Banishing Bacteria
Grade 5
Presentation
Hypothesis
If rubbing alcohol is added to the bacteria then it will be the most effective for killing bacteria because rubbing alcohol is strong enough to break down bacterial cell structure.
Research
BLEACH
Bleach is a cleaning product that is often used to remove color and whiten things, like clothes or surfaces. It is very effective at killing molds, germs, and bacteria, which makes it useful for disinfecting and sterilizing. Bleach can come in different forms, such as a liquid or a powder, depending on how it's used. The bleach used in this experiment was made from a chemical called sodium hypochlorite, which is the main ingredient that gives bleach its strong cleaning power. Sodium hypochlorite (NaOCl) is a powerful oxidizing agent. When it is mixed with water, it breaks down into hypochlorous acid (HOCl), which is highly effective at destroying microorganisms. The hypochlorous acid works by oxidizing the cell walls of bacteria, viruses, and molds. This breaks the chemical bonds in the membrane of the cell, causing it to rupture and exposing the cell’s internal components, which ultimately leads to the destruction of the microorganism. The release of chlorine from the bleach contributes to this disinfecting action by further breaking down the structure of pathogens. Because of its ability to kill harmful microorganisms, bleach is commonly used in households, hospitals, and other places that need to stay clean and safe.
WATER
Water is something we use every day for washing, cooking, and drinking. It is made up of two hydrogen atoms and one oxygen atom, which is why its chemical formula is H₂O. Water can exist in three different forms: as a liquid, solid (like ice), or gas (like steam). This ability to change states is due to water's unique molecular structure and the forces between water molecules. Water is found all over the world in places like lakes, rivers, oceans, and even underground aquifers. It is essential for life, as our bodies are made up of about 60% water. Water is necessary for many vital functions in the body, including digestion, temperature regulation, and nutrient transport. It also plays a key role in cellular processes, such as maintaining hydration, enabling chemical reactions, and helping to remove waste from our bodies. Additionally, water is vital for plants, as it is needed for photosynthesis, which allows them to produce food and oxygen. Animals depend on plants for food and oxygen, creating a balance in ecosystems that supports all life. Research has shown that water itself doesn’t grow or kill bacteria, but it can carry bacteria from one place to another. Water plays a crucial role in maintaining ecosystems, as it supports plants, animals, and human life. It is needed for survival and growth, making it a key part of life on Earth!
DISH SOAP
Dish soap is used for removing food and grease from dirty dishes. It usually comes in a liquid form and helps break down oils and other messes by reducing the surface tension. Surface tension is the force that causes the surface of a liquid to behave like a stretched elastic membrane. It occurs because water molecules are attracted to each other, creating a "skin" on the surface that resists external force. When soap is added to water, it lowers the surface tension by disrupting the forces between water molecules. This allows the water to spread more easily and interact with oils and grease on the dishes. The soap works through a process called emulsification, which involves soap molecules that have two different parts: a hydrophobic (water-repelling) tail and a hydrophilic (water-attracting) head. The hydrophobic tail binds to oils and grease, while the hydrophilic head binds to water molecules. As a result, the oils become surrounded by the soap molecules and are lifted away by the water. This unique structure of soap molecules, combined with its ability to reduce surface tension, makes it effective at breaking up greasy substances that water alone cannot remove. The label on dish soap doesn’t mention anything about killing or growing bacteria, but it is still used for cleaning. Dish soap is an important part of keeping our kitchenware clean and free from food residue.
VINEGAR
Vinegar is made up of 5% acetic acid (CH₃COOH) and the rest is water. It is often used for cleaning because it has antibacterial properties, which means it can help kill germs. Vinegar is made when alcohol goes through a process called fermentation. During fermentation, yeast converts the alcohol into acetic acid, which gives vinegar its sour taste and cleaning power. Vinegar is a good cleaner because the acetic acid helps break down dirt, grease, and mineral deposits. It also works well at removing stains and can even dissolve soap scum. People also use vinegar in many foods, like salad dressings and sauces. It is good for preserving foods too, like pickles, by keeping them fresh longer. However, just because vinegar is used to clean, it doesn't mean it can always kill all bacteria. Its ability to kill germs is not as strong as some other disinfectants, like bleach.
RUBBING ALCHOHOL
Rubbing alcohol, with the chemical formula C₃H₈O (isopropyl alcohol), kills bacteria by breaking down their proteins in a process known as denaturation. During denaturation, the bonds in the proteins of the bacteria are broken, causing them to unravel and lose their structure, which ultimately destroys the bacteria. In this experiment, 99% isopropyl alcohol was used for its high concentration, making it very effective at denaturing proteins and killing harmful microorganisms. However, rubbing alcohol is toxic and highly flammable, so it should always be handled with care to avoid accidents.
