Correlating Autoxidation Trends of Expanded Polystyrene Foam in Tenebrio Molitor Larvae with Gut Bacterial Biodiversity
Grade 12
Presentation
Hypothesis
Introduction
While it has been recognized that some insects can biodegrade plastic polymers, there is a knowledge gap regarding the specific mechanisms involved in insect-mediated plastic degradation (Siddiqui et al., 2024). Specifically, expanded polystyrene (EPS) is a widely used synthetic polymer that persists in the environment due to its resistance to natural decomposition—requiring centuries to autoxidize with prominent visual changes such as yellowing and brittleness, and only with drastically reduced molecular weight (Klemchuk, 1990; Nakatani et al., 2024). Studies have found that the larval form of the beetle species Tenebrio molitor (mealworms) is capable of EPS degradation, with research suggesting that their digestive system may be responsible for the breakdown of polystyrene into biodegradable byproducts (Yang et al., 2015; Brandon et al., 2018; Yang et al., 2018; Lou et al., 2021). It has been seen that this breakdown is partially a result of autoxidation within the mealworm gut, confirmed with Fourier Transform infrared (FT-IR) spectral analysis and pyrolysis–gas chromatography–mass spectrometry (py-GC/MS) analysis of autoxidation byproducts in mealworm frass (Nakatani et al., 2024). However, the extent to which this process is dependent on gut microbiota remains unclear (Yang et al., 2015; Liu et al., 2023). Some studies indicate that bacteria within the mealworm gut are responsible for the autoxidation of polystyrene, while others suggest that the mealworm itself may possess intrinsic enzymatic degradation mechanisms (Liu et al., 2023; Nakatani et al., 2024; Park et al., 2023; Tsochatzis et al., 2021). This project aims to investigate the role gut bacteria plays in mealworm-facilitated polystyrene degradation by comparing autoxidation levels in frass samples from both ampicillin-treated and untreated mealworms using FT-IR spectroscopy. Ampicillin was chosen on the basis of its general inhibition of bacterial, moderate extent of such, and accessibility. Other antibiotic inhibitors such as ciprofloxacin and norfloxacin, although noted as most effective in reducing gut bacteria, have been found to impair insect growth, development, and fecundity (Zhang et al., 2022; Kafil et al., 2013). Additionally, previous studies have confirmed that ampicillin is a viable antibiotic to be used in this experiment (Mamtimin et al., 2023). Concerns were raised on the mostly gram negative nature of gut bacteria compared to ampicillin’s ability to inhibit primarily gram positive bacteria, and moderately inhibit gram negative bacteria. To address this, ampicillin will be fed to the experimental group of mealworms in both the wheat bran diet phase, and the PS diet phase of the experiment to increase the gut’s exposure to the antibiotic. The criteria for which the autoxidation levels will be determined by the intensity of peaks in the FT-IR spectrums corresponding with hydroperoxide (at ~3550 cm⁻¹), alcohol and phenol groups (~3250 cm⁻¹), and carbonyl groups (~1900-1600 cm⁻¹) (Ranby & Lucki, 1980; Crabtree & Habib, 1991; Nakatani et al., 2024; Smith, 2017). These parameters were determined as most critical as they are produced as products of benzene ring cleavage, resulting from the introduction of oxygen functional groups by radical oxidative reactions (Tolinski, 2009). Presence of α,β-unsaturated ketones (~1670 cm⁻¹) as noted by Nakatani et al. will also be investigated, as it may indicate the occurrence of additional reactions other than oxidation in PS degradation (Nakatani et al., 2024). This study addresses a question incited from previous studies (Yang et al., 2015; Liu et al., 2023; Nakatani et al., 2024): How is the polystyrene-degrading–ability of mealworms (Tenebrio molitor) affected by the inhibition of gut microbial activity via ampicillin?
Question
How is the polystyrene-degrading–ability of mealworms (Tenebrio molitor) affected by the inhibition of gut microbial activity via ampicillin?
Hypothesis
If gut bacterial activity within mealworms (Tenebrio Molitor) is inhibited via an ampicillin treatment, the breakdown of polystyrene foam (PS) will be reduced compared to untreated mealworms, as observed through changes in the FT-IR spectra of the mealworms’ frass at specific wavenumbers indicative of autoxidative byproducts.
Rationale
The polystyrene degradation that mealworms are seen to promote is hypothesized to occur through a combination of both the activity of gut microbes and autoxidation: a process driven by reactive oxygen species (ROS). Studies have suggested that within the mealworm, polystyrene (PS) undergoes chain scission and benzene ring modification, with some key oxidation products being those of hydroperoxides, alcohols (specifically phenols), and α,β-unsaturated ketones. Thus, should key IR landmarks of these be less pronounced after inhibiting the gut bacteria with ampicillin, it would be seen that microbial processes do significantly contribute to these PS transformations.
Expected Effects
If gut bacterial activity does play a significant role in the degradation of PS, then the ampicillin-treated mealworms should exhibit:
- Reduced carbonyl/ketone formation, indicated by a reduced intensity in the ~1900–1600 cm-1 region, suggesting PS chain scission from hydroperoxides.
- Prominent presence of α,β-unsaturated ketones at ~1675 cm-1 indicate evidence for non-autoxidative decomposition reactions as a means for PS degradation.
- Reduced alcohol and phenol formation, indicated by a reduced intensity in the 3250 cm⁻¹ region.
- Reduced hydroperoxide formation, indicated by a weaker peak at ~3550 cm⁻¹, suggesting decreased oxidative chain scission.
Presence of α,β-unsaturated ketones (~1670 cm⁻¹) will also be investigated, as it may indicate the occurrence of additional reactions other than oxidation in PS degradation
If the observed IR spectra between the ampicillin-treated and untreated mealworms contain no significant differences, then it would be evidenced that digestive enzymes are the primary driver of PS breakdown, independent of any microbial activity.
