Lithium Engine
Grade 7
Presentation
Hypothesis
Hypothesis
The solution of water lithium is placed in will influence the reaction.
Research
- Our world primarily uses fossil fuels, oil, and gas to power everything, but we are slowly moving to renewable energy sources.
- Renewable energy sources come from nature, such as solar or wind energy, however this can be inconsistent based on the weather or environment.
- While lithium is not a renewable energy source, it is an alternative energy source, in fact, it is used today in EV batteries and rechargeable and non-rechargeable batteries. It can store energy.
- Phone batteries have rechargeable lithium batteries, if the electricity in your house comes from solar panels, or wind energy, then lithium is a clean energy storage.
- Lithium also has a lot of chemical potential energy that can be released using its reactive nature with halogens and water.
One of the most prevalent types of Lithium extraction is mining for Spodumene, a lithium rich ore found in the Andes and China. The ore contains around 2 to 3 percent lithium with upwards of 5 to 6 percent, making it the most viable way to obtain lithium pure enough to be used in batteries, sometimes even in pottery and supplements. To collect Spodumene, open pit mining is favored but underground mining is sometimes used for deeper reserves; after mining and extraction, the ore is crushed and grinded down, and then roasted to change the Alpha-spodumene to its more expensive counterpart, Beta-spodumene. After that, the lithium rich ore is treated with Acid and then crystalized to remove the acid impurities, and packaged a little after that. Most companies will look for and think about environmental care and act (cough, usually) upon that.
In its pure state, Lithium is very toxic, except in very small doses. Lithium is mostly used for making long term rechargeable batteries for conveniences like hybrid and full electric cars, phones and other electronic devices including laptops, PCs, tablets and many more. Sometimes, lithium is used in non-rechargeable batteries, (The batteries we used in our experiment were of this type).
Lithium is the third element on the periodic table, it is an alkali metal, like sodium and potassium, with an atomic mass of 6.941 u, it has a melting point of 180.50°C and a boiling point of 1342°C. Lithium does not occur naturally in its metal form, however it is found in small amounts in nearly all igneous rock and in waters of some mineral springs. Since lithium is an alkali metal, it reacts with halogens such as fluorine and chlorine. Lithium has an exothermic reaction with water (Hydrogen and Oxygen) which creates a byproduct of hydrogen gas, and produces a lot of heat energy. We tried to use this heat energy in our experiment to power our engine model. Most electronics should be kept away from water because they contain lithium in their batteries, which reacts with water.
- Manufacturing of rechargeable batteries for electronics, electric vehicles, and electric grid storage is the largest global use for lithium, representing 80% of total usage
- The Government of Canada has identified lithium as a critical mineral because it is a key material in the renewable energy transition.
Lithium Consumption has changed a lot over the years as demands have varied wildly since even the mid 2010’s, mostly with the upturn for electric vehicles. In 2010, the majority of lithium extracted was implemented in ceramics, pottery and glass, which together accounted for around a quarter of the lithium use. 23% of lithium was used in batteries, mostly non rechargeable batteries, and the remaining half were used in various cases including lubricating greases, steel and aluminium production and alloys, pharmaceuticals and other items.
Pie chart of lithium use
Variables
Method 2
Manipulated Variable: Type of water Responding Variable: Reaction time and Temperature increase Controlled Variables: Same Place (University Lab), Controlled size of Lithium sample
Method 2A
Manipulated Variable: Weight of lithium Sample Responding Variable: Reaction time and Temperature increase Controlled Variables: Same Place (University Lab), Controlled type and Amount of Water
Procedure
Method 1 - Extracting Lithium
Remove the outside plastic wrap
Remove the metal cap and case, slide out the core
Quickly put the core in mineral oil
Remove the lithium sheet from the core of the battery
Cut the lithium sheet into the desired size of pellets, or sheets.