BACTERIA
Bacteria are living organisms, but they are very simple and made up of just one cell. They don't have most of the parts that other living organisms have, like a nucleus. Bacteria are so small that you can usually only see them with a microscope. There are different types of bacteria, and they come in various shapes. The main shapes are cocci (spherical), bacilli (rod-shaped), and spirilla (spiral-shaped). Bacteria can also have different structures, like a cell wall that helps protect them, and some bacteria have a flagellum, which allows them to move. They take in food and get rid of waste through their cell walls. Bacteria reproduce by splitting into two cells, and each of those cells splits again, allowing them to grow into billions of bacteria very quickly. Some bacteria can cause diseases, such as strep throat or food poisoning. Heat (over 60°C) and 70% alcohol can kill bacteria by breaking down their cell structures and stopping them from working.
Variables
|
Manipulated Variable |
Type of liquid |
|
Controlled Variables |
Location temperature, bacteria sample location, amount of liquid |
|
Responding Variable |
Amount of bacteria (square mm) |
Procedure
SET UP
- Pour 20g nutrient agar into a heat proof glass jar.
- Add 350ml of distilled water to the jar.
- Put the jar in the microwave for 1 minute.
- Continue to microwave the jar for 1 minute at a time until dissolved.
- Once the the agar is dissolved fill each petri dish about half way with agar.
- Let the petri dishes cool for 1 hour or more.
- Label the petri dishes using the names of the liquids and number them. Make sure that there are 2 petri dishes labeled for each liquid.
- Draw a line on the petri dish that equally divides them in half.
EXPERIMENT
- Swab the kitchen sponge. Make sure to go in a zigzag formation when swabbing.
- Using the same swab used on the kitchen sponge swab the first petri dish.
- Repeat steps 1 and 2 for all the petri dishes.
- Place the petri dishes in a warm area.
- Let the petri dishes set and do not disturb the bacteria.
- From here make sure to record quantitative and qualitative data throughout the experiment.
- Wait 5 days for the bacteria to grow. Take lots of pictures and observations.
- On the 5th day add ¼ tsp of the liquid for that petri dish. Make sure to add the liquid only on the side of the petri dish with the label.
- Let the petri dishes sit and grow for 4 days. Take daily observations and photos.
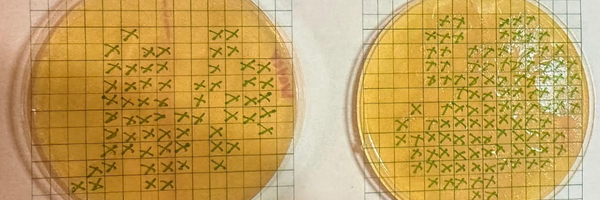
- On the 4th day put a 15x15 grid over the photos from the day the liquids are put on & the day the experiment ends.
- Draw an x in every box that is more than halfway full with bacteria.
- Count the number of squares that have xs in them and analyze.
Observations
he bacteria began to grow within 24 hours after incubation. Initially, the colonies appeared as small, white splotches scattered across the surface of the agar, arranged in a distinct zigzag pattern. As time passed and the bacterial growth continued, the zigzag shapes began to thicken and spread outwards, forming more defined and clustered regions. The overall texture of the bacterial colonies started to resemble lumpy, dried nasal mucus, often described as "boogers," giving it an unappealing, irregular surface.
As the bacteria proliferated, the agar also began to emit a strong, unpleasant odor. Interestingly, the foul smell was noticeable even before the agar was swabbed. The odor seemed to intensify as the colonies developed, suggesting that the bacteria were actively metabolizing and producing volatile compounds. This unpleasant scent contributed to an overall gross atmosphere surrounding the culture, signaling the presence of potentially pathogenic or decomposing organisms.
QUALITATIVE OBSERVATIONS
|
Observations November 2nd |
Observations November 4th |
Observations November 9th |
|
|
Water |
Cloudy, not clear any more |
Fogy, cloudy |
Cloudy, most bacteria still there |
|
Dish soap |
Flat, no longer bumpy |
Bacteria gone |
Bacteria gone |
|
Rubbing alcohol |
Shiny, smooth |
Bacteria still there, no changes |
Drying out, no differences |
|
Vinegar |
Turning white and looking lifeless |
Grey, cloudy |
No differences |
|
Bleach |
Clear coating |
Yellow coating; wiped of cloudiness |
Most bacteria gone |
QUANTITATIVE OBSERVATIONS
|
Water |
Vinegar |
Rubbing Alcohol |
Bleach |
Dish Soap |
||||||
|
Sample |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
|
Area of bacteria before adding liquid (mm2) |
64 |
28 |
19 |
25 |
76 |
30 |
28 |
33 |
62 |
68 |
|
Area of bacteria after adding liquid (mm2) |
46 |
36 |
28 |
28 |
35 |
26 |
18 |
14 |
0 |
0 |
|
Percentage remaining (%) |
72 |
129 |
147 |
112 |
46 |
87 |
64 |
42 |
0 |
0 |
Analysis
Dish Soap was the most effective because there was no remaining bacteria after the liquid was applied. On the other hand Vinegar was the least effective because the bacteria kept growing after the liquid was applied. Water was inconclusive because one sample increased in the amount of bacteria & the other sample decreased in the amount of bacteria. Bleach & Rubbing Alcohol were the runner ups to Dish Soap because both of them basically halved the amount of bacteria they had from the beginning.