Research
Understanding Polystyrene Structure and Properties
Polystyrene (PS) is a synthetic polymer consisting of repeating styrene monomers. Each monomer contains a benzene ring—a 6 carbon structure with conjugating (alternating) single and double bonds. This benzene ring in PS makes it highly stable and resistant to decomposition and benzene ring cleavage without disrupting processes such as the introduction of oxygen functional groups as a means to break the benzene ring structure (Montoya Sánchez & de Klerk, 2018). This inherent stability of PS, aided by its hydrophobic nature, means that natural decomposition processes such as autoxidation occur at extremely slow rates, or if molecular weight is significantly reduced (Klemchuk, 1990). However, Yang et al. has confirmed that larvae of Tenebrio molitor (mealworms) are capable of degrading polystyrene, with evidence suggesting that both gut microbiota and intrinsic enzymatic activity contribute to the PS’ breakdown (Yang et al., 2015; Liu et al., 2023). Fourier-transform infrared (FT-IR) spectroscopy and pyrolysis gas chromatography/mass spectrometry (py-GC/MS) analyses have revealed that radical oxidative reactions occur within the mealworm digestive system, leading to the formation of oxidation byproducts (Nakatani et al., 2024). Such discoveries have identified a specific type of oxidation, noted as autoxidation, to occur in the mealworm gut. This process occurs spontaneously but slowly in the presence of oxygen at normal temperatures and pressures, resulting in the cleavage of polymeric chains by free radicals (Tolinski, 2009).
Mechanisms of Oxidation: Reactive Oxidative Reactions and Reactive Oxygen Species
To understand the oxidative degradation of polystyrene in mealworms, the idea of free radicals, specifically reactive oxygen species, must be investigated:
First, it can be understood that autoxidation is a chemical reaction where a substance (usually an organic compound like plastic, fats, or oils) reacts with airborne oxygen without heat, light, or enzymes to start the process. Results of this process include changes in colours, structure, function, and molecular weight of a substance (Tolinski, 2009). Autoxidation is a specific form of oxidation where the breaking of bonds via chain scission is “self-propagating.”
During oxidation, reactive oxygen species (ROS), including hydroperoxides and hydroxyl radicals, initiate chain scission of the PS backbone. A ROS refers to a free radical—a highly reactive atom or molecule with at least one unpaired electron—that has reacted with oxygen. It can be understood that ROS are required for autoxidation to occur:
- Initiation: A molecule loses an electron, forming a highly reactive free radical. This can occur via oxygen exposure, ultraviolet (UV) radiation, or other chemical interactions. In autoxidation, initiation can stem from the formation of carbonyl and hydroxyl groups (Ranby & Lucki, 1980).
- Propagation: The free radical reacts with oxygen, forming ROS such as hydroperoxides and hydroxyl groups. These species result in further degradation, as this step prompts a cascade of chain scissions. The creation of ROS repeats like a chain reaction as hydroperoxides decompose further into ketones and alcohols (Crabtree & Habib, 1991).
- PS chain scission refers to the breaking of long polymer chains into shorter fragments via processes such as oxidation. The molecular weight of PS is reduced when this occurs. Klemchuk notes that a drastic reduction of molecular weight can result in the potential for plastic biodegradation (Klemchuk, 1990).
- Termination: The reaction terminates when two radicals combine or when the polymer is fully degraded into smaller byproducts and cannot be degraded further.
Radical oxidative reactions introduce oxygen functional groups into the benzene ring, leading to its cleavage into smaller, more reactive compounds. This cleavage process can transform the benzene ring into hydroperoxides, alcohols, or carbonyl groups (specifically simple ketones), which are more biodegradable and are indicated by changes in PS colour and strength. Further explanation of this degradation points to quinonization, and the addition of oxygen functional groups to a benzene ring to increase its instability.
Benzene Cleavage and Byproducts
Various oxidative byproducts can be observed in PS autoxidation in the mealworm gut. The extent of autoxidation in mealworm frass investigated in this experiment will be determined by the peak intensity of the following:
- Alcohol and Phenol Formation:
Oxidation introduces hydroxyl (-OH) groups into the molecular structure from benzene ring cleavage, forming phenols and alcohols. The presence of hydroxyls suggest that oxidation has partially broken down the polymer structure, making it more susceptible to further degradation via chain scission, as hydroxyls are a ROS. In specific, phenol groups (a specific alcohol: a benzene ring with a hydroxyl group) peak intensity detected on FT-IR spectra would indicate PS degradation via a phenolic intermediate (Nakatani et al., 2024). It has been confirmed previously that PS degradation relies partially on quinonization via this temporary phenolic product.
- Quinonization refers to the transformation of benzene rings or phenols (the identified temporary intermediate by Nakatani et al.) into quinones through oxidation. This process significantly increases the reactivity of the benzene, and its susceptibility to breakdown (Fang et al., 2005).
- A quinone is a compound derived from a benzene ring where two carbonyl (=O) groups replace hydrogen atoms in a para (opposite) or ortho (adjacent) position. Quinones derived from benzene makes for highly toxic ROS when in a redox cycle with semiquinones radicals—a special type of phenoxyl radical (Devi & Mehendale, 2014).
- Studies have also recognized that quinone presence aid in the yellowing decomposition of plastic products by autoxidation reactions (Allen, Edge, & Hussain, 2022).
- A quinone is a compound derived from a benzene ring where two carbonyl (=O) groups replace hydrogen atoms in a para (opposite) or ortho (adjacent) position. Quinones derived from benzene makes for highly toxic ROS when in a redox cycle with semiquinones radicals—a special type of phenoxyl radical (Devi & Mehendale, 2014).
Alcohols and phenols can be observed at wavenumber (~3250 cm⁻¹) on FT-IR spectra (Nakatani et al., 2024; Reusch, 2013; UCLA, 2001).
- Hydroperoxide Formation:
Hydroperoxide (ROOH) species are formed as oxidation products, and are a ROS necessary for PS chain scission. Hydroperoxide compounds contain the hydroperoxy functional group (ROOH), where R represents an organic group, and are unstable intermediates in processes such as oxidation. Hydroperoxide decomposition allows for carbonyl (specifically ketones) and alcohols to be derived (Crabtree & Habib, 1991). Hydroperoxides can be observed at wavenumber ~3550 cm⁻¹ (Nakatani et al., 2024; Reusch, 2013; UCLA, 2001).
- Studies have also recognized that hydroperoxides aid in the yellowing decomposition of plastic products by autoxidation reactions (Allen, Edge, & Hussain, 2022).
