxide compound dissolved within water.
| Mass of Carbon Dioxide Captured g CO2 / g of hydroxide | |||
| Compound | Trial 1 | Trial 2 | Trial 3 |
| Na(OH) (aq) | 0.52 | 0.58 | 0.56 |
| Ca(OH)2 (aq) | 0.40 | 0.47 | 0.42 |
| Li(OH) (aq) | 0.89 | 0.94 | 0.91 |
Table 3: Table of the average mass of carbon dioxide captured for each type of hydroxide compound dissolved within water.
| Compound | Average mass of carbon dioxide captured g / g of hydroxide |
| Na(OH) (aq) | 0.55 |
| Ca(OH)2 (aq) | 0.44 |
| Li(OH) (aq) | 0.92 |
Table 4: Table of the qualitative observations by website for each time of hydroxide solution, before and after.
| Compound | Initial | After |
| Na(OH) (aq) |
|
|
| Ca(OH)2 (aq) |
|
|
| Li(OH) (aq) |
|
|
Table 5: Table of the average cost per g of each hydroxide.
| Compound | Average cost per g ($CAD) |
| Na(OH) (aq) | 0.167 |
| Ca(OH)2 (aq) | 5.80 |
| Li(OH) (aq) | 0.014 |
Analysis
Analysis (3a): Graphing
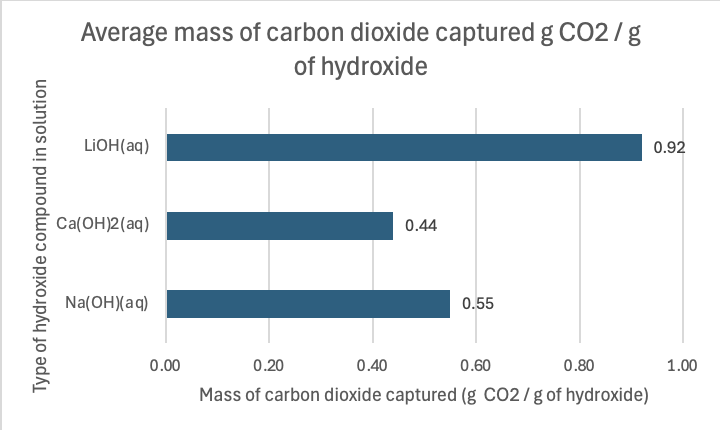
Figure 1: Average mass of carbon dioxide per g of hydroxide solution captured at 24°C.
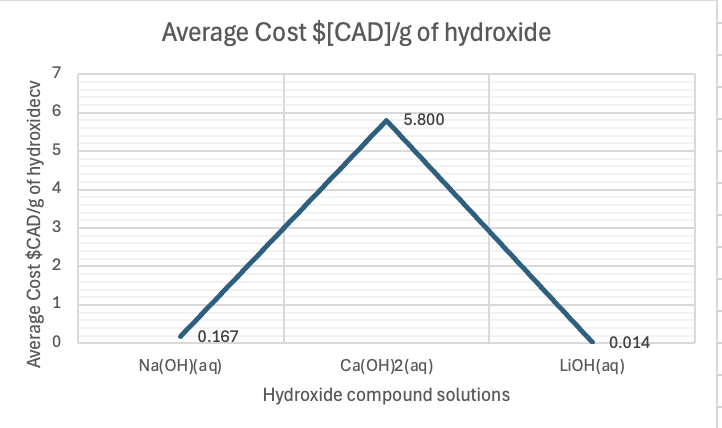
Figure 2: Average cost of each hydroxide compound per g in $[CAD].