I learned that the bacteria grown looks like a very bad infected wound & smells horrible. I also learned that it is best not to put agar on hot surfaces for the agar will evaporate & liquify. If this experiment was done again it is recommended that special attention is dedicated to being precise with the measurement of liquids.
Conclusion
This experiment aimed to test the hypothesis that Rubbing Alcohol would be the most effective for getting rid of bacteria because Rubbing Alcohol is believed to break down bacterial cell structure. This hypothesis was disproven seeing as Dish Soap ended up with the least amount of bacteria at the end of this experiment.
The order of effectiveness from best to worst was:
- Dish Soap
- Bleach
- Rubbing Alcohol
- Water
- Vinegar
Application
Understanding the science behind how different liquids impact the growth of bacteria is connected to the real world in many ways. First of all, understanding the best ways to get rid of bacteria is very important. This is important because some bacteria are helpful and protect humans & animals, but other bacteria can make animals, plants, and humans sick!
Some examples of bacteria-borne illnesses that can make humans sick include food poisoning caused by Salmonella or E. coli, respiratory infections from Streptococcus pneumoniae, and tuberculosis caused by Mycobacterium tuberculosis.
According to MIT News, scientists are already making little robots called phagemid plasmids. How these little engineered bugs work is they get sent into the body and dig inside the targeted bacteria. Once they’re inside, they release toxic liquids that kill the bacteria. This method of killing bacteria sounds very effective, but it is still not safe to do this yet.
This directly relates to the project on how liquids impact the growth of bacteria. By exploring how different liquids affect bacterial growth, the goal is to better understand how liquids, whether natural or engineered, can be used to either promote or prevent the growth of harmful bacteria in the human body or the environment.
Knowing what kills bacteria the most can help protect both humans and animals by enabling more effective methods to prevent infections and control outbreaks. By identifying liquids or substances that effectively kill harmful bacteria, better cleaning agents, antibiotics, and treatments can be designed to safeguard public health and protect animal populations from bacterial diseases.
A cost analysis of the different cleaners used, including dish soap, water, bleach, vinegar, and rubbing alcohol, would also be helpful. Here’s a rough estimate of their costs:
- Dish soap: About $2–$5 for a 16–32 oz bottle.
- Water: Typically free or low cost, depending on location.
- Bleach: Around $2–$5 for a gallon.
- Vinegar: Approximately $2–$3 for a gallon.
- Rubbing alcohol: Around $1–$4 for a 16 oz bottle.
This analysis would help determine which of these cleaners are not only effective at killing bacteria but also which are the most affordable and accessible for use in households, hospitals, and farms.
Sources Of Error
There are many things that could have impacted the results of this experiment, especially the fact that there was not a way to grow the same amount of bacteria on each petri dish. Also, the measurements of the area of bacteria in each petri dish were not very precise. There is also a very high chance that there was some contamination throughout the experiment, especially if:
- The liquids accidentally mixed.
- Different types of bacteria were growing.
- There was improper handling of the petri dishes or equipment, such as cross-contamination from the tools used for transferring the bacteria.
- The petri dishes were not properly sealed.
- The environment was not sterile enough, or the samples were exposed to airborne contaminants.
This could have led to the growth of unintended bacteria. Explaining this, contamination occurs when bacteria from an outside source, such as another petri dish, air, or even from human contact, is introduced into the experiment. Even tiny traces of bacteria can thrive in the warm, nutrient-rich environment of the petri dish, causing unexpected growth and skewing the results.
Citations
- Researchers develop a new means of killing harmful bacteria. (2015, June 25). MIT News | Massachusetts Institute of Technology. https://news.mit.edu/2015/engineered-particles-kill-harmful-bacteria-0625
- Professional, C. C. M. (2024, May 1). Germs. Cleveland Clinic. https://my.clevelandclinic.org/health/articles/24495-germs
- bacteria. (n.d.). Britannica Kids. https://kids.britannica.com/kids/article/bacteria/352814
- Vandergriendt, C. (2021, June 30). What to know about using alcohol to kill germs. Healthline. https://www.healthline.com/health/does-alcohol-kill-germs
- Wikipedia contributors. (2024, May 29). Rubbing alcohol. Wikipedia. https://en.wikipedia.org/wiki/Rubbing_alcohol
- Vinegar. (2024, November 7). The Nutrition Source. https://nutritionsource.hsph.harvard.edu/food-features/vinegar/
- Does vinegar kill germs? - David Suzuki Foundation. (2022, October 31). David Suzuki Foundation. https://davidsuzuki.org/living-green/does-vinegar-kill-germs/
- vinegar. (n.d.). Britannica Kids. https://kids.britannica.com/kids/article/vinegar/435794
- Dishwashing liquid Facts for Kids. (n.d.). https://kids.kiddle.co/Dishwashing_liquid
- water. (n.d.). Britannica Kids. https://kids.britannica.com/kids/article/water/390625
Bleach Facts for kids. (n.d.). https://kids.kiddle.co/Bleach