- Ketone Formation—Simple Ketones and α,β-Unsaturated Ketones:
Ketones is a type or organic compound containing a carbonyl group bonded to two carbon atoms with various other branches (C=O). Simple ketones are known oxidation byproducts that form when hydroperoxides decompose, leading to PS chain scission (Crabtree & Habib, 1991). The presence of simple ketones indicates oxidation of PS. Simple ketones can be detected in FT-IR spectra at wavenumber ~1900-1600 cm⁻¹ , corresponding to the characteristic carbonyl group (C=O) stretching vibration (Nakatani et al., 2024; Reusch, 2013; UCLA, 2001; Smith, 2017). Alpha and beta ketones are characterized by the position of the carbonyl group relative to the rest of the chemical structure—defined by the carbons’ distance from the carbonyl bond. The carbon directly next to the C=O is recognized as the alpha carbon, and the carbon one step away from that position is known as the beta carbon. Alpha ketones have relative chemical groups attached to the alpha carbon, while beta ketones have additional groups attached to the beta carbon.
- α,β-unsaturated ketones are a special type of ketone and are formed during more complex reactions, compared to simple ketones. Unlike simple ketones, α,β-unsaturated ketones contain a double bond (C=C) adjacent to the carbonyl (C=O), forming a conjugated system. As such, other chemical groups are attached to both the alpha and beta carbon that is supported by the C=C bond. Presence of α,β-unsaturated ketones suggest additional biochemical transformations, such as dehydrogenation or enzymatic modification of PS degradation intermediates, to be responsible for PS degradation (Nakatani et al., 2024). α,β-Unsaturated ketones can be detected at ~1675 cm⁻¹ in FT-IR spectra, where C=O stretching occurs with additional contributions from C=C vibrations (Nakatani et al., 2024; Reusch, 2013; UCLA, 2001).
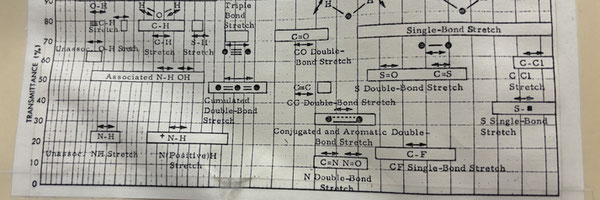
Understanding FT-IR Spectrometry
Fourier-transform infrared (FT-IR) spectrometry is an analytical technique for identifying molecular structures based on their IR energy absorption and measuring the amount at which a specific wavelength is absorbed. It operates by exposing a sample to a broad spectrum of infrared light , and observing the wave returned (CITE). A mathematical operation called the Fast Fourier Transform (FT) is applied to convert the raw data into an absorbance spectrum, wherein the returned values are separated into the transmittance per component infrared (IR) wavelength. The resulting spectrum shows peaks or dips, indicating the characteristic vibrational frequencies of potential specific functional groups in the sample. These signals can be used to identify structural changes occurring in a material, as dips and peaks at various regions on the IR spectra may correspond with different functional groups (CITE).
In the context of this experiment. the emergence of hydroxyl (-OH), carbonyl (C=O), and hydroperoxide (ROOH) groups indicative of oxidative cleavage of the benzene rings and main polymer chains (Crabtree & Habib, 1991; Ranby & Lucki, 1980) will be observed, and transmittances compared between the frass samples of control and experimental groups.
Quinonization
Quinonization refers to the chemical process by which quinones—a class of organic compounds characterized by a benzene ring with two ketone functional groups—are formed from other molecules. This transformation often occurs through autoxidation, initiated by ROS such as hydroxyl groups among others. This process is a significant step in the oxidation of benzene rings, as Nakatani et al. suggests that phenolic intermediates form as the PS polymer undergoes autoxidation, leading to the benzene ring-opening reactions and formation of quinones (Nakatani et al., 2024). The mechanisms of quinone formation in PS degradation in mealworms can be summarized into the following:
- Initial Oxidation: ROS attack the benzene rings in PS, forming hydroxyl (alcohol group) intermediates such as phenols. This phenol is recognized to be a temporary byproduct of oxidation that can be broken down further.
- Further Oxidation: Phenols undergo additional oxidation, resulting in its conversion to quinones.
- Dimer Fragmentation: Quinones and phenols can subsequently form dimer fragments, detected using pyrolysis-GC/MS (py-GC/MS) analysis. These fragments indicate additional polymer cleavage and oxidation. (Nakatani et al., 2024).
The detection of phenols in mealworm frass provides evidence that mealworms facilitate the oxidation of PS at low temperatures. This can be compared directly to conventional thermal degradation methods of plastics, which require high temperatures (>500°C) for efficient PS breakdown (Achilias et al., 2007; Mistry, Pandian, & Choksi, 2024).
Previous Studies and Known Discoveries
In 2015, Yang et al. confirmed that mealworms were able to digest PS, and Brandon et al. found that mealworms can consume PS containing hexabromocyclododecane (HBCD) flame retardants, with minimal retention of toxic additives (Yang et al., 2015; Brandon et al., 2019). Such a discovery incited many other researchers to study the degradation of various other plastics such as polyethylene and other forms of PS by mealworms (Brandon et al., 2018; Yang et al., 2018; Lou et al., 2021). Nakatani et al. recognized autoxidation to be responsible for this degradation, similar to that of lignin by white rot fungi, because it occurs at a low temperature, such as in the body (Watanabe, et a., 2003; Nakatani et al., 2024). In addition, the decrease in molecular weight of PS via chain scission and the low amount of cycloalkyl compounds such as HBCD in the mealworm body suggest that benzene and cycloalkyl ring degradation occurs in mealworms (Ranby & Lucki, 1980; Nakatani et al., 2024). Carbonyl, hydroxyl, and hydroperoxide group formation was determined as indicative of oxidation occurrence in PS on IR spectra due to the cascading nature of oxidation (Ranby & Lucki, 1980). These compounds were recognized as potential initiators to photochemical and oxidative reactions of PS (based on ROS). Chain branching in photo-oxidative reactions of PS were found to stem from the decomposition of hydroperoxides into free radicals that take part in the formation of carbonyl and hydroxyl groups (Ranby & Lucki, 1980). These understandings determined our dependent variables for this experiment.
Liu et al. has questioned the separation of gut microbiota from the gut microenvironment in past studies of the mealworm-mediated PS degradation. The idea of synergy between gut bacteria and intrinsic mealworm ability has been investigated as a result (Liu et al., 2023). The presence of enzymes responsible for initiating autoxidation reactions that result in benzene ring cleavage has also been speculated, however no reported cases have been confirmed (Nakatani et al., 2024). Currently, the notion that mealworm gut microbiota as responsible for PS degradation is accepted, as many strains of potential mealworm gut bacteria have been identified as PS degraders (Tsochatzis et al., 2021; Park et al., 2023). The aim of our study is to provide insight on this conflict through the use of a moderate antibiotic inhibitor, and the analysis of autoxidation byproducts on IR spectra.