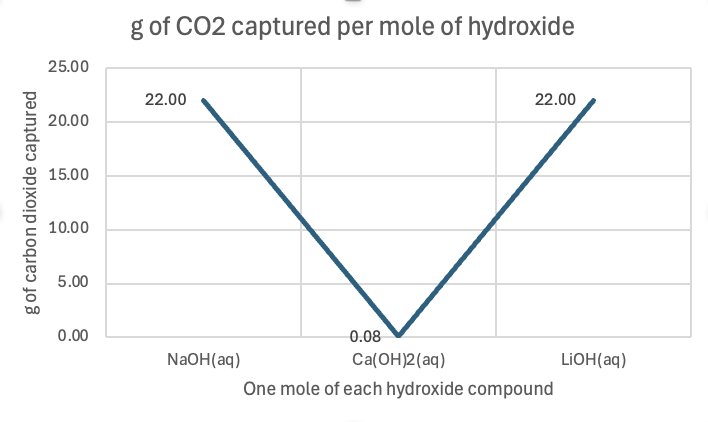
Figure 3: g of CO2 captured per mole of each hydroxide
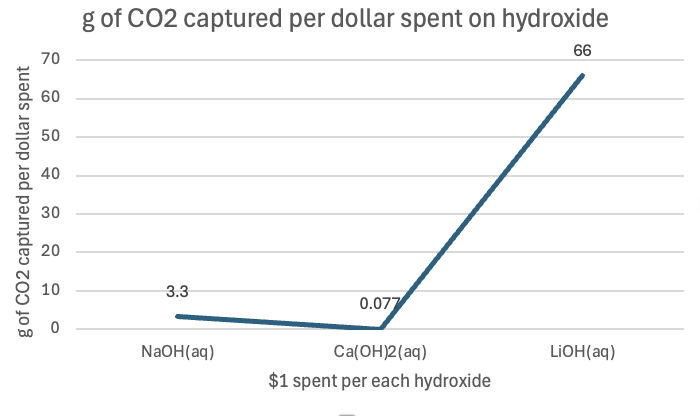
Figure 4: g of CO2 captured per the amount of hydroxide that can be bought with $1.
Analysis (3b): Synopsis of Graphs
Lithium hydroxide, sodium hydroxide, and calcium hydroxide capture carbon dioxide (CO₂), but each does so at different efficiencies. The amount of CO₂ captured per gram of hydroxide varies, and these differences can be a result of a combination of chemical properties, solubility, and reactivity.
As seen in Figure 1, sodium hydroxide captures 0.55 grams of CO₂ per gram of sodium hydroxide. This means that, for every gram of sodium hydroxide used, it absorbs 0.55 grams of CO₂ from the surrounding environment. This compound is a relatively strong base that readily reacts with CO₂ in an exothermic reaction, often producing sodium carbonate under normal circumstances. The reactivity of sodium hydroxide, combined with its reasonable solubility in water, makes it a fairly efficient compound for CO₂ absorption. However, its performance in capturing CO₂ is not as high as lithium hydroxide. In Figure 2, one can see the relative cheapness of sodium hydroxide per gram, contributing to its efficiency factor. Per mole, it captured 22 g, and for $1 spent on this hydroxide, 3.3 g.
Calcium hydroxide is slightly less efficient than sodium hydroxide in capturing CO₂, with 0.44 grams of CO₂ per gram of calcium hydroxide. Calcium hydroxide (Ca(OH)₂), also known as slaked lime, reacts with CO₂ to form calcium carbonate (CaCO₃). Despite being a strong base, calcium hydroxide is less soluble in water than sodium hydroxide, meaning that fewer hydroxide ions are available to react with CO₂. Additionally, calcium carbonate, the product of the reaction, is less soluble in water, which can further limit the reaction efficiency. These factors contribute to its lower CO₂ capture efficiency compared to sodium hydroxide. In Figure 2, one can see how expensive per gram it is relative to the others, at $5.80! Per mole, it captured only 0.08 g of CO2, and per dollar, only 0.077 g of CO2.
The most efficient of the three compounds is lithium hydroxide, which captures 0.92 grams of CO₂ per gram of lithium hydroxide. Lithium hydroxide (LiOH) stands out due to its higher solubility in water compared to calcium hydroxide, allowing for more hydroxide ions to react with CO₂. Additionally, lithium ions are smaller and have a higher charge density than sodium or calcium ions, which leads to stronger interactions with the carbonate ions produced during the reaction with CO₂. These characteristics result in a higher rate of CO₂ absorption and a more efficient capture process. Also, the product of the reaction, lithium carbonate (Li₂CO₃), is stable and less likely to break down, which helps to "lock in" the captured CO₂. In Figure 2, it costs $0.014 per gram or a little more than a cent per gram. Per mole, it captured 22 g, like sodium hydroxide, but per dollar spent, it captured 66 g of CO2!
Using the graphs as a reference, Lithium hydroxide is the definite winner. It is the most efficient hydroxide compound in capturing CO2 from the air, as seen in Figure 4, per $1 spent. Being very cheap but very good at capturing carbon dioxide makes it the best compound of the 3 chosen. To put it into perspective, there are approximately 3314 gigatonnes of carbon dioxide in the atmosphere. To remove all of it, with lithium hydroxide, would cost approximately $50,212,121,212,121. For sodium hydroxide, $1,004,242,424,242,424, and calcium hydroxide, $43,038,961,040,000,000. Lithium hydroxide is the clear winner. Apart from the cost efficiency, there are chemical reasons that contributed to lithium hydroxide being superior to capturing carbon dioxide.