Method 2 - Testing different water solutions
Prepare a beaker of 100 ml distilled water
Test temperature of water before
Put lithium in the water
Use a heat measuring device to record the heat of the reaction of the lithium in the water
Repeat this process 3 times
Repeat steps 1 through 5 with tap water and saline
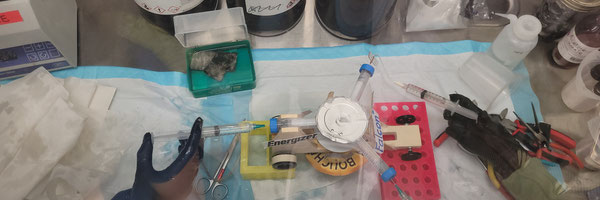
Method 3 - Using Lithium to Power a Car
Prepare a radial engine car (See fig. 1)
Extract lithium (See method 1)
Cut three pieces of lithium and put them in the piston chambers
Put water inside the piston chambers, one at a time
Allow space for lithium car to move forward
Repeat the process of steps 1 through 5, three times, with increasing in size lithium pieces
Observations
- The biggest temperature increase was in tap water
- Could be due to other chemicals and solutes in tap water
- The fastest reaction reaction was in distilled water
- Perhaps the impurities of saline (salt) and tap (minerals) prevented quick movement of the lithium by friction
- In the end, the thing that changed reaction time and temperature the most was the amount of lithium
Analysis
- The biggest temperature increase was in tap water
- Could be due to other chemicals and solutes in tap water
- The fastest reaction reaction was in distilled water
- Perhaps the impurities of saline (salt) and tap (minerals) prevented quick movement of the lithium by friction
- In the end, the thing that changed reaction time and temperature the most was the amount of lithium
Conclusion
We concluded that multiple types of water had differing benefits. On one hand, Distilled water proved to be the least heat productivity but also the shortest reaction time and on the other hand saline had the longest reaction time and middle of the pack heat production, and tap water had middle of the pack reaction speeds and the highest exothermic capability. Overall, we conclude that, in theory, Lithium could power a car, though we did not have the adequate materials or contructing ability to actually prove this theory.
Application
These expirements were all conducted to determine whether the exothermic reaction between raw lithium and dihydrogen monoxide would be capable to propel a model car, applying to real life as a substitute to a gasoline reaction.
Sources Of Error
- Weighing the lithium before the reaction may have given a more accurate result
- Lithium tarnishes quickly in air, changing the chemical composition of it
- Reaction is so quick it is hard to time accurately
- Next time we would need a better car model for our third part of the experiment (timing, valves, materials, etc…)
- The engine is the most important part of the car, however it was difficult to create because we only had some fragile materials and a lot of hot glue
- Even though our car did not go, we proved the concept of a lithium car by making the engine spin one and a half strokes (180 degrees)
Citations
References
Renjith Krishnan, Gokul Gopan, (2024) A comprehensive review of lithium extraction: From historical perspectives to emerging technologies, storage, and environmental considerations, Cleaner Engineering and Technology, 20, https://doi.org/10.1016/j.clet.2024.100749.
Royal Society of Chemistry, (2025, Jan 19) Lithium, https://periodic-table.rsc.org/element/3/lithium
Government of Canada, (2024, Dec 20) Lithium Facts, https://natural-resources.canada.ca/minerals-mining/mining-data-statistics-analysis/minerals-metals-facts/lithium-facts
David, R. R. (2021, Dec 14) Strategic Materials and Energy Transition: Lithium, Energy Industry Review https://energyindustryreview.com/metals-mining/strategic-materials-and-energy-transition-lithium/
Egan, T. (2022, Jan 13) What is lithium used for in renewable energy? https://energyx.com/blog/what-is-lithium-used-for-in-renewable-energy/
PyroChemMiner (2019, July 28) How to Disassemble a Battery to Extract Lithium [Video]. Youtube. https://www.youtube.com/watch?v=3cP65_2JyjU
Acknowledgement
I acknowledge and thank my dad for providing materials and lending me and my partner his lab.