Antibiotic Inhibition and Selection
Previously, it has been determined that antibiotics such as ciprofloxacin and norfloxacin are most effective in inhibiting gut biodiversity in insects such as Grapholita molesta (oriental fruit moth) and Eurygaster integriceps (sunn pest) (Zhang et al., 2022; Kafil et al., 2013). However, impaired insect growth, development, and fecundity were noted as a result of the highly-effective inhibition of bacterial symbionts necessary for healthy insect behaviour. It was inferred that a similar result would be observed if such antibiotics were applied to mealworms as a means of bacteria inhibition based on their shared insecta class and basic physical traits. It was hypothesized that such developmental differences between treated and un-treated mealworms would introduce additional behavioural factors that would hinder PS degradation abilities. Instead, we deemed that an alternative, more moderate, antibiotic inhibitor would be more suitable for this experiment. Ampicillin was chosen on the basis of its bacterial-inhibition properties, moderate extent of such, and accessibility. Previous studies confirmed that ampicillin is a viable antibiotic to be used in this experiment (Mamtimin et al., 2023). Concerns were raised on the mostly gram negative nature of gut bacteria compared to ampicillin’s ability to inhibit primarily gram positive bacteria, and moderately inhibit gram negative bacteria. To address this, ampicillin was fed to the experimental group of mealworms in both the wheat bran diet phase, and the PS diet phase of the experiment to increase antibiotic exposure to the gut.
Due to the uncertainty of the effectiveness of the ampicillin, a glucose solution of wet-autoclaved water and anhydrous dextrose will be provided in order to facilitate sugar consumption even when cellulose-degrading bacteria may be inhibited. Wheat bran is composed partially of cellulose, which can only be broken down by gut bacteria (Fujimori, 2021). No enzyme has been observed to have this trait. The addition of a glucose solution to the wheat bran will attempt to mitigate this possibility. Wheat bran was chosen as the main food source of the mealworms during the initial phase of the experiment for its alignment to a conventional mealworm diet.
Variables
Independent Variable
- The ampicillin treatment to inhibit gut bacterial activity in mealworms, or lack thereof.
Dependent Variable
- The degree to which the consumed polystyrene is digested, measured through the amount of autoxidative byproducts seen in FT-IR spectral changes at key wavenumbers. Such landmarks include reduced carbonyl/ketone formation (~1900-1600 cm⁻¹), reduced alcohol and phenol formation (3250 cm⁻¹), and reduced hydroperoxide formation (~3550 cm⁻¹). Presence of α,β-unsaturated ketones (~1670 cm⁻¹) will also be observed.
Controlled Variables
- Insect species (Tenebrio molitor)
- Environmental conditions (temperature, light exposure)
- Fluid amount available to the mealworms
- Polystyrene amount and type
- Mealworm diet other than and prior to polystyrene (i.e. wheat bran)
- Presence of external bacteria in the wheat bran
- Duration of time spent on the controlled wheat bran diet
- Duration of exposure to polystyrene
- Ampicillin concentration and dosage (within the experimental group)
- FT-IR analysis equipment and method
Procedure
Phase 1: Treating the Mealworms with Ampicillin
To each of six ethanol-sterilized containers, 20 grams of dry-autoclaved wheat bran (Purchased fromSave on Foods, brand Roger’s Mill) was added to serve as bedding and feed. For the three control containers, 8000 µL of wet-autoclaved water was introduced. Additionally, 2000 µL of 5% (5 g/100 mL) anhydrous dextrose (BDH9230) solution was incorporated to ensure that the mealworms could digest the cellulose-based wheat bran in the event that gut bacteria were inhibited by the antibiotic. The mixture was then homogenized via mixing using a spatula (Fig. 1).
Figure 1. 20 g wheat bran, 8000 µL water, 2000 µL dextrose solution, and 20 mealworms.
Figure 2. The container in Fig. 1, along with the other control and experimental groups.
For the experimental containers, the same procedure was followed, except that 1000 µL of water was replaced with 1000 µL of 10% (100 mg/mL) ampicillin (Sigma-Aldrich A5354) solution. Thus, each container received a total of 10 000 µL of fluid via pipette.
Of the 200 mealworms obtained from PetSmart, 120 were selected based on visual health then divided randomly into 6 groups of 20. Each group was placed into a designated container. The containers were then covered with dry-autoclaved paper towels and fitted with an askew lid to allow ventilation (Fig. 2). The mealworms were allowed to rest for 96 hours to facilitate ingestion of the antibiotic or lack thereof.
Phase 2: Obtaining Frass Samples
After 120 hours, the mealworms were extracted using autoclaved tweezers and kept separate. The wheat bran was disposed of, and all six containers were ethanol-sterilized. Each container was then supplied with a block of cut polystyrene foam and 10 plies of dry-autoclaved paper towel. The paper towels were moistened with 3000 µL of water (Fig. 3), with 300 µL of the water being substituted with 300 µL of ampicillin solution for the experimental groups.
Figure 3. Paper towels being doused with 3000 µL of water via pipette.
After both 24 and 48 hours, an additional 3000 µL of water was pipetted onto the paper towels (Fig. 3).
72 hours after the mealworms were introduced to the new containers, the mealworms were ethically disposed of. The polystyrene and paper towels were shaken to dislodge excess frass, which was collected and transferred onto clean sheets of weigh-boat paper. The paper was creased to facilitate the transfer of frass into six separate, labeled microcentrifuge tubes.
Phase 3: Analyzing Frass Samples
After 24 days, the frass samples were analyzed using a Cary 630 FT-IR Spectrometer (Fig. 4, Fig. 5). Depositing the samples onto the spectrometer plate, the frass from each of the 6 groups was analyzed and compared to reference libraries for spectral analysis. The plate and tip of the spectrometer arm were both disinfected via 70% ethanol between the analyses of each group.
Figure 4. Frass samples on the FT-IR spectrometer plate.
Figure 5. A Cary 630 FT-IR spsectometer.
Observations
Approximate Mass of Polystyrene Blocks Before and After Mealworm Consumption
|
Mass, hour 0 (g) |
Mass, hour 72 (g) |
|
|
Control #1 |
0.30 |
0.29 |
|
Control #2 |
0.30 |
0.26 |
|
Control #3 |
0.28 |
0.28 |
|
Experimental #1 |
0.29 |
0.25 |
|
Experimental #2 |
0.25 |
0.25 |
|
Experimental #3 |
0.30 |
0.27 |
Figure 6. Mass of polystyrene before and after the experiment. It must be noted that the scale was only accurate to 2 significant digits, and that although the blocks were cut to the same dimensions, differences in mass and density are present.
Number of Mealworms Within Each Container
|
Count, hour -120 |
Count, hour 0 |
Count, hour 72 |
|
|---|---|---|---|
|
Control #1 |
20 |
20 |
20 |
|
Control #2 |
20 |
20 |
20 |
|
Control #3 |
20 |
16 |
16 |
|
Experimental #1 |
20 |
20 |
20 |
|
Experimental #2 |
20 |
15 |
15 |
|
Experimental #3 |
20 |
16 |
16 |
Figure 7. Number of mealworms within each container at key points during the experiment. Several mealworms had died during the wheat bran consumption phase. Although the deaths were concentrated in C3, E2, and E3, which were closer to the back of the desk, it is unclear whether they could be amounted to coincidence or discrepancies in environmental conditions—of which we saw none. However, since it is the composition of frass and not the amount of it that is the dependent variable of this experiment, the amount of mealworms as long as they produce a usable amount of frass is largely irrelevant.
Figure 8. FT-IR spectrum of control group 1. Analyzed using a Cary 630 FT-IR spectrometer, at the University of Calgary under supervision.
Figure 9. FT-IR spectrum of control group 2.
Figure 10. FT-IR spectrum of control group 3.
Figure 11. FT-IR spectrum of experimental group 1.
Figure 12. FT-IR spectrum of experimental group 2.
Figure 13. FT-IR spectrum of experimental group 3.
Analysis
For PS degradation to occur, it is understood that the PS’ polymers must be cleaved. Reduction in polymer molecular weight is indicative of PS chain scission and benzene ring cleavage. This has already been proven in previous studies by analysing the molecular weight curves of PS pieces before and after degradation (Nakatani et al., 2024). Polymer cleavage can also be observed via comparisons of FT-IR spectra of treated and untreated mealworms shown in Figure 20, 21, and 22.
Through direct comparison of the FT-IR spectra in Figure 22, a drop in transmittance percentage at ~3550 cm-1 can be observed—the turning point attributed to hydroperoxide presence. The isolated presence of hydroperoxide at this region on the spectra is noted to be slightly larger in the experimental samples, demonstrated by Figure 24’s separation of transmittance percentages at specified regions: 3550 cm-1 for hydroperoxides, 3250 cm-1 for phenols, 1640 cm-1 for α,β-unsaturated ketones, and 1900-1600 cm-1 for other ketones. The slope decrease recognized at the region associated with hydroperoxides is notably more steep in the FT-IR spectra displaying the experimental samples, indicating an increase of hydroperoxide presence in experimental samples. This slope overlaps directly with the peaks observed at 3250 cm-1, indicative of OH groups characterized by the broad peak at this region. A notable difference at this wavenumber can be observed in Figure 22, which shows greater hydroxyl group presence in the antibotically-treated mealworm groups. Both peaks observed in this higher wavenumber region (4000-3000 cm-1) provide evidence which directly refutes our original hypothesis. The FT-IR spectra at both these peaks clearly indicate that there is higher ROS presence in the forms of hydroperoxides and hydroxyl groups in ampicillin-inhibited mealworm-mediated PS degradation compared to mealworm-mediated PS degradation without bacterial inhibition. Similar trends can be recognized in the 1900-1600 cm-1 region for carbonyl groups such as ketones. While the entirety of this region peaks greater with the experimental spectra compared to the control spectra, a drastic peak at ~1640 cm-1 can be observed and associated with α,β-unsaturated ketones presence in the frass samples (the location of this peak is noted to deviate slightly from other IR chart locations of ~1675-1670 (Nakatani et al., 2024; Reusch, 2013; UCLA, 2001)). Prominent α,β-unsaturated ketone presence in the FT-IR spectra indicate that additional reactions, outside of autoxidation, significantly contribute to the PS degradation process (Nakatani et al., 2024). A greater α,β-unsaturated ketone, and overall ketone peak, in the experimental spectra reveal a higher extent of PS breakdown in ampicillin treated mealworms. Such findings suggest more complex mechanisms of PS degradation than previous literature research indicated.
Through understanding these FT-IR spectra, evidence indicates that our original rationales for our expected outcome are proven to be incorrect. It can be recognized that exposure to ampicillin—an inhibitory antibiotic—increased polymer cleavage in mealworm-mediated PS degradation. The absorption of all noted oxidation byproducts (hydroperoxides, alcohols, ketones) is greater on all experimental spectras compared to control spectras. Additionally, the dramatic peaks at 1640 cm-1 indicate that autoxidation alone is not wholly responsible for PS breakdown in the mealworm gut. This new perspective can explain the library comparisons observed in Figures 14, 15, 16, 17, 18, and 19. Control spectra 1, 2, and 3, display top matches with analysed PS and synthetic samples from various accredited libraries—shown as 71.57 and 77.56 PS sample matches in C1 and C3 groups, and a C2 match with 4-phenyl-1-butanol at 59.61: a molecule with an intact benzene ring. In direct contrast, experimental spectra 1, 2, and 3, show library matches with organic samples associated with a local library—63.20 match in E1, 69.73 match in E2, both with flour starch, and an E3 match with yeast at 37.55. This trend clearly indicates a more effective transformation of PS, among other inorganic substances, to mealworm frass that should more closely resemble organic structures such as flour starch and yeast.
A Mann-Whitney U test was performed on the transmittances from all groups at 1640, 3250, and 3550 cm-1, as
- a Mann-Whitney U does not require any parametric assumptions,
- a two-sample t-test was not applicable to the data given that the FT-IR spectra do not follow a normal distribution, and
- any individual wavenumber did not have enough samples (n1 = 3, n2 = 3) to make a meaningful conclusion from a Mann-Whitney U. Thus, the key wavenumbers’ data was combined for the analysis.