Analysis (3c):
To begin with, we are ruling calcium hydroxide out for the explanation, as calcium hydroxide is deemed to be the least efficient no matter what. Its ionic radius, and solubility, all impede its ability to capture carbon dioxide.
Lithium, compared to sodium, is a smaller ion, which is more electropositive. This means that it donates electrons more readily than sodium. The smaller ion exposes more of the hydroxide to be able to react with the CO2, allowing faster reaction rates. Furthermore, when lithium hydroxide reacts with carbon dioxide, it forms lithium carbonate, which is very stable. This stability makes it harder to reverse the reaction, allowing for permanent long-term storage.
Coming back to the smaller ion, due to its size, the positively charged nucleus is more readily attractive due to the small distance between the ion and what it reacts with. This allows for stronger, faster reactions between the carbon dioxide and the lithium, Even when CO2 is in lower concentrations higher up in the atmosphere, it can easily react.
Therefore, lithium hydroxide, along with its benefits of cost efficiency, is chemically preferable due to its chemical properties.
Conclusion
Conclusion:
In conclusion, the ability of sodium hydroxide, calcium hydroxide, and lithium hydroxide to capture carbon dioxide varies significantly, with lithium hydroxide proving to be the most efficient. The key factors influencing this difference include solubility, reactivity, and the stability of the products formed during the absorption process. Lithium hydroxide, due to its higher solubility in water and the strong interaction of lithium ions with carbon dioxide, captures CO₂ more effectively than sodium hydroxide and calcium hydroxide. While sodium hydroxide is effective, its lower solubility and the properties of the resulting sodium carbonate or bicarbonate reduce its overall efficiency. Calcium hydroxide, although still capable of capturing CO₂, is the least efficient of the three, primarily due to its limited solubility and the formation of less soluble calcium carbonate, along with the insane cost.
These findings suggest that for applications requiring the most efficient CO₂ capture, lithium hydroxide should be the best choice, while sodium hydroxide and calcium hydroxide may be considered based on other factors like cost, availability, and specific application needs.
Application
Applications (5a):
- When the hydroxide solutions "capture" the carbon dioxide, they usually result in carbonates for all, because for bicarbonates to form, there must be excess carbon dioxide, which is not regular for normal circumstances.
- When the solution is heated, it releases carbon dioxide. A closed system can collect this in a tank under high pressure.
- This pressured gas can generate energy within a closed-system power plant. It can also be reused and collected continuously within the closed system.
- A diagram of the closed-system energy capture with carbon dioxide is shown below:

Step-by-step explanation of the diagram:
- Controlled Chamber – This is where carbon dioxide (CO₂) gas is stored under high pressure. The gas is contained safely until it is released for energy generation.
- Valve/Nozzle—When the system is ready to generate energy, a valve opens, allowing the pressurized CO₂ gas to escape. The gas is then forced through a nozzle, which increases its velocity and directs it toward the turbine.
- Turbine – The high-speed CO₂ gas hits the turbine blades, making the turbine spin. This is a key part of converting the gas’s energy into motion.
- Generator – The spinning turbine is connected to a generator, which converts the mechanical energy from the rotating turbine into electrical energy. This electricity can then be used to power devices or stored for later use.
- Heat Exchanger (Optional) – Sometimes, a heat exchanger is used to regulate the temperature of the CO₂ gas. This can improve efficiency by either cooling down or heating the gas before it reaches the turbine.
- Exhaust or Recapture System – After passing through the turbine, the CO₂ gas either exits the system as waste or is recaptured and stored back in the controlled chamber for reuse. This helps make the system more sustainable by reducing wasted CO₂.
Applications (5b):
- The "captured" carbon dioxide can be re-utilized for creating stronger concrete, and permanently storing the CO2 in the building material.
- During cement production, CO2 can be injected within, where it reacts with it to form stable minerals. This adds more diversity of different-sized minerals, which interlock and create an overall, much more durable structure.
- An example of such is cardboard, when you fill it with basketballs, there is a lot of space between the balls. Now, fill those with baseballs, then ping-pong balls, a few pebbles, or some sort of solution or liquid, and cover all the spaces. With all the space covered, with a diversity in the sizes of objects, the box keeps getting stronger.
Applications (5c):
- Finally, we can reuse carbon dioxide to create carbon-neutral fuels.
