Lava Lamp Experiment
Grade 6
Presentation
Hypothesis
Mfoniso:
Null: I think trial #1 and trial #3 will not have a massive difference in being the best type of lava lamp because in both experiments they include sodium - which is one of the main ingredients in both experiments.
Positive: I think trail #2 will create the best lava lamp because with the coke, more carbon dioxide is produced in this trial making larger carbon dioxide bubbles creating a more satisfying lava lamp effect. I also think this will be the best lava lamp because with more carbon dioxide produced the experiment can go on for longer making a long and satisfying lava lamp effect.
Negative: I think trial #3 will be the worst lava lamp because with a salt lava lamp, it doesn’t produce any carbon dioxide but instead sodium. Though sodium does react with water a lot, it’s not the best with forming bubbles making it lose the satisfying lava lamp effect. And i think the sodium won’t have a long lasting effect because in order to keep a long lasting effect, you need to add more carbon dioxide - which salt does not have.
If there is enough carbon dioxide produced, then a longer lava lamp effect is created because, without enough carbon dioxide the lava lamp effect will only last for a short amount of time.
Emaan:
Null: I agree with Mfoniso that trial #1 and trial #3 will not have a massive difference because in both experiments it includes sodium which is a crucial ingredient in both trials.
Positive: I think trial #1 ill be the best lava lamp because it produces a balanced amount of both sodium and carbon dioxide.
Negative: I agree that with Mfoniso that trial #3 will have the worst lava lamp because it does not produces enough carbon dioxide which play a huge role in a making a homemade lava lamp
If there is no carbon dioxide, a lava lamp effect will not be created because, you need carbon dioxide in order to make a lava lamp.
Research
1st Trial
In the first experiment it is a alka seltzer and water chemical reaction. Alka seltzer = sodium bicarbonate (NaHCO3) + citric acid (C6 H8 O7). Alka Seltzer in its solid form does not produce enough energy to form a chemical reaction. In the alka seltzer, the two chemicals are in its solid form. The particles don’t move. When the alka seltzer reaches water, the chemicals are released and can react to each other. They form a chemical reaction which includes: Sodium citrate(Na3C6H5O7), Liquid water (H2O), and Carbon Dioxide (CO2). Then, in the water, the alka seltzer tablet can now produce carbon dioxide bubbles.
In the first trial, the alka seltzer makes carbon dioxide bubbles mixed with water. Since these bubbles are denser than both oil and liquid water, it floats to the top. After the bubble reaches the surface, the bubble immediately pops. When enough bubbles pop, the water and the remaining gas become denser than the liquid water and the oil, and eventually sinks to the bottom.
2nd Trial
Coke and the mentos (serving as the fizzy tablet), has no chemical reaction. It causes a physical reaction. This is because the carbon dioxide in the coke only changes from a liquid state to a gaseous state. When we add oil, the reaction creates carbon dioxide gas, and the gas bubbles gets trapped in the oil - making a cool lava lamp effect!
In this trail, one mento won’t produce enough energy to keep the lava lamp going on, so in this trial we are using about 5 mentos.
3rd Trial
To keep every experiment fair, we did the math and figured out that ½ of a teaspoon of salt is as close as we can get to the amount of sodium in the alka seltzer tablet. In this experiment, when salt (NaCl) is added to water (H2O) and oil, it sinks to the bottom of the mixture carrying some oil with it because it is denser than both liquids. When the salt reaches the water it dissolves. As the salt dissolves, the water releases the oil that came with the salt. That oil now floats to the top (since it is denser than water. This cycle repeats - creating the lava lamp effect.
Variables
Controlled Variable: timing, cups, measurements of the amount of liquids poured in each cup - will all be about the same.
Manipulated Variable: liquids, chemicals, fizzy tablets, materials, amount of fizzy tablets.
Responding Variable: the length of time each trial lasted, size of bubbles, speed of bubbles, the length of time used to start each reaction.
Procedure
Procedure:
Trial #1:
- Get ingredients and materials - a large cup, oil, water, food colouring, spoon, and alka seltzer tablets.