The statistical analysis via a Mann-Whitney U Test revealed that, combined, the results from 1640, 3250, and 3550 cm-1 were not statistically significant. Using the following mathematical procedure:
|
Treated |
Untreated |
|
|
61.77913 |
1 |
|
|
65.79573 |
2 |
|
|
73.56097 |
3 |
|
|
74.9733 |
4 |
|
|
76.10325 |
5 |
|
|
79.52663 |
6 |
|
|
81.14297 |
7 |
|
|
81.58897 |
8 |
|
|
81.64367 |
9 |
|
|
88.06007 |
10 |
|
|
88.88923 |
11 |
|
|
89.2796 |
12 |
|
|
90.25195 |
13 |
|
|
90.49468 |
14 |
|
|
90.59111 |
15 |
|
|
92.1327 |
16 |
|
|
93.6126 |
17 |
|
|
94.18995 |
18 |
|
|
R1 = 65 |
R2 = 106 |
, it was determined that the U value of the test was 20. The critical value for n1 = 9 and n2 = 9 for an α of 0.05 is U <= 17. But due to the failure of U = 20 to satisfy this condition, the null hypothesis H0—that the differences in data are not statistically significant—is not rejected.
Highlighted regions in Figure 22 can also be considered. Evident peaks at ~2925 cm-1, ~1030 cm-1, and ~695 cm-1 can be noted on the control spectra (Figure 20), and peaks at ~2925 cm-1, ~1030 cm-1 can be observed on the experimental spectra (Figure 21). However, these specific regions were not defined to serve as criteria that determine the extent of PS degradation via autoxidation. The peaks at ~2925 cm-1 on the spectra can be hypothesized to represent a weak -C-H stretch, which may suggest that the hydrocarbon backbone of PS remains partially intact, even after degradation (UCLA, 2001). Greater peak intensity shown in the experimental spectra in Figure 25 can not be conclusively explained (average control transmittance percentage at ~86%, and average experimental transmittance percentage at ~78%), however may indicate that the PS degradation process does not remove all C-H groups. Similar observations can be recognized at ~1030 cm-1, a wavenumber region associated with a strong -C-OH stretch (UCLA, 2001). Analysis presented in Figure 25 shows the average transmittance percentage of the experimental spectra to be ~56%, compared with the average found in the control spectra: ~75%. It can be hypothesized that at this wavenumber region, strong presence of -C-OH bonds reflect the formation of hydroxyl groups that allow for further polymer breakdown via ROS (UCLA, 2001). Furthermore, additional comments can be made regarding the narrow peak observed at ~695 cm-1 on seemingly only the control spectra (Figure 20). Closer examination of the y-axis scale of transmittance percentage in Figure 22 reveals that the unique peak perceived in the control samples is actually shared in the experimental samples as well. Figure 25 evidences this, as the average control transmittance percentage at ~695 cm-1 is ~64%, while the average experimental is ~66%. Michigan State University notes that vibrations along this wavenumber are indicative of vibrational bending, specially in the form of -C-H deformation or bending. It can be hypothesized that such a peak is characteristic of mono-substituted benzene rings, which are present in intact polystyrene (Smith, 2021). As such, a potential explanation for the peaks, now understood to be present in both the control samples and experimental samples, can be hypothesized: some of the PS’ structure is not lost during mealworm-facilitated PS degradation.
Figure 14. Library analysis of control group 1.
Figure 15. Library analysis of control group 2.
Figure 16. Library analysis of control group 3.
Figure 17. Library analysis of experimental group 1.
Figure 18. Library analysis of experimental group 2.
Figure 19. Library analysis of experimental group 3.
Figure 20. FT-IR spectra of control groups 1, 2, and 3, superimposed
Figure 21. FT-IR spectra of experimental groups 1, 2, and 3, superimposed.
Figure 22. Superimposed FT-IR spectra of all groups, with key peaks marked. Dependent variables are marked with arrows and circles, while points of interest are highlighted
Figure 23. A graphic depicting the process of autoxidation and chain scission to fully digest polystyrene within a mealworm. (Nakatani et al., 2024)
Figure 24. Data results separated by treatment at specific wavenumbers. The wavenumbers of 1640, 3250, and 3550 cm-1 are used for statistical analysis via Mann-Whitney U test.
Figure 25. IR Transmittance at select wavenumbers of interest. Means of treatment are also shown.
Conclusion
The objective of this experiment was to investigate the role of gut bacteria in the mechanisms of mealworm-mediated PS degradation. It was hypothesized that inhibition of gut bacteria via ampicillin exposure to an experimental group would result in greater intensity of FT-IR peaks of autoxidation byproducts observed in an untreated control group. The results of the FT-IR spectra completely refuted this hypothesis; a positive correlation between bacteria inhibition and indicators of PS degradation was recognized, although this trend was not deemed statistically significant by a Mann-Whitney U test (U = 20). FT-IR spectra of the samples reveal consistently greater peak intensities in wavenumber regions that detect the presence of carbonyl groups, hydroxyl groups, hydroperoxides, and α,β-unsaturated ketones. Comparison of FT-IR spectra with reference libraries are also indicative of this trend—observed in the matches of the control spectra with inorganic compounds (including PS), and the matches of the experimental spectra with complex organic compounds such as flour starch and yeast. From this, the inference can be made that PS degradation in experimental groups was more effective than in control groups. Additional peaks at ~1030 cm-1, ~695 cm-1, and ~2925 cm-1 were also noted in all FT-IR spectra and suggest the notion of potential PS structure retention in degradation. The results of this experiment cannot be conclusively explained, as previous literature studies have confirmed PS breakdown via gut bacteria as a known and recognizable degradation pathway (Yang et al., 2015; Brandon et al., 2018; Yang et al., 2018; Lou et al., 2021; Tsochatzis et al., 2021; Park et al., 2023; Nakatani et al., 2024). Therefore the interpretation that mealworm gut bacteria inhibits PS degradation cannot be suggested or supported. The findings of this study suggest the complex nature of the mechanisms of mealworm-mediated PS degradation, and the potential for synergy between bacteria and other degradation pathways in mealworms (Liu et al., 2023). Repeated testing would be necessary to reliably confirm any of these results.
Application
Previous studies have emphasized the potential applications of the mealworm digestion of PS in plastic waste management, such as via effectively implementing methods to isolate the responsible bacterium for their observed contribution to the PS degradation (Tsochatzis et al., 2021; Park et al., 2023). Such potential applications are dependent on the idea that the mealworm gut microbiota is responsible, to an extent, for PS degradation processes such as autoxidation. This study attempted to fill a knowledge gap present in the literature regarding mealworm-mediated PS degradation: the extent to which gut microbiota and intrinsic mealworm abilities is responsible for PS autoxidation. The results of this experiment are inconclusive and fail to suggest that this process is primarily reliant in the mealworm gut. A Mann-Whitney U test confirms that our results are not statistically significant, and suggests a more complex mechanism for PS degradation than originally assumed. It can be hypothesized that there may be the occurrences of additional reactions other than autoxidation responsible for PS degradation. The source of these reactions was not confirmed. Other studies have noted that a lack of research has been conducted regarding the synergy between the gut microbiota and gut microenvironment, as a majority of literature focuses on the separation of PS-digesting gut bacterial species from the mealworm (Liu et al., 2023). The results of this project aids in this proposal, providing new insight into the mechanisms of mealworm-facilitated PS degradation for further research and future attempts of novel plastic waste management solutions.
Sources Of Error
Many of this project’s limitations pertain to the small sample size in its low n value. In total, the frass of 120 mealworms was collected: 60 per treatment category and 20 per group, over 3 trials. Although visually heterogeneous, the individual mealworms’ frass were combined and recognized as one inseparable substance to be analysed with the Cary 630 FT-IR Spectrometer: a spectrometer requiring a sample of large enough size to operate accurately. It can be understood from this that only 6 points of IR transmittance data are available at any specific wavenumber, rendering meaningful statistical analysis at such wavenumber infeasible. Therefore, a Mann-Whitney U test was performed such that data could be accumulated from multiple wavelengths (McClenaghan, 2024). General trends were able to be observed, but further extrapolation was not possible. This effect was compounded by potential losses in frass during transfers from the containers to the bullet tubes and to the spectrometer, as there is a minimum number of mealworms per group required to attain a usable quantity of frass; thus, more experimental and control groups could not be created lest the needed amount of frass collected be compromised.
- Intrinsic differences between Tenebrio molitor larvae may have also contributed to potential error in this experiment. Although 20 mealworms per group was likely enough to avoid significant deviations from an expected result, this would have been ensured through a higher sample size per container. It is recognized that more groups, larger groups, and more trials would have led to a more accurate and repeatable experiment.
The initial hypothesis of this experiment also fails to consider the nuance in that gut microbial activity likely impacts the mealworms’ intrinsic digestive processes and vice versa. As such, by inhibiting one, noted to be the bacteria in this study, it would have likely have had an unseen impact on the processes of the mealworm, and therefore the FT-IR results independent of solely the inhibition of gut bacteria. Liu et al. speaks about the common separation of the mealworm gut microbiota in research regarding mealworm-mediated PS degradation (Liu et al., 2023). A synergistic relationship between the gut microbiota and gut microenvironment (including enzymes) can be considered.
Uniformity of frass samples can also be recognized as an experimental source of error in the FT-IR analysis stage by their impact on the spectrometer results. Evidently, the frass samples of all groups were heterogeneous in their composition: indicated by colour differences observed between frass pellets. As such, it can be understood that the FT-IR spectra derived may inaccurately represent the amount, and concentration of functional groups, crushed under the spectrometer’s arm, observed in the entire sample. However, even with this limitation, a trend can still be drawn, as the transmittance observed on the IR spectra indicates a consistency shared between control and experimental groups at specific key points on the spectra. To mitigate this error, powdering the frass samples and mixing them with KBr before the FT-IR analysis could be considered in future experiments.
Citations
References
Achilias, D. S., Kanellopoulou, I., Megalokonomos, P., Antonakou, E., & Lappas, A. A. (2007). Chemical recycling of polystyrene by pyrolysis: Potential use of the liquid product for the reproduction of polymer. Macromolecular Materials and Engineering, 292(8), 923–934. https://doi.org/10.1002/mame.200700058
Allen, N. S., Edge, M., & Hussain, S. (2022). Perspectives on yellowing in the degradation of polymer materials: Inter-relationship of structure, mechanisms and modes of stabilisation. Polymer Degradation and Stability, 201, 109977. https://doi.org/10.1016/j.polymdegradstab.2022.109977
Brandon, A. M., El Abbadi, S. H., Ibekwe, U. A., Cho, Y.-M., Wu, W.-M., & Criddle, C. S. (2019). Fate of Hexabromocyclododecane (HBCD), a common flame retardant, in polystyrene-degrading mealworms: Elevated HBCD levels in egested polymer but no bioaccumulation. Environmental Science & Technology, 54(1), 364–371. https://doi.org/10.1021/acs.est.9b06501
Brandon, A. M., Gao, S.-H., Tian, R., Ning, D., Yang, S.-S., Zhou, J., Wu, W.-M., & Criddle, C. S. (2018). Biodegradation of polyethylene and plastic mixtures in mealworms (larvae of Tenebrio molitor) and effects on the gut microbiome. Environmental Science & Technology, 52(11), 6526–6533. https://doi.org/10.1021/acs.est.8b02301
Crabtree, R. H., & Habib, A. (1991). Comprehensive organic synthesis: Oxidation by Chemical Methods (Vol. 7). Pergamon Press.
Devi, S. S., & Mehendale, H. M. (2014). Quinone. Encyclopedia of Toxicology, 26–28. https://doi.org/10.1016/c2010-1-66187-1
Fang et al. (2005). Toxicology and Pharmacology (Vol. 205).