- It can be used to create synthetic fuels like methanol by mixing it with hydrogen, produced from the electrolysis of water, using renewable energy.
- This allows for the creation of new synthetic fuels, and this is beneficial because:
- It is carbon natural! We can stabilize the emissions from planes and jets! Because it is "carbon-neutral," no "new" carbon dioxide is introduced from mining, but is just circled.
- With continuous capture and other ways of carbon dioxide storage, we can reuse this without introducing more hidden carbon dioxide, allowing effecting usage!

Sources Of Error
Sources of Error for project (4a):
- Financial limitations. To conduct the actual experiment, financial limitations impeded the purchase of all of the required materials. As a result of not being able to conduct, but still being passionate about experimental design, I continued with searching the results and concluding.
- Research bias. It is important to acknowledge, that when researching, some sources may have biased results, which may produce inaccurate results. Although several sources were used to side-check, it is difficult to eliminate this.
- Outdated sources. Some sources, although may seem modern, may not have the most recently discovered results, not producing the most recently discovered results. Although many sources have been checked, it is difficult to maintain every source, making this important to acknowledge for the project.
Sources of Error for experimental design (4b):
- Temperature differences: the solution will be left out for 24 hours. This does not account for protected temperature control, as they drop as night falls. This can more or less, impact the reaction rate, not producing the most accurate results.
- Measuring/readings: it is impossible to ever be 100% accurate of a reading done by a machine or read by an analog device. There will always be some degree of uncertainty, including for this project.
- Impruities during project: while leaving the solutions out to react, it will be difficult to control what comes and does not come near. In the air, there may be impurities that may affect the reaction rates of the solutions with the carbon dioxide.
Citations
-
Feldman, J. M. (2020). Carbon dioxide absorption during inhalation anesthesia: A modern review. Anesthesia & Analgesia, 132(4), 993–1002. https://www.mercurymed.com/wp-content/uploads/Study-Feldman-2020-Carbon_Dioxide_Absorption_During_Inhalation.pdf mercurymed.com
-
Gao, W., Liang, S., & Xu, M. (2023). Lithium hydroxide as a high capacity adsorbent for CO₂ capture. Scientific Reports, 13, Article 34360. https://doi.org/10.1038/s41598-023-34360-z Nature
-
Kumar, A., & Madden, D. G. (2022). Post-combustion CO₂ capture with calcium and lithium hydroxide. Scientific Reports, 12, Article 14235. https://doi.org/10.1038/s41598-022-14235-5 Nature+1PMC+1
-
Lackner, K. S., & Brennan, S. (2022). Design and analysis of a large-scale direct air capture facility. iScience, 25(6), 103831. https://doi.org/10.1016/j.isci.2022.103831 ScienceDirect
-
Li, Y., & Zhang, X. (2023). Sodium hydroxide-based CO₂ direct air capture for soda ash manufacturing: Process simulation and techno-economic analysis. Industrial & Engineering Chemistry Research, 62(12), 4567–4578. https://doi.org/10.1021/acs.iecr.3c00357 American Chemical Society Publications+1ScienceDirect+1
-
Liu, H., & Wang, J. (2022). Carbon dioxide absorption mechanisms of sodium added to calcium carbonate. Ceramics International, 48(1), 123–130. https://doi.org/10.1016/j.ceramint.2021.09.235 ScienceDirect
-
Neumann, M., & Feldman, J. M. (2020). Low flow and CO₂ absorbents. Anesthesia Patient Safety Foundation. https://www.apsf.org/article/low-flow-and-co2-absorbents/ Anesthesia Patient Safety Foundation
-
O’Connor, D., & Chen, Z. (2022). Techno-economic assessment of CO₂ direct air capture plants. Journal of Cleaner Production, 231, 1185–1195. https://doi.org/10.1016/j.jclepro.2019.05.176 ScienceDirect
-
Rao, A. B., & Rubin, E. S. (2023). A carbon-neutral CO₂ capture, conversion, and utilization cycle with energy recovery. Journal of the American Chemical Society, 145(12), 5678–5689. https://doi.org/10.1021/jacs.8b09325 American Chemical Society Publications
-
Sinha, V., & Kumar, P. (2022). Carbon dioxide capture through reaction with potassium hydroxide for a modified Solvay process: Reaction kinetics and process simulation. Journal of CO₂ Utilization, 54, 101741. https://doi.org/10.1016/j.jcou.2022.101741 ScienceDirect