- Pour ¼ of a cup of water in the large cup.
- Squeeze a few drops of food colouring into the water.
- Mix the food colouring and the water with a spoon.
- Pour in ¾ of a cup of oil in the large cup.
- Drop in 1-2 alka seltzer tablets.
- Enjoy the satisfaction of your homemade lava lamp!
Trial #2
- Get ingredients and materials - A large cup, coke, five mentos, and oil.
- Pour in ¼ of a cup of coke in the large cup.
- Now, pour in ¾ of a cup of oil in the large cup also.
- Drop in the 5 mentos all at once.
- Enjoy!!
Trial #3
- Get ingredients and materials - A large cup, food colouring, salt, water, oil, and a spoon.
- Pour in ¼ of a cup of water in the large cup.
- Squeeze in a few drops of food coloring.
- Mix the food coloring and water with the spoon.
- Pour in ¾ of a cup of oil in the large cup.
- Pour in ½ of a teaspoon of salt in the large cup.
- Enjoy the satisfaction of your homemade lava lamp!
Observations
Observations:
1st Trial:
As soon as we added the alka seltzer tablet, the mixture started bubbling. We saw multiple tunnel-like structured effects in this lava lamp. We saw this in the two other experiments that we ran. The lava lamp effect continued for about 1 minute and 8 seconds. During the end of this experiment, so many bubbles came together. More and more bubbles were being made until the experiment ended. This lava lamp created such a satisfying effect.
2nd Trial:
When we added the mentos, we waited a few seconds until the experiment started. The coke and oil were rushing up and down a lot, and many of the tunnel-like structured effects were taking place. The experiment slowly started to stop until the coke and oil were back to normal again. This experiment lasted 59 seconds.
3rd Trial:
As soon as we added the salt, many of the tunnel-like structures came about. No actual bubbles fell from top to bottom, but they formed inside of the water, during when the effect was taking place. Throughout the end of the experiment, bubbles slowly stopped forming and only a couple tunnel-like structured effects happened until the experiment came to a complete end. This experiment lasted 7 seconds
Analysis
What Happened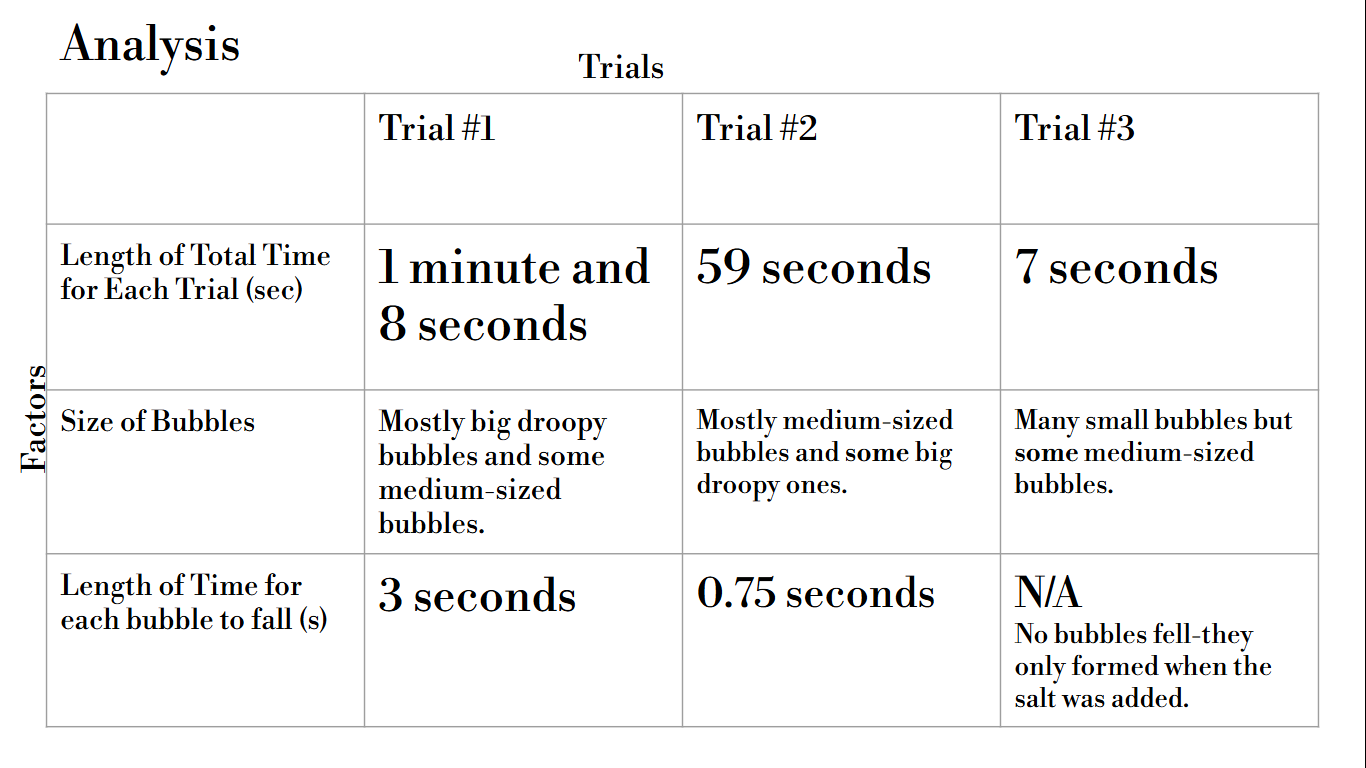 What Is a Chemical Reaction?
What Is a Chemical Reaction?
Analysis Comparison Of Each Experiment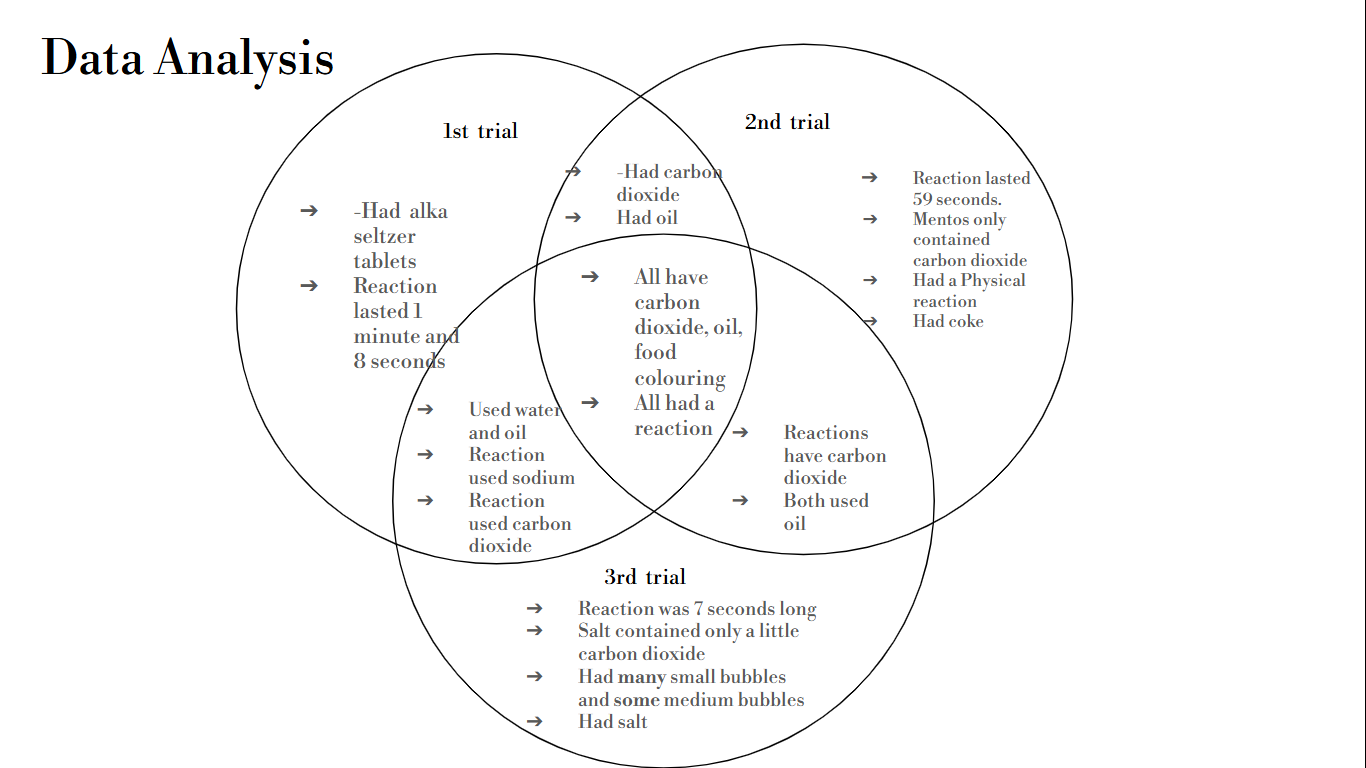 Trial 1
Trial 1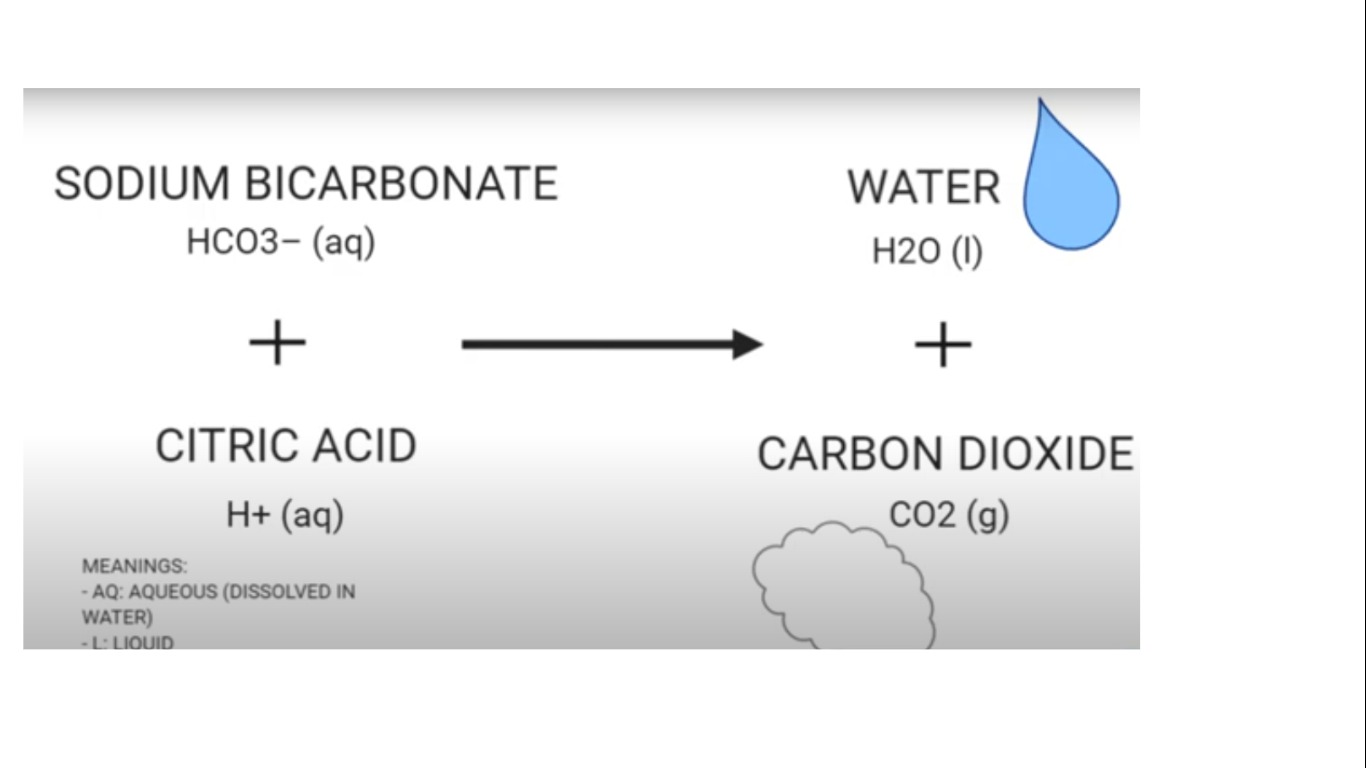
Conclusion
Mfoniso: The purpose of this experiment was to find out which chemical reaction was the most similar to the original lava lamp. The results showed that my hypothesis was slightly correct when i quoted “If enough carbon dioxide is produced, then a longer lava lamp effect is created.” This is because that even though you do need enough carbon dioxide to create an overall lava lamp effect, it also depends on how much carbon dioxide is released in a certain amount of time. If the carbon dioxide is released more slowly and gradually like trail 1’s experiment, more carbon dioxide is saved and the process continues for a longer period of time as the tablet dissolves. Also, the effect gave enough time for bubbles to form making the bubbles be on the bigger side. Compared to trail 2’s experiment, the carbon dioxide was released way to quickly and