Regenerative Mechanisms: Decoding Axolotl Regeneration for Human Healing
Grade 9
Presentation
No video provided
Problem
Imagine a world where humans are able to grow back an entire limb that's been lost in an accident or repair a spinal cord after severe injury. This idea may sound impossible at first, but modern regenerative medicine is aiming to make this a reality. While humans are capable of regenerating tissues such as skin, parts of our liver, and fingertips in young children, we still lack the ability to regenerate more complex structures and our regenerative abilities are even more limited when compared with other species in the animal kingdom.
In the past, scientists have tried and failed repeatedly to find a definite solution to this problem. We have tried stem cell therapies, gene editing and various tissue engineering but these methods have still been generally inconclusive and vague. This medical limitation has been a major challenge that affects millions of people who suffer from conditions like amputations, spinal cord injuries, and organ failure. However, in recent years, humans have developed a growing interest in a small yet remarkable species of salamander who may hold the future to regenerative medicine: the axolotl.
This study explores the extraordinary regenerative abilities of the axolotls that are capable of regrowing almost any part of its body. By studying the axolotl’s regeneration in detail, scientists will be able to revolutionize modern medicine. From regrowing various damaged tissues, to potentially being able to cure cancer, the information we can extract from this small axolotl is so vast. In this project, I hope to be able to shed some light on how we can one day overcome the limitation of our regeneration and open new possibilities for human healing.
Method
The main question that this project revolves around is “How are the regenerative abilities of axolotls able to provide insights on potential human regeneration?” When researching this topic I first focused on the background of regeneration in general, explaining what it is and why it is important for human functions and what are the limitations that we face. Afterwards, I did more research on axolotl regeneration, investigating their regenerative capacity and specific processes that allow regeneration to occur. I then compared this to how humans heal wounds and learned why the axolotl regenerative process is much more efficient and effective. Later on, I focused more on the possible human implications of my research and finding in depth past studies that hold potential to future medicine.
I followed a similar process when writing my research report, providing background information for regenerative medicine and their potential. Next, introducing the axolotl and their regenerative mastery in detail, I then compared the axolotl regeneration to a human’s limited healing. Lastly, I included the human applications from the research and finding possible future solutions for people with spinal cord injuries, amputees, and cancer patients. The following will draw conclusions for my initial question of the potential of axolotl regenerative for modern medicine.
Research
Regeneration refers to the process in which the body restores or renews the cells within a tissue. There are two types of regeneration that happen in the human body: physiological regeneration, which occurs to keep up with the regular replacement of cells, and reparative regeneration, which is triggered by injury. Our reparative regeneration abilities are extremely limited and incomplete with only partial restoration of the former structure. However, this is not the case for some amphibians, including the axolotl whose regeneration can be complete in a way that the new tissue is identical to the old tissue.
The axolotl is an amphibian whose natural habitat is located in lakes of Mexico. Unlike most salamanders who transition from water onto land after metamorphosis, axolotls keep their juvenile features and never leave the water. This phenomenon is known as neoteny, this allows the axolotl to retain its regenerative abilities that would typically fade in other species after they mature. This is due to their lack of thyroid-stimulating hormone: thyroxine. Although an injection of thyroxine or iodine can stimulate their metamorphosis, their regenerative capacities are affected and they will live a much shorter lifespan. Unlike humans, axolotls are infamous for being able to regenerate complex body parts including eyes, heart and brain tissue, gills, spinal cord segments, and entire limbs. Not only are the regenerated tissues precisely identical to the original, the whole process is completed with leaving a single scar.
Regardless of regeneration, all organisms must have grown their limbs at some point during infancy, similar to the axolotl. Limb development all starts with the formation of tiny buds called limb buds, these buds are full of progenitor and stem cells. These cells are undifferentiated meaning that they have potential of becoming various specialized cell types. They soon differentiate and multiply rapidly to eventually form a full infant limb. When axolotls lose a limb, they aim to replicate this process again. The process follows a series of specified steps to ensure that there will be no complications (Fig. 1).
FIGURE 1 Shows the different stages of limb regeneration in an axolotl (“dpa” refers to “days post amputation”). Following the steps of wound healing, dedifferentiation, blastema formation, and redevelopment.
- Wound Healing: When an axolotl’s limb is first amputated, the wound site is covered rapidly through fibrosis with epidermal cells (skin cells) to form the wound epidermis instead of forming scar tissue like humans would. With nerve stimulation, the wound epidermis thickens and forms an apical epithelial cap (AEC) over the limb stump. The AEC is critical for signaling the cells beneath it to begin the regeneration process.
- Dedifferentiation: Once the wound is covered, nerve cells at the injury site release neurotrophic factors, which trigger surrounding cells (like muscle and skin cells) to revert to a more primitive state, known as progenitor cells. The Wnt signaling regulates this process called cell dedifferentiation.
- Blastema Formation: The blastema is a mass of undifferentiated progenitor cells that forms at the site of injury after dedifferentiation. The major nerve signal is the anterior gradient protein (AGP) that supports blastema development while the FGF (fibroblast growth factor), BMP (bone morphogenic protein), and TGFβ (transforming growth factor β) promotes the formation of the blastema and regulates the growth of the new limb. The blastema is arguably the most essential part of regeneration because it has the ability to become any tissue required for the limb to regenerate—muscle, bone, skin, and nerves. The blastema mimics the early stages of limb bud development during embryonic growth. The limb bud and blastema have identical goals and the key difference is that the blastema is made up of recycled and repurposed cells instead of entirely new ones.
- Redevelopment: This limb stump undergoes a period of rapid ontogenetic allometric growth (the rate of growth of a structure) over the next couple of weeks. Because the axolotl is also growing during the period of limb regeneration, there is not a fixed size for the regrown limb so cell proliferation must be tightly regulated. For the first few weeks after blastema formation, the growth progress is rapid (approximately 0.04 cm/day). Afterwards, progress slows gradually over the next couple of weeks (approximately 0.02 cm/day) until growth rate is isometric with the other limb so the resulting limb is proportionate with the rest of the body. The speed of the axolotl regeneration relies on the age of the organism as shown in Fig. 2. Once the regeneration is complete, the regenerative nervous signals are immediately switched off to prevent uncontrolled cell growth or tumor formation.
(A)
(B)
FIGURE 2 Axolotl limb regeneration speed from amputation to full restoration at 6 months old (A) and 10 months old (B). The time it takes for limb regeneration is affected by the age of the organism, with the rate of restoration decreases with age.
Other injuries in the body are healed and regenerated in a similar way to how axolotls regenerate their limbs. Their unique way of regenerating damaged and amputated tissues provides helpful insights to future applications for modern medicine.
Data
So why aren’t humans unable to replicate this regenerative process? We first need to look into how humans heal after injury. After an injury, our nervous system has an inflammation response, this is the cause of swelling, heat, and pain around the wounded area. Inflammation combats infections with pathogens and triggers the healing response. However, this response can also be risky and harm the healthy parts of your body and cause a variety of chronic illnesses. Additionally, our stem cells are unable to dedifferentiate like an axolotl’s and they lose their regenerative abilities with age and therefore lacking the ability to create blastemas. The scar tissue formed at the injury site also prevents regeneration as a potential evolutionary trait to suppress rapid cell proliferation as a method to prevent uncontrolled cell division and cause cancer.
An axolotl’s genome is extremely complex with 14 chromosome pairs and a size of 32 GB, approximately 10 times the size of the human genome (Nowoshilow et al, 2018). They have highly repetitive sequences that have yet to be deciphered but there are key genes for regeneration. Although scientists theorize that it may not be the genomes themselves that make an axolotl have such extensive functions but the way that they regulate them. This could be the key reason why they are able to execute the regenerative process with such precision and perfection. Unfortunately, there is still not enough research done on this subject matter for us to safely incorporate an axolotl’s genes into our own. Nonetheless, there is still so much innovative information that we can extract from the research we have done on axolotls that could be implemented into advanced regenerative medicine.
Unlike humans, axolotls have an anti-inflammatory response when healing wounds. Instead axolotls use macrophages to cell debris and old tissue to prevent infection, they also stimulate angiogenesis (capillary growth) and cell proliferation to heal the injury. Macrophages play a crucial role in tissue regeneration in axolotls and macrophage depletion disrupts the gene pathways that are essential for the regenerative process (Godwin et al., 2013). In humans, macrophages play an important role during our fetal development period and the embryo is still able to repair injury without scar formation. Although macrophages are present during the earlier stages of wound healing to regulate inflammation, macrophage depletion later on leads to less efficient wound repair and scarring. The early secretion of anti-inflammatory macrophages in axolotls could lead to potential therapeutic strategies for preventing scarring and promoting tissue regeneration in humans following tissue injury.
One of the most extraordinary aspects of axolotl regeneration is their ability to fully regenerate damaged spinal cords, which is a significant area of interest for treating human spinal cord injuries. In mammals including humans, spinal cord injury is often irreversible, leading to paralysis and severe disabilities due to the inability of the central nervous system to regenerate. However, axolotls overcome this limitation through a highly efficient and fascinating process. After a spinal cord injury the axolotl’s first response is to send signals for the ependymal radial glial (spinal cord lining) cells at the injury site to proliferate and form a tube of glial cells, which functions as a foundation of the new spinal cord. The terminal vesicle at the end of this tube acts as a source for new spinal cord tissue, and epithelial-mesenchymal transition (EMT) allows the cells to migrate, proliferate, and eventually form functional neurons to restore normal nerve function. The MicroRNA miR-200a plays a pivotal role in spinal cord regeneration by ensuring that stem cells differentiate correctly into neurons and glial cells, instead of mesodermal cells such as muscle and bone cells which can block the neurological pathways (Fig. 3) (Walker et al., 2022). In humans, after a spinal cord injury, ependymal glial cells primarily form astrocytes and along with the pre-existing astrocytes at the injury site, leads to the formation of a glial scar. This scar acts as a barrier to nerve regeneration, allowing little neurogenesis to occur (Tazaki et al., 2017). However, axolotls are able to avoid this scar formation by creating a more favorable environment for neurogenesis through their unique regenerative processes. Understanding how axolotls manage to bypass the scar formation by encouraging new spinal cord tissue growth could help researchers develop therapies that prevent or reverse the scar tissue formation in human injuries. By reprogramming human cells to mimic axolotl regeneration, spinal cord injuries could potentially be treated through gene therapy aimed to promote proper neurogenesis. By harnessing epigenetic reprogramming techniques, scientists are exploring methods to enable mammals, including humans, to regenerate their spinal cords similarly to axolotls.
FIGURE 3 A small group of spinal cord cells from an axolotl were fluorescently labelled for this study. The control cells (A-D) were labelled green and were left unbothered over the course of 12 days, the cells proliferated and grew into spinal cord tissue as described above. However, the other group of cells (E-J) were injected with a miR-200a inhibitor to restrict its regular function. These cells were found to have dedifferentiated into muscle cells which disrupted the regenerative process of the spinal cord. This experiment further highlights the importance of the miR-200a microRNA in preventing improper dedifferentiation from happening in the spinal cord. https://cdn.ncbi.nlm.nih.gov/pmc/blobs/bd16/8918811/84eb27788782/develop-149-200033-g3.jpg
As previously described, the blastema is a mass of progenitor cells that form after injury, capable of differentiating into a multitude of tissue types including muscle, skin, and bone. The formation of a blastema is a key feature in axolotl regeneration, making it the subject of great interest for human regenerative medicine. In mammals, injured tissues typically form scar tissue, which is non-functional and prevents proper regeneration. Unlike axolotls, humans and mammals lack the cellular plasticity (ability of adult cells to transform into different types of cells when needed) and regenerative signaling pathways needed to regenerate entire limbs or organs. Even if regenerative therapies succeed in creating a blastema-like structure, there are challenges in integrating these cells into existing tissue networks known as tissue integration. Nerve regeneration and vascularization (formation of blood vessels) are particularly difficult areas for blastema implantation to succeed in humans. Researchers are looking into ways to overcome these challenges by introducing the correct signaling patterns into injured tissue to induce blastema formation and enhance regeneration. For example, fibroblast growth factor (FGF), Wnt signaling, and bone morphogenetic proteins (BMPs). These signals (Fig. 4) guide the blastema to dedifferentiate and proliferate to form the correct tissue types, ensuring that bone, skin, or muscle cells regenerate where needed, hoping to one day implement this process in human regeneration (Simkin et al., 2015). Regenerative therapies could potentially use blastema-like cells to promote the regeneration of tissues lost to injury, aging, or disease. Understanding the formation of the blastema in mammals could lead to medical breakthroughs in limb prosthetics and biomimetic tissue engineering, where bioengineered tissue is used to replace lost or damaged tissues.
|
Pathway Name |
Purpose |
|
Fibroblast growth factor (FGF) |
Supports blastema growth and regulates limb patterning, present in during the formation of the AEC and the blastema |
|
Wnt signalling |
Regulates cell dedifferentiation and potentially regulates nerve integration in the new limb as well as bone growth, present during blastema formation and limb stump growth |
|
Bone morphogenetic proteins (BMPs) |
Promotes cell condensation and blastema development, regulates apoptosis (programmed cell death), present during the early limb bud development |
|
Transforming growth factor β (TGF β) |
Encourages tissue integration into the blood network as well as initial wound healing, present during the initial injury |
|
Anterior gradient protein (AGP) |
The major nerve signal that supports blastema development and continued growth, present during blastema formation and growth |
FIGURE 4 Explains the role of the different pathways that are present during regeneration in more detail. Acts as an aid to better understand the signals involved in blastema formation and tissue growth.
Unlike humans who are extremely susceptible to cancer, axolotls have remarkable cancer resistance due to their ability to regenerate tissues with precision without forming tumors. The key to this process lies in the axolotl p53 gene, which regulates the cell cycle and prevents uncontrolled cell growth which usually leads to the development of a cancerous tumor. p53 upregulation is a crucial part of the axolotl’s regenerative process. When axolotls regenerate limbs or organs, their p53 gene works to ensure that only healthy, regulated cell growth occurs, preventing the formation of tumors. Researchers are studying how axolotls can maintain tumor suppression during regeneration. Although the axolotl’s p53 gene is similar to the human p53 gene, the two function differently at different temperatures. When the human p53 gene was compared to its axolotl counterparts it was found the p53 in human cells were stimulated at a temperature of 37°C whereas the axolotl p53 was fairly inactive. The same experiment was conducted again at a temperature of 30°C and 25°C and it was observed that the axolotl p53 was twice as active as the human p53 at 25° meaning the axolotl p53 gene becomes significantly more active at cooler temperatures (Fig. 5). This study sheds light on the environmental requirements for efficient regeneration (Villiard et al., 2007). By understanding how axolotls' p53 gene works, scientists aim to develop therapies that promote tumor-suppressing pathways in human tissues. Studies have been progressing and has shown that axolotl limb tissue extracts can induce cell cycle arrest in human cancer cells, particularly in acute myeloid leukemia (Schraverus et al., 2022). This study was conducted in a controlled environment with a sustained temperature of 21°C, providing further insights on the environmental requirements to trigger the tumour growth control signals. Axolotl oocyte extracts have also been proven to be able to reprogram breast cancer cells through a process called DNA demethylation (Schraverus et al., 2022). This process regulates gene expression within a cell by activating the tumor suppressor genes in cancer cells. With current technology, gene editing to mimic axolotl cancer resistance would be extremely risky leaving with lots of room for error. This has led to further investigations into axolotl proteins as a potential anti-cancer therapy. More extensive studies will be needed to decipher their genes that are able to suppress tumor growth, hoping to one day apply these methods onto human cancer patients.
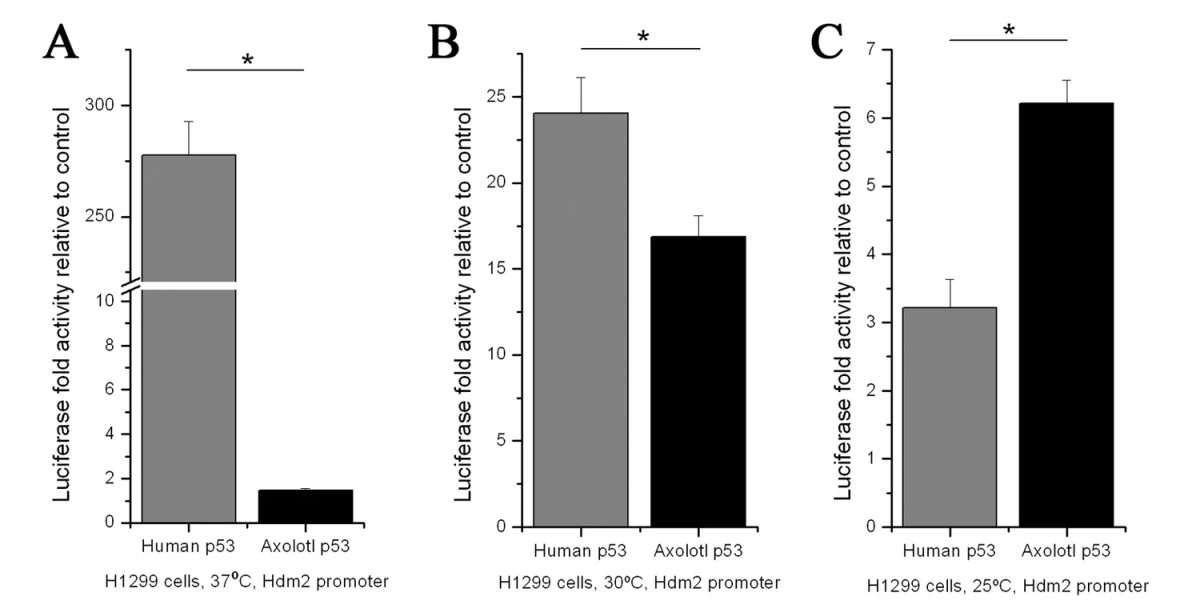
FIGURE 5 A comparative graph of the activation of the human and axolotl p53 gene in three separate temperatures 37°C in (A), 30°C in (B), and 25°C in (C). It is shown that decreasing the temperature increases activation of the axolotl p53 gene. The results of the graph is justified because the human p53 gene should be the most active at the human body temperature, whereas the axolotl p53 activation should increase as the temperature decreases which more closely resembles their environment.
Conclusion
The study of axolotls provides hope for the future of regenerative medicine. Through their extraordinary ability to regenerate complex tissues, axolotls offer key insights into how we might one day be able to heal human bodies in ways we never thought possible. By understanding the genetic and cellular processes behind axolotl regeneration, we might unlock new ways to treat spinal injuries, limb regrowth, organ failure, and even cancerous tumors. As research progresses, it may only be a matter of time before we can regenerate our own tissues with the precision and grace of an axolotl.
Citations
Adamson, C. J., Morrison-Welch, N., & Rogers, C. D. (2022, June). The amazing and anomalous axolotls as scientific models. Developmental dynamics : an official publication of the American Association of Anatomists. https://pmc.ncbi.nlm.nih.gov/articles/PMC9536427/
Bölük, A., Yavuz, M., & Demircan, T. (2022, July 30). Axolotl: A resourceful vertebrate model for regeneration and beyond. American Association For Anatomy. https://anatomypubs.onlinelibrary.wiley.com/doi/10.1002/dvdy.520
Choi, Y., Meng, F., Cox, C. S., Lally, K. P., Huard, J., & Li, Y. (2017, April 10). Regeneration and regrowth potentials of digit tips in amphibians and mammals. International journal of cell biology. https://pmc.ncbi.nlm.nih.gov/articles/PMC5402240/
Faisal, M., Mehreen, A., Hays, D., Yaseen, F., & Liang, Y. (2024, December 12). The Genetic Odyssey of Axolotl Regeneration: Insights and Innovations. The International Journal of Developmental Biology. https://ijdb.ehu.eus/article/240111yl#
Godwin, J. W., Pinto, A. R., & Rosenthal, N. A. (2013, June 4). Macrophages are required for adult salamander limb regeneration. Proceedings of the National Academy of Sciences of the United States of America. https://pmc.ncbi.nlm.nih.gov/articles/PMC3677454/
John W. McArthur, Z. K., Nicol Turner Lee, M. S., & Matt Kasman, R. A. H. (2016, July 28). Regenerative Medicine: A Future Healing Art. Brookings. https://www.brookings.edu/articles/regenerative-medicine-a-future-healing-art/
Nowoshilow, S., Schloissnig, S., Fei, J.-F., Dahl, A., Pang, A. W. C., Pippel, M., Winkler, S., Hastie, A. R., Young, G., Roscito, J. G., Falcon, F., Knapp, D., Powell, S., Cruz, A., Cao, H., Habermann, B., Hiller, M., Tanaka, E. M., & Myers, E. W. (2018, January 24). The axolotl genome and the evolution of key tissue formation regulators. Nature News. https://www.nature.com/articles/nature25458
Schraverus, H., Larondelle, Y., & Page, M. M. (2022, December 14). Beyond the lab: What we can learn about cancer from wild and domestic animals. Cancers. https://pmc.ncbi.nlm.nih.gov/articles/PMC9776354/
Simkin, J., Sammarco, M. C., Dawson, L. A., Schanes, P. P., Yu, L., & Muneoka, K. (2015, June 9). The mammalian blastema: Regeneration at our fingertips. Regeneration (Oxford, England). https://pmc.ncbi.nlm.nih.gov/articles/PMC4895320/
Song, F., Li, B., & Stocum, D. L. (2010, September). Amphibians as research models for Regenerative Medicine. Organogenesis. https://pmc.ncbi.nlm.nih.gov/articles/PMC2946045/
Tazaki, A., Tanaka, E. M., & Fei, J.-F. (2017, October 10). Salamander spinal cord regeneration: The ultimate positive control in vertebrate spinal cord regeneration. Developmental Biology. https://www.sciencedirect.com/science/article/pii/S0012160616305140
Vieira, W. A., Wells, K. M., & McCusker, C. D. (2019, November 8). Advancements to the axolotl model for regeneration and aging. Gerontology. https://pmc.ncbi.nlm.nih.gov/articles/PMC7214127/
Villiard, Éric, Brinkmann, H., Moiseeva, O., Mallette, F. A., Ferbeyre, G., & Roy, S. (2007, September 28). Urodele p53 tolerates amino acid changes found in p53 variants linked to human cancer - BMC ecology and evolution. BioMed Central. https://bmcecolevol.biomedcentral.com/articles/10.1186/1471-2148-7-180
Walker, S. E., Sabin, K. Z., Gearhart, M. D., Yamamoto, K., & Echeverri, K. (2022, February 1). Regulation of stem cell identity by mir-200a during spinal cord regeneration. Development (Cambridge, England). https://pmc.ncbi.nlm.nih.gov/articles/PMC8918811/
Wells, K. M., Kelley, K., Baumel, M., Vieira, W. A., & McCusker, C. D. (2021, November 15). Neural control of growth and size in the axolotl limb regenerate. eLife. https://elifesciences.org/articles/68584
Whited, J. (2024, September). How do animals regrow their limbs? and why can’t humans do it? Jessica Whited: How do animals regrow their limbs? And why can’t humans do it? | TED Talk. https://www.ted.com/talks/jessica_whited_how_do_animals_regrow_their_limbs_and_why_can_t_humans_do_it
Zambrano, L. (2021, June). These salamanders snack on each other (but don’t die). Luis Zambrano: These salamanders snack on each other (but don’t die) | TED Talk. https://www.ted.com/talks/luis_zambrano_these_salamanders_snack_on_each_other_but_don_t_die
Figure 1 https://ijdb.ehu.eus/article/img/240111yl/Fig-03
Figure 2A https://ijdb.ehu.eus/article/img/240111yl/Fig-06
Figure 2B https://ijdb.ehu.eus/article/img/240111yl/Fig-07
Figure 3 https://cdn.ncbi.nlm.nih.gov/pmc/blobs/bd16/8918811/84eb27788782/develop-149-200033-g3.jpg
Header Image https://www.tissyou.com/wp-content/uploads/2019/11/img-blog-axolotl.jpg
Project Image https://ichef.bbci.co.uk/images/ic/1280x720/p0jmmr0y.jpg
Acknowledgement
I would like to thank Mr. Thompson, who coordinated the science fair at my school this year. Thank you so much for answering my numerous quesitons and providing me with the opportunity to participate in this event!