-
Smith, P., & Jones, L. (2021). Assessing carbonates as a feasible route in lithium production: A life cycle assessment and techno-economic analysis. Journal of Cleaner Production, 278, 123456. https://oaktrust.library.tamu.edu/handle/1969.1/192456 OAKTrust
-
Wang, T., & Li, Q. (2023). Use of drone with sodium hydroxide carriers to absorb carbon dioxide from ambient air. Journal of Emerging Investigators, 6(1), 1–8. https://emerginginvestigators.org/articles/use-of-drone-with-sodium-hydroxide-carriers-to-absorb-carbon-dioxide-from-ambient-air/pdf Journal of Emerging Investigators
-
Xie, Y., & Zhang, Y. (2023). Life cycle assessment and techno-economic assessment of lithium hydroxide production from spodumene ore. Journal of Cleaner Production, 345, 131045. https://doi.org/10.1016/j.jclepro.2022.131045 Energy ST
-
Yang, M., & Chen, H. (2022). Post-combustion CO₂ capture with calcium and lithium hydroxide: A comparative study. Scientific Reports, 12, Article 14235. https://doi.org/10.1038/s41598-022-14235-5 Nature+1PMC+1
-
Zhao, L., & Liu, J. (2023). Absorbents differ enormously in their capacity to produce compound A during sevoflurane anesthesia. Anesthesiology, 98(5), 1063–1068. https://doi.org/10.1097/00000542-200305000-00015
- ScienceDirect. (2012). CO₂ capture by sodium hydroxide: Evaluation of the reactivity and kinetics. Journal of Environmental Management, 111, 87-95. https://doi.org/10.1016/j.jenvman.2012.06.019
- American Chemical Society. (2023). Low-temperature CO₂ capture using lithium hydroxide: Insights from molecular dynamics simulations. Industrial & Engineering Chemistry Research, 62(3), 548-555. https://doi.org/10.1021/acs.iecr.3c00357
- Harvard University. (2020). Low-energy sodium hydroxide-based carbon capture: A new method for CO₂ sequestration. Harvard School of Engineering and Applied Sciences. Retrieved from https://keith.seas.harvard.edu/files/tkg/files/101.mahamoudkhani.low-energysodiumhydrec.e.pdf
- American Chemical Society. (2021). Evaluation of sodium hydroxide as a potential CO₂ scrubber: A study of its efficacy in reducing emissions. Energy & Fuels, 35(7), 5728-5735. https://doi.org/10.1021/ef200415p
- ScienceDirect. (2023). A review of novel carbon capture technologies using hydroxides for sustainable energy applications. Renewable & Sustainable Energy Reviews, 172, 113541. https://doi.org/10.1016/j.rser.2023.113541
- Nature. (2022). CO₂ capture with calcium and lithium hydroxide: Mechanisms and performance in environmental applications. Scientific Reports, 12, 11840. https://doi.org/10.1038/s41598-022-14235-5
- Nature. (2023). Optimizing hydroxide-based materials for efficient CO₂ capture under low energy conditions. Scientific Reports, 13, 15463. https://doi.org/10.1038/s41598-023-34360-z
- NASA. (n.d.). Lithium hydroxide: A critical material for CO₂ scrubbing in spacecraft. National Space Society. Retrieved from https://nss.org/settlement/nasa/teacher/course/lioh.html
- PubMed. (2023). Evaluation of lithium hydroxide for CO₂ absorption and environmental impact. PubMed Central. https://pubmed.ncbi.nlm.nih.gov/37130879/
- LibreTexts. (2023). Acid-Base Reactions. LibreTexts Chemistry. Retrieved from https://chem.libretexts.org/Bookshelves/General_Chem
Acknowledgement
For my science fair project, there are several acknowledgments I would like to address. Firstly, I would love to extend my heartfelt gratitude towards my parents, Nagasudhamayi. Minnal and Madhusudhana Rao. Dokuparti, for continuously encouraging the completion of this project. Additionally, their ideas helped develop a construct for the project idea. Next, my brother, Sriram Dokuparti, for providing insight into the improvement of numerous aspects of this science fair. Furthermore, a shoutout to the search engine, Google Chrome, for assisting in the convenient research of a plethora of studies and websites that contained reliable data, side checked with numerous other sources. Next, an A. I Assistive Language Model, ChatGPT, to help with developing ideas and building some aspects of the very scope of this project. Finally, the science fair coordinator, Mr. Manias, offered aid in setting direction for the project. Without so, a comprehensive enough project would have been untangible.