Fujimori, S. (2021). Humans have intestinal bacteria that degrade the plant cell walls in herbivores. World Journal of Gastroenterology, 27(45), 7784–7791. https://doi.org/10.3748/wjg.v27.i45.7784
Kafil, M., Bandani, A. R., Kaltenpoth, M., Goldansaz, S. H., & Alavi, S. M. (2013). Role of symbiotic bacteria in the growth and development of the sunn pest,eurygaster integriceps. Journal of Insect Science, 13(1), 1–12. https://doi.org/10.1673/031.013.9901
Klemchuk, P. P. (1990). Degradable plastics: A critical review. Polymer Degradation and Stability, 27(2), 183–202. https://doi.org/10.1016/0141-3910(90)90108-j
Liu, Q., Wu, H., Sun, W., Lu, Y., & Zhang, H. (2023). Cooperation between Tenebrio Molitor (mealworm larvae) and their symbiotic microorganisms improves the bioavailability of polyethylene. Journal of Polymers and the Environment, 31(9), 3925–3936. https://doi.org/10.1007/s10924-023-02843-9
Lou, Y., Li, Y., Lu, B., Liu, Q., Yang, S.-S., Liu, B., Ren, N., Wu, W.-M., & Xing, D. (2021). Response of the yellow mealworm (Tenebrio molitor) gut microbiome to diet shifts during polystyrene and polyethylene biodegradation. Journal of Hazardous Materials, 416, 126222. https://doi.org/10.1016/j.jhazmat.2021.126222
Mamtimin, T., Han, H., Khan, A., Feng, P., Zhang, Q., Ma, X., Fang, Y., Liu, P., Kulshrestha, S., Shigaki, T., & Li, X. (2023). Gut microbiome of mealworms (Tenebrio molitor larvae) show similar responses to polystyrene and corn straw diets. Microbiome, 11(1). https://doi.org/10.1186/s40168-023-01550-w
McClenaghan, E. (2024, March 25). Mann-Whitney U test: Assumptions and Example. Informatics from Technology Networks. https://www.technologynetworks.com/informatics/articles/mann-whitney-u-test-assumptions-and-example-363425
Mistry, C. R., Pandian, S., & Choksi, H. (2024). Investigating the impact of microwave and conventional heating sources on the co-pyrolysis of plastics with non-biomass materials. Journal of Analytical and Applied Pyrolysis, 177, 106364. https://doi.org/10.1016/j.jaap.2024.106364
Montoya Sánchez, N., & de Klerk, A. (2018). Autoxidation of Aromatics. Applied Petrochemical Research, 8(2), 55–78. https://doi.org/10.1007/s13203-018-0199-4
Nakatani, H., Yamaura, Y., Mizuno, Y., Motokucho, S., Dao, A. T., & Nakahara, H. (2024). Biodegradation mechanism of polystyrene by mealworms (Tenebrio Molitor) and nutrients influencing their growth. Polymers, 16(12), 1632. https://doi.org/10.3390/polym16121632
Park, J.-W., Kim, M., Kim, S.-Y., Bae, J., & Kim, T.-J. (2023). Biodegradation of polystyrene by intestinal symbiotic bacteria isolated from mealworms, the larvae of Tenebrio Molitor. Heliyon, 9(6). https://doi.org/10.1016/j.heliyon.2023.e17352
Ranby, B., & Lucki, J. (1980). New aspects of photodegradation and photooxidation of polystyrene. Pure and Applied Chemistry, 52(2), 295–303. https://doi.org/10.1351/pac198052020295
Reusch, W. (2013, May 5). Group Frequencies. Infrared Spectroscopy. https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/infrared/infrared.htm
Siddiqui, S. A., Abdul Manap, A. S., Kolobe, S. D., Monnye, M., Yudhistira, B., & Fernando, I. (2024). Insects for plastic biodegradation – a review. Process Safety and Environmental Protection, 186, 833–849. https://doi.org/10.1016/j.psep.2024.04.021
Smith, B. C. (2017). The Carbonyl Group, Part I: Introduction. Spectroscopy, 32(9), 31–36.
Smith, B. C. (2021). The infrared spectra of polymers III: Hydrocarbon polymers. Spectroscopy, 22–25. https://doi.org/10.56530/spectroscopy.mh7872q7
Tolinski, M. (2009). Additives for polyolefins: Getting the most out of polypropylene, polyethylene and TPO. William Andrew Pub.
Tsochatzis, E., Berggreen, I. E., Tedeschi, F., Ntrallou, K., Gika, H., & Corredig, M. (2021). Gut microbiome and degradation product formation during biodegradation of expanded polystyrene by mealworm larvae under different feeding strategies. Molecules, 26(24), 7568. https://doi.org/10.3390/molecules26247568
UCLA. (2001, March 9). Infrared Spectroscopy Table. IR table. https://www.chem.ucla.edu/~bacher/General/30BL/IR/ir.html
Watanabe, T., Katayama, S., Enoki, M., Honda, Y., & Kuwahara, M. (2003). Formation of acyl radical in lipid peroxidation of linoleic acid by manganese‐dependent peroxidase from ceriporiopsis subvermispora and Bjerkandera Adusta. European Journal of Biochemistry, 267(13), 4222–4231. https://doi.org/10.1046/j.1432-1033.2000.01469.x
Yang, S.-S., Brandon, A. M., Andrew Flanagan, J. C., Yang, J., Ning, D., Cai, S.-Y., Fan, H.-Q., Wang, Z.-Y., Ren, J., Benbow, E., Ren, N.-Q., Waymouth, R. M., Zhou, J., Criddle, C. S., & Wu, W.-M. (2018). Biodegradation of polystyrene wastes in yellow mealworms (larvae of Tenebrio Molitor Linnaeus): Factors affecting biodegradation rates and the ability of polystyrene-fed larvae to complete their life cycle. Chemosphere, 191, 979–989. https://doi.org/10.1016/j.chemosphere.2017.10.117
Yang, Y., Yang, J., Wu, W.-M., Zhao, J., Song, Y., Gao, L., Yang, R., & Jiang, L. (2015). Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 1. Chemical and physical characterization and isotopic tests. Environmental Science & Technology, 49(20), 12080–12086. https://doi.org/10.1021/acs.est.5b02661
Zhang, X., Wang, X., Guo, Z., Liu, X., Wang, P., Yuan, X., & Li, Y. (2022). Antibiotic treatment reduced the gut microbiota diversity, prolonged the larval development period and lessened adult fecundity of Grapholita Molesta (Lepidoptera: Tortricidae). Insects, 13(9), 838. https://doi.org/10.3390/insects13090838
Acknowledgement
We would like to thank the following for their support throughout this project:
- Mr. Brant Pohorelic, MSc at the University of Calgary, who provided us the lab space, chemicals, and equipment for our experiment.
- Ms. Michelle Forgeron, PhD at the University of Calgary, who organized access to the lab and its equipment, using her budget to purchase antibiotics. We would also like to thank Ms. Forgeron for connecting us with appropriate expertise during the various phases of our project.
- Mr. Jianjun Li, Instrumentation Technician at the University of Calgary, who taught and assisted us with the FT-IR spectrometers.