the carbon dioxide ran out too quickly which resulted in a shorter lava lamp effect. And when the carbon dioxide runs out, there will be no more lava lamp. With the more quick and rapid effect that this trial had, it made bubbles form too quickly leading to the bubbles being in smaller sizes. And lastly trial 3’s experiment, the reaction didn’t work because there was not enough carbon dioxide produced which defends my hypothesis. With not enough carbon dioxide produced, a long lasting lava lamp effect will not be created. Instead, the sodium in the salt only alters the density and the behavior of the liquids -not creating much bubbling. Overall, though my hypothesis was slightly correct, it does need to have more clarification and accuracy in order to be 100% correct.
Emaan: The results showed that trial 1 is the best lava lamp effect due to the balanced effect this lava lamp had. The production of the carbon dioxide and how it was released really benefited the length of time this lava lamp effect went for. It also gave enough time for the bubbles to form making them bigger which resulted in a slow fall. Though my hypothesis is correct my reasoning wasn’t. When i said “I think trial 1 will be the best lava lamp because it produces a balanced amount of sodium and carbon dioxide” this actually isn’t true. The only reason this trial worked the best is because of the way the carbon dioxide was released - slowly and gradually. In addition, yes, my hypothesis was correct but i wouldn't say my reasoning helped me get to that point.
Application
To replicate the satisfying and soothing effect of the original lava lamp effect, so that you don't have to buy a $60 lava lamp but instead create one at home.
We also think it is important when it comes mathamatically to measuring because the amount of oil in each experiment really determines if you can see what is going on in the experiment. The amount of oil also effects the behavior of the overall lava lamp effect.
Sources Of Error
- Forget to time the experiments (we had to redo them).
- Having our hypothesis wrong.
- Salt Lava Lamp (Trial #3) tremedously failing.
- Having the measurements be un-accurate.
Citations
https://www.youtube.com/watch?v=T2Om-ag3IOw
https://www.youtube.com/shorts/KgRYxJuH0gM
Kitchen Science: Lava Lamp Experiment | Birmingham Museums
physical reaction
Because carbon dioxide changes from a liquid state to a gaseous state in the Diet Coke and Mentos experiment but does not change chemical makeup, it is a physical reaction.
Diet Coke & Mentos Experiment | Steps & Results - Study.com (will not let you view due to subscription reasons, though i quoted what i found when searching up. from google.)
https://www.youtube.com/watch?v=joT50SYQctc
What Happens When Sodium Reacts with Water? - Unacademy
The Amazing Science Behind Coke + Mentos - Solugen
Alka-Seltzer® Original Flavor Effervescent Tablets
Glowing Lava Lamp - Science Fun
https://www.youtube.com/watch?app=desktop&v=lVkgDUkF6QE
Acknowledgement
Thanks to:
Parents - for supporting us during the experiment
Friends - for briefly helping us with our experiment
Partner : for being so amazing and helping with the entire experiment
Teachers: for giving feedback and helping enhance our